Abstract
Insulin is known to attenuate septic shock-induced myocardial depression. Possible mechanisms include an anti-inflammatory or inotropic effect of insulin. The objective of this study was to determine whether the mechanism of action of insulin in attenuating septic shock-induced myocardial depression is through an immunomodulatory effect. Fourteen pigs were assigned to one of two groups. Both groups received a 4-h infusion of lipopolysaccharide endotoxin from Escherichia coli 0111:B4. Group 2 additionally received insulin at 1.5 U/kg/h with infusions of D50 normal saline and KCl to maintain normal serum glucose and potassium levels. Cardiac function was measured with shortening fraction using transthoracic echocardiogram. Plasma TNF-α, IL-1β, and IL-6 levels were obtained every 30 min. Postmortem cytokine analysis and histomorphology were performed on the heart tissue. Although insulin attenuated septic shock-induced myocardial depression, this was not due to an anti-inflammatory effect and, therefore, likely resulted from an inotropic effect of insulin.
Keywords: high-dose insulin, severe sepsis, cardiac dysfunction, heart failure, myocardial dysfunction
INTRODUCTION
Insulin has been shown to improve hemodynamics and cardiovascular function by ameliorating myocardial depression associated with the systemic inflammatory response [1–3]. It is not known if these beneficial cardiovascular effects of insulin are related to the anti-inflammatory effect of insulin in terms of decreasing circulating cytokines, which are known to be cardiac depressants, or if insulin improves cardiovascular function in sepsis through improving inotropy of the myocytes, or if there is another mechanism that has not yet been defined. In this study, we evaluated the immunomodulatory effect that high-dose insulin has on the heart in a porcine model of septic shock to determine whether decreasing the effect of the inflammatory profile plays a role in the improved hemodynamics seen with insulin during severe sepsis. Given the fact that a number of the inflammatory cytokines contribute to myocardial depression in sepsis [4–8] and that insulin decreases the production of these cytokines [9–13], it is our hypothesis that the immunomodulating effect of insulin is responsible for attenuating the myocardial depression seen with sepsis.
MATERIALS AND METHODS
The protocol in this study was approved by our Institutional Animal Care and Use Committee. Fourteen Landrace–Yorkshire pigs weighing 12–16 kg were used in this study.
Anesthesia
The pigs received initial sedation with two intramuscular injections of 1 ml/kg of KAX, an anesthetic cocktail containing ketamine 23 mg/ml, azepromazine 0.58 mg/ml, and xylazine 0.8 mg/ml, administered 10 min apart. Electrocardiogram (ECG) electrodes and a pulse oxymeter probe were placed for cardiopulmonary monitoring.
Following placement of arterial and venous catheters using standard cut-down techniques, the pigs were then intubated orally or though a tracheostomy using a cuffed endotracheal tube (5.0–6.0 mm internal diameter). Using an Puritan Bennett 840 (Nellcor Puritan Bennett, Carisbad, CA, USA) or VIP Bird ventilator (Bird Products, Palms Spring, CA, USA), the animals were ventilated using volume control mode ventilation, with a tidal volume of 5–8 ml/kg, rate of 25–35 breaths per minute, positive end expiratory pressure of 5 cm H2O, and FiO2 of 1.0. Arterial blood gasses were obtained at 30-min intervals using an i-STAT 1 blood chemistry analyzer (Abbott, Princeton, NJ, USA). Minute ventilation was adjusted to maintain PaCO2 35–45 mmHg.
Continuous infusions of KAX were initiated at a dose of 9.2 mg/kg/h of ketamine, 0.23 mg/kg/h of azepromazine, and 0.32 mg/kg/h of xylazine. Additional IM doses of 5 ml of KAX were given as needed for sedation. Sufentanil was also started at 2–3 μg/kg/h and was titrated to ensure adequate anesthesia.
Experimental Protocol
Arterial blood gas and a serum cytokine level were obtained at baseline. A baseline transthoracic echocardiogram was obtained, and the shortening fraction was calculated from the parasternal short axis view at the papillary muscle level (Fig. 1). A single attending pediatric cardiologist, who was blinded to the animals’ grouping, reviewed all echocardiographic studies.
Fig. 1.
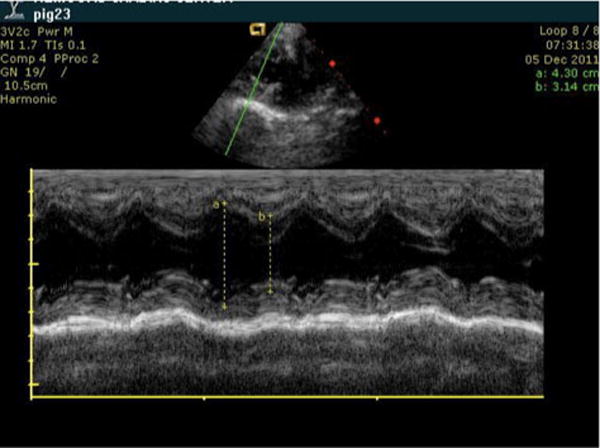
Representative echocardiographic image demonstrating how the measurements were obtained for the shortening fraction. After obtaining maximum and minimum distances of the left ventricle along the short axis, the shortening fraction was calculated using the equation [(max−min)/max]. Measurements were taken using parasternal short axis view at the level of the papillary muscles.
The pigs were randomly assigned to one of two groups: group 1 (control group, n=7) or group 2 (insulin group, n=7). Both groups received a continuous intravenous infusion of lipopolysaccharide (LPS) from Escherichia coli serotype 0111:B4 (Sigma-Aldrich, St. Louis, MO, USA), which has been shown to induce a hypodynamic cardiovascular state that mimics the hemodynamic effects of septic shock typically seen in the pediatric patient population [14–16]. The infusion began at 0.625 μg/kg/h and was doubled every 15 min to a maximum dose of 5 μg/kg/h. The infusion was maintained at this maximum rate for 1.25 h and then dropped to the initial dose of 0.625 μg/kg/h for the remainder of the study (total=300 min). Both groups received an infusion of 0.9% normal saline at a rate of 20 ml/kg/h that was started simultaneously with the toxin infusion to maintain the intravascular volume during the toxin infusion. Additional fluid boluses of 10–20 ml/kg of 0.9 % normal saline were administered as needed to maintain systolic blood pressure above 70 mmHg and diastolic blood pressures above 30 mmHg. Group 1 also received 0.9 % normal saline with 10 % dextrose and 20 mEq/l of potassium chloride (KCl) at a maintenance rate. Group 2 received dextrose containing fluids as described in the algorithm below.
In addition to the LPS, group 2 received regular insulin (Novolin R, Novo Nordisk, Princeton, NJ, USA) at an infusion rate of 1.5 U/kg/h. This dose has been shown in prior studies to improve cardiac function in states of impaired cardiac function and to provide an anti-inflammatory effect [1, 9]. Bedsides, glucose checks were performed on both groups every 30 min. Group 2 received continuous fluid infusions from two different solutions: one bag containing 0.9 % normal saline and a second bag containing normal saline with 50 % dextrose (D50) and 20 mEq/l of potassium chloride (KCl). The fluids were titrated between the two solutions to achieve a target blood glucose of 100–200 according to the following algorithm:
The fluids were started with the 50 % dextrose solution infused at a maintenance rate and the 0.9 % normal saline infused at a rate of 20 ml/kg/h minus the volume of the insulin.
If blood glucose dropped below 100 mg/dl on the bedside glucose test, the D50 normal saline solution was increased by 20 % with a parallel decrease in the 0.9 % normal saline.
If blood glucose was greater than 200, the D50 normal saline solution was decreased by 20 % with a parallel increase in the 0.9 % normal saline.
This algorithm was followed with each blood glucose check.
Serum electrolytes were analyzed every 30 min using an i-STAT blood chemistry analyzer (Abbott). When the serum potassium fell below 3 mEq/l, a continuous infusion of KCl was started at 1 mEq/kg/h. The rate of the KCl infusion was adjusted throughout the study to maintain the serum potassium greater than 3 mEq/l.
Plasma levels of TNFα, IL-1β, and IL-6 were obtained at 30-min intervals throughout the protocol. The shortening fraction was reevaluated using transthoracic echocardiogram 2 h after the LPS infusion was initiated, using the same parameters as described earlier.
At the end of the 300-min protocol, the animals were humanely killed with an intravenous bolus of ketamine (30 mg/kg) as well as 3 μg/kg of sufentanil. This was followed by thoracotomy and pulmonary exsanguination by isolating the pulmonary artery and flushing the lungs with Millonig buffer solution (37.42 g NaH2PO4:H20, 9.66 gm NaOH, 17.4 g glucose, and 220 U heparin).
Samples (approximately 2 cm3) of the left and right ventricles were harvested and placed in 10 % buffered formalin for subsequent paraffin embedding, sectioning, and staining with hematoxylin and eosin for histological evaluation. Additional samples of both ventricles were instantly obtained and snap frozen in liquid nitrogen. The samples were stored at −70 °C until assaying for quantitative cytokine analysis.
Histology Analysis
Formalin-fixed cardiac tissue was processed following paraffin embedding. Sections of 5 μM were cut for slide preparation. Tissue was stained with hematoxylin and eosin and visualized at 10–200× magnification using an Eclipse 80i light microscope (Nikon, Tokyo, Japan) equipped with a digital camera (Digital Sight DS-SM; Nikon).
Cytokine Analysis
Plasma was obtained at 30-min intervals throughout the study protocol. These samples were centrifuged immediately at 2000 RCF for 20 min at 4 °C. The plasma was separated from the supernatant, quick frozen in liquid nitrogen, and placed in a freezer at −70 °C where they were stored until the cytokine studies were performed.
Quantitative cytokine analysis was performed on tissue homogenates of the right and left ventricles. The previously frozen samples were removed from the −70 °C freezer and placed on dry ice. Right ventricle and left ventricle tissues (200 mg) were separated from the stock into a microcentrifuge tube and crushed using scissors and a pellet pestle attached to a motor. The sample was then homogenized in 1 ml of radioimmunoprecipitation assay (RIPA) buffer (Sigma-Aldrich) with protease inhibitor cocktail (Sigma-Aldrich) at 1:100 dilution. Five microliters of RIPA buffer was used for 1 mg heart tissue. The sample was then incubated on ice for 5 min, after which it was centrifuged at 12,000g for 10 min at 4 °C. The supernatant containing the soluble protein was transferred to tubes for storage, quick frozen in liquid nitrogen, and stored at −70 °C for future use.
The levels of TNF-α, IL-6, and IL-1β in the plasma and tissue homogenates were measured with quantitative ELISA using commercially available porcine TNF-α, IL-6, and IL-1β ELISA kits (R&D Systems, Minneapolis, MN, USA). All samples were appropriately diluted to fall within the detection range of each assay, and all samples and standards were assayed in duplicate.
The test sensitivity for respective immunoassays was as follows: TNF-α (2.8–5.0 pg/ml), IL-6 (0.68–4.30 pg/ml), and IL-1β (2.65–13.6 pg/ml). Inter- and intra-assay coefficients of variance were less than 10 %.
Statistical Analysis
Baseline and postbaseline measurements are summarized by mean and standard error (SE) of mean presented as a function of group and time. A two-sample t test was used to compare the mean baseline characteristics between the control and insulin groups. An analysis of covariance model was performed to compare the mean hemodynamic parameters (heart rate, mean arterial pressure [MAP], mean airway pressure [MAWP], PIP, PaO2/FiO2, base deficit, and shunt fraction) at 2 h postinjury between the control and insulin groups after adjusting for baseline. The change from baseline measurement was used as the response, the group (control vs. insulin) as a factor, and the baseline measurement as a covariate. A mixed-model, repeated-measures analysis of variance was used to compare the mean differences of natural log-transformed cytokines (TNF-α, IL-1β, and IL-6) in plasma between the control and insulin groups over time. The natural log-transformed heart tissue cytokine (of left ventricle and right ventricle) measurements (TNF-α, IL-1β, and IL-6) of the control and insulin groups were compared using a two-sample t test. All analyses were two-tailed at 5 % level of significance and were performed using the statistical package SPSS version 19.0 (SPSS, Chicago, IL, USA). Significance was accepted at P>0.05 (n=7) for each group.
RESULTS
The continuous intravenous infusion of LPS as described above resulted in observable changes in vital signs and ventilator support in several pilot studies. These studies were performed to establish the timeframe and current septic shock-induced myocardial depression in a porcine model. In the current prospective study, three of the animals died suddenly within the last hour, before the end of the study, because of development of a pneumothorax, which was related to an unforeseen ventilator malfunction. Because of the attrition of animals and the acute nature of this model (pilot studies), we elected to report our hemodynamic parameters and shortening fraction data for each group at baseline and at 2 h to capture a good sample size of viable animals. As shown in Table 1, at baseline, there was little difference between groups with respect to hemodynamics with the exception of MAP (one animal had a large drop in MAP. We did not consider this difference in baseline MAP between the two groups to be a clinically significant finding, given the fact that this parameter was affected by a transient drop of baseline MAP in one animal.) At 2 h, there was a trending deterioration in hemodynamics (increase in heart rate, MAWP, PIP, and shunt fraction; decrease in PaO2/FiO2) in both groups.
Table 1.
Hemodynamics Comparison at Baseline and 2 h for Each Group
| BL | Control | Insulin | P | 2 h | Control | Insulin | P |
|---|---|---|---|---|---|---|---|
| HR (min−1) | 98.4 (8.6) | 107.9 (7.4) | 0.42 | HR (min−1) | 116 (6.8) | 113.4 (11.0) | 0.774 |
| MAP (mmHg) | 85 (6.4) | 65.9 (3.9) | 0.03 | MAP (mmHg) | 69.4 (5.6) | 67.6 (2.0) | 0.973 |
| MAWP (cm H2O) | 9.8 (0.5) | 10.0 (0.6) | 0.77 | MAWP (cm H2O) | 11.7 (0.8) | 11.0 (1.2) | 0.575 |
| PIP (cm H2O) | 19.3 (1.6) | 19.4 (1.7) | 0.95 | PIP (cm H2O) | 26.1 (2.6) | 25.3 (2.6) | 0.816 |
| PaO2/FiO2 | 445.6/45.9 | 438.4/14.0 | 0.87 | PaO2/FiO2 | 283.8/86.9 | 355/57.6 | 0.47 |
| Shunt fraction | 11.9 (2.0) | 12.5 (0.7) | 0.76 | Shunt fraction | 18.5 (3.8) | 15.7 (2.3) | 0.464 |
Physiological parameters (means ± SEM) measured as a function of group at baseline and 2 h
BL baseline, HR heart rate, MAP mean arterial pressure, MAWP mean airway pressure, PIP peak inspiratory pressure, P is the P value to compare baseline-adjusted mean hemodynamics between control and insulin group
Shortening Fraction
Results from the mean shortening fraction analysis are presented in Fig. 2 as a function of time (baseline and 120 min) and group (control and insulin). As shown, the mean shortening fraction decreased 27 % for the animals in the control group (P=0.019) but not for the animals in the insulin group (decline of 12 %, P=0.213).
Fig. 2.
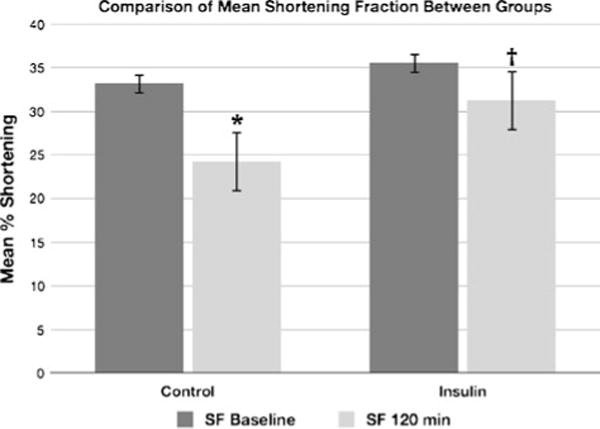
Difference in shortening fraction from baseline measurements to measurements obtained 2 h after initiation of the toxin infusion in the insulin and control groups. *P=0.019. †P=0.213.
Serum Cytokine Levels
The differences in mean serum cytokine levels between the two groups, looking at both time and group effects, are presented in Figs. 3, 4, and 5. As noted in Fig. 3b, the mean IL-1β concentrations in plasma for the control and insulin groups are presented as a function of time during the experiment. Natural log-transformed mean plasma IL-1β was different between the groups at 2, 2.5, 3, and 3.5 h (P>0.05). In addition, there was an increase in natural log-transformed mean IL-1β (time effect) for the control group at 2.5, 3, and 3.5 h and for the insulin group at 3.5 h.
Fig. 3.
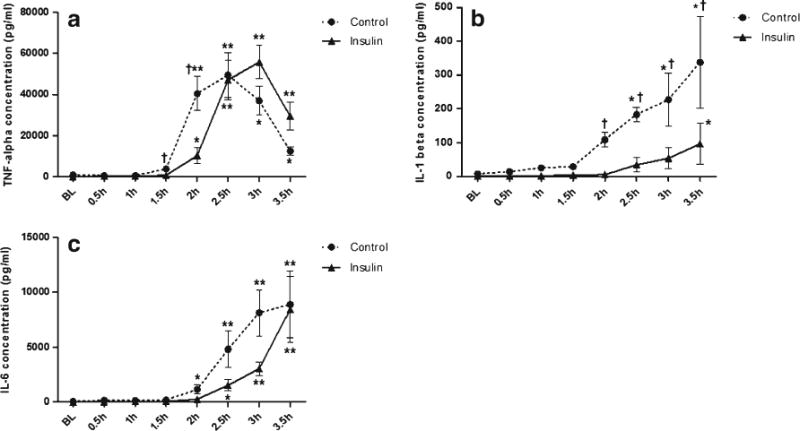
a TNF-α concentrations in plasma for the control and insulin groups during the experiment. Mean natural log-transformed plasma TNF-α was significantly different between groups at 1.5 and 2 h (P>0.05); TNF-α increased for both groups over time and peaked at 2.5 h for the control group and 3 h for the insulin group. Data are mean ± SEM and n=7 for each condition. *P>0.05 for time; **P>0.001 for time; †P>0.05 for group. b IL-1β concentrations in plasma for the control and insulin groups during experiment. Natural log-transformed mean plasma IL-1β was significantly different between groups at 2, 2.5, 3, and 3.5 h (P>0.05); there was a significant increase in natural log-transformed mean IL-1β (time effect) for the control group at 2.5, 3, and 3.5 h and for the insulin group at 3.5 h. Data are mean ± SEM and n=7 for each condition. *P>0.05 for time; †P>0.05 for group. c IL-6 concentrations in plasma for the control and insulin groups during the experiment. Plasma IL-6 showed no significant difference between groups (P<0.05). IL-6 increased over time for both groups. Data are mean ± SEM and n=7 for each condition. *P>0.05 for time; **P>0.001 for time.
Fig. 4.
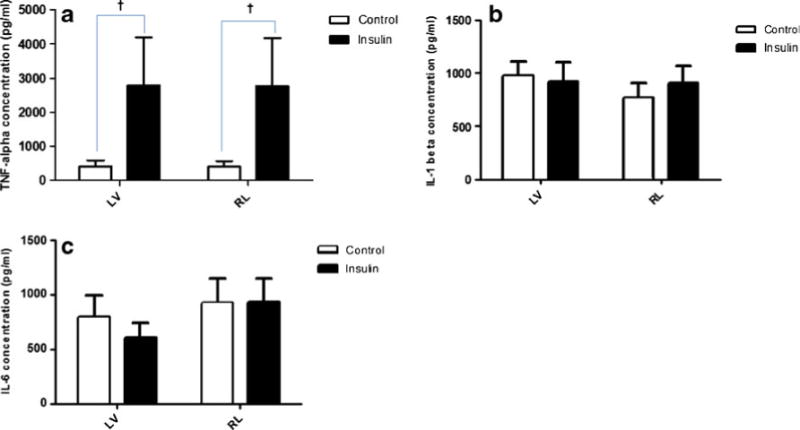
TNF-α (a), IL-1β (b), and IL-6 (c) concentrations in heart homogenates from left and right ventricles at the endpoint of the experiment for the control and insulin groups. The mean TNF-α concentration of the insulin group was greater than that of the control group (P>0.05). There was no significant difference between the groups in mean IL-1β and IL-6. Data are mean ± SEM and n=7 for each condition. †P>0.05 for group.
Fig. 5.
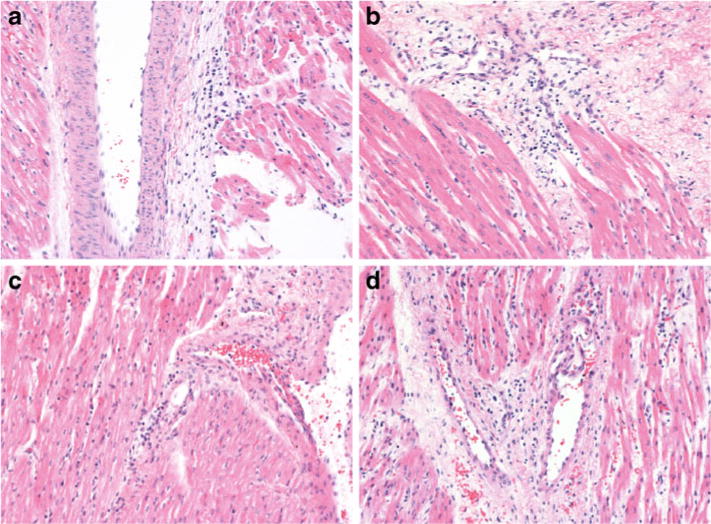
Representative samples of slides used for histological evaluation of the left and right ventricles in the control and insulin groups. a and b Hematoxylin and eosin stain of left ventricle from control group and insulin group, respectively. c and d Hematoxylin and eosin stain of right ventricle from control group and insulin group, respectively. Slide thickness is 5 μm. Magnification is 200×.
Mean TNF-α concentrations in plasma for the control and insulin groups during the study are presented in Fig. 3a. Natural log-transformed plasma TNF-α concentration was different between the groups at 1.5 and 2 h (P>0.05). In addition, TNF-α concentration increased for both groups over time and peaked at 2.5 h for the control group and 3 h for the insulin groups.
As illustrated in Fig. 3c, mean IL-6 concentrations in plasma for the control and insulin groups showed no difference between groups (P<0.05); however, IL-6 increased over time for both groups. Also, it is noteworthy that both groups reached the same plasma level at 3.5 h.
Right and Left Ventricle Cytokine Levels
The mean cytokine profiles for heart homogenates from left and right ventricles (end point of the experiment) for the control and insulin groups are presented in Fig. 4. As shown, the mean TNF-α concentration of the insulin group was 85 % greater in both the right and left ventricles compared to the control group (P>0.05). There was no difference in IL-1β or IL-6 between groups.
Histology
Representative images of tissue from the left and right ventricles stained with hematoxylin and eosin are shown in Fig. 5. Evaluation of the slides reveals a similar degree of inflammation in the heart tissue samples from both groups.
DISCUSSION
The purpose of this study was to assess the role that the immunomodulating effect of insulin plays in attenuating septic shock-induced myocardial depression. As demonstrated by both the changes in the hemodynamic data from baseline to the 2-h mark and the rise in the serum cytokine concentrations, the LPS infusion produced septic shock in these animals with an associated systemic inflammatory response syndrome. When subjected to septic shock induced by E. coli LPS endotoxin, the animals in the group that were treated with high-dose insulin did have attenuation in the decline of their shortening fraction compared with the control group. Fluid boluses with normal saline were administered to maintain adequate hemodynamic profiles in both groups to demonstrate that changes in shortening fraction between the groups are, in fact, due to changes in contractility from the myocardial depression rather than due to compensatory changes in response to abnormal hemodynamics. This effect of insulin was independent of its effect on serum glucose concentration, as the dextrose infusions in both groups were adjusted to maintain the serum glucose level within a similar range in both groups. However, even though the serum levels of TNF-α, IL-1β, and IL-6 demonstrated a high level of inflammation in both groups, analysis of the right and left ventricle IL-6 and IL-1β concentrations showed an insignificant difference in cytokine levels between the two groups. Analysis of the TNF-α concentration in the right and left ventricles demonstrated that the insulin group actually had a higher concentration of TNF-α than the control group. Based on previous studies, which demonstrate that the myocardial depressive effects of the inflammatory cytokines are due to their direct effect on the myocytes and not to soluble serum receptors, we attributed greater importance to the tissue cytokine levels compared with the serum levels [17–19].
The etiology of septic shock-induced cardiac dysfunction can be subdivided into three categories as follows: mechanical causes, metabolic causes, and inflammatory causes. In terms of mechanics, myocardial edema due to vascular leakage caused by the inflammatory state can decrease cardiac compliance, contractility, and overall function [7]. From a metabolic standpoint, the myocardial depression can be attributed to decreased production of ATP, as the myocytes of septic patients have a decreased uptake of glucose, ketone bodies, and free fatty acids, all while the heart is in a hypermetabolic state [7, 20]. Mitochondrial dysfunction is also present in sepsis with the inflammatory state causing mitochondrial structural damage and decreased activity of the electron transport chain leading to decreased ATP production [7]. Finally, there is also an inflammatory component to the myocardial depressive effects of septic shock, which is considered to be the most significant cause for the myocardial dysfunction induced by sepsis [4].
Parrillo et al. [21] demonstrated that serum taken from patients in the acute phase of sepsis had a depressive effect on the contractile property of isolated rat myocytes [21]. It was subsequently shown that TNF-α, IL-1β, and IL-6 were responsible for these myocardial depressive effects [4, 6, 7]. Both endotoxin and cytokines lead to a suppression of L-type calcium current, with an accompanied reduction in systolic intracellular calcium concentration and decreased cell contractility [7, 22–24].
A number of studies have demonstrated improved hemodynamics following administration of high-dose insulin in septic shock. Bronsveld et al. [1] reported their experience administering high-dose insulin (1.5 U/kg) together with glucose and potassium to 15 patients with hypotension and elevated serum lactate levels that were refractory to fluid resuscitation and vasoactive medications. Following the administration of the insulin, they observed an increase in the cardiac index as well as a decrease in the systemic vascular resistance [1]. In a porcine model of septic shock using LPS endotoxin, Holger et al. [15] demonstrated an improvement in cardiac output, SVO2, oxygen delivery, systemic vascular resistance, and pulmonary vascular resistance in a group that was treated with high-dose insulin plus fluid resuscitation and compared with a group receiving fluid resuscitation alone. Although the precise mechanism through which insulin improves cardiac performance in septic shock has not been identified, speculation has focused on two mechanisms as follows: the anti-inflammatory effect and an inotropic effect.
One of the primary positive inotropic effects that insulin has on the cardiac myocyte is its effect on calcium flux. Aulbach et al. [25] demonstrated that insulin increases the intracellular calcium concentration of the myocytes by increasing conductance of calcium through the L-type calcium channels and by increasing intracellular concentration of cAMP [25]. Insulin also leads to an increased influx of calcium into the myocyte through enhanced stimulation of the Na+–Ca2+ exchanger’s reverse mode, which brings calcium into the cell in exchange for sodium, which is transported out of the cell. This effect is mediated either directly, through the activation of PI-3-kinase [26, 27], or indirectly, through increased activation of the Na+–H+ exchanger, which transports Na+ into the cell in exchange for H+. By enhancing the activity of the Na+–H+ exchanger, thereby increasing the intracellular sodium concentration, the reverse mode of the Na+–Ca2+ exchanger is activated, which will extrude Na from the cell in exchange for calcium [26, 28]. Insulin also promotes a positive inotropic effect through enhancing the SR Ca2+ uptake of calcium into the sarcoplasmic reticulum [26–28]. These effects may be significant in combating the myocardial depressive effect of the various cytokines that are active in sepsis. In addition to increasing the intracellular calcium concentration, insulin also enhances the responsiveness of the myocytes to calcium [26].
Insulin also has significant anti-inflammatory effects that may benefit patients with septic shock-induced myocardial depression [9]. Insulin has been shown to decrease the concentration of circulating TNF-α, IL-6, and IL-1β [9, 11, 29]. Because these cytokines are known to be myocardial depressants [30], the cytokine-lowering effect of insulin is a potential etiology for the mechanism through which insulin ameliorates the myocardial depression in sepsis [2]. It is likely that the effect that insulin has on these inflammatory cytokines is related to its effect on NF-κβ, as insulin has been shown to decrease significantly the intranuclear NF-κβ concentration and to increase the NF-κβ inhibitor, IκB, which binds to NF-κβ in the cytosol, preventing its translocation into the nucleus. Insulin also inhibits the expression of intracellular adhesion molecule-1 (ICAM-1) in cultured human epithelial cells, likely through enhanced production of nitric oxide. ICAM-1 plays a central role in the inflammatory cascade by allowing monocyte adhesion to the epithelium, thereby initiating the inflammatory process. Decreasing the expression of ICAM-1 could have an impact on attenuating a potential inflammatory response [31]. Because this present study failed to demonstrate a greater degree of inflammation in the control group compared with the insulin group in tissue cytokine levels, a finding corroborated by the histological analysis, it is our conclusion that although insulin does appear to lessen the myocardial depressive effects of septic shock, it does not appear to do so through an anti-inflammatory effect on the cardiac myocytes. It is, therefore, likely that this effect seen with high-dose insulin is due to its positive inotropic qualities. These findings add to our understanding regarding the mechanism of action of insulin in this role, as a potential therapy for hypodynamic septic shock.
This study had a number of limitations. Although the use of LPS endotoxin as a continuous infusion with titration in the dose of endotoxin units is a well-established model of septic shock, it may not reflect physiologic conditions seen in sepsis, which typically has a large release of endotoxin that is not sustained throughout the period of sepsis. Secondly, the purpose of this study was to evaluate the mechanism through which insulin improves the hemodynamics in sepsis. As such, we felt initiating the insulin infusion at the beginning of the protocol would allow us to produce this effect in a controlled manner. This does not reflect the way that insulin would be used for these purposes in a clinical setting, where it is used as a rescue therapy. Additionally, we decided to measure heart function with transthoracic echocardiograms rather than with invasive hemodynamic monitoring. This decision was made to mimic what is done clinically, as invasive hemodynamic monitoring is not routinely used in pediatric patients with septic shock [32, 33]. All animals that survived 4 h did receive a third echocardiogram at that time; however, due to high loss of animals at that point in the protocol, data collected from the third echocardiogram were not amenable to meaningful statistical analysis. Finally, the focus of this study was to delineate the mechanism through which insulin attenuates septic shock-induced myocardial depression. Follow-up studies are warranted to determine the impact that this intervention has on overall survival in patients with septic shock.
CONCLUSIONS
In this porcine model of septic shock, the use of high-dose insulin did appear to lessen the degree of myocardial depression seen with septic shock. Analysis of both heart tissue cytokine levels, as well as histology of the tissue, pointed to a mechanism other than an anti-inflammatory effect as being the cause of this attenuation in myocardial depression. It is likely that an inotropic effect of high-dose insulin is what led to the improved cardiac function seen in this and other septic shock models. Further studies to evaluate the mechanism by which insulin improves cardiac function, focusing on a potential primary inotropic effect, are warranted.
Acknowledgments
This study was supported by the Nemours Biomedical Research and the NIH COBRE P20 GM 103464-8. Dr. Rodriguez is supported by the NIGMS T32 (GM08562) pediatric clinical pharmacology fellow funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) under the Best Pharmaceuticals for Children Act of the National Institutes of Health (NIH).
Footnotes
Conflicts of Interest. None of the authors listed have any financial disclosures or conflicts of interest related to this publication.
This study was performed at the Nemours/Alfred I. duPont Hospital for Children, Wilmington, DE, USA.
References
- 1.Bronsveld W, van den Bos GC, Thijs LG. Use of glucose-insulin-potassium (GIK) in human septic shock. Critical Care Medicine. 1985;13(7):566–570. doi: 10.1097/00003246-198507000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Hamdulay SS, Al-Khafaji A, Montgomery H. Glucose-insulin and potassium infusions in septic shock. Chest. 2006;129(3):800–804. doi: 10.1378/chest.129.3.800. [DOI] [PubMed] [Google Scholar]
- 3.Holger JS, Engebretsen KM, Marini JJ. High dose insulin in toxic cardiogenic shock. Clinical Toxicology (Philadelphia, Pa) 2009;47(4):303–307. doi: 10.1080/15563650802701929. [DOI] [PubMed] [Google Scholar]
- 4.Favory R, Lancel S, Marchetti P, Mordon S, Chopin C, Formstecher P, Neviere R. Endotoxin-induced myocardial dysfunction: evidence for a role of sphingosine production. Critical Care Medicine. 2004;32(2):495–501. doi: 10.1097/01.CCM.0000109452.36271.FA. [DOI] [PubMed] [Google Scholar]
- 5.Kumar A, Thota V, Dee L, Olson J, Uretz E, Parrillo JE. Tumor necrosis factor alpha and interleukin 1beta are responsible for in vitro myocardial cell depression induced by human septic shock serum. The Journal of Experimental Medicine. 1996;183(3):949–958. doi: 10.1084/jem.183.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pathan N, Hemingway CA, Alizadeh AA, Stephens AC, Boldrick JC, Oragui EE, McCabe C, Welch SB, Whitney A, O’Gara P, Nadel S, Relman DA, Harding SE, Levin M. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet. 2004;363(9404):203–209. doi: 10.1016/S0140-6736(03)15326-3. [DOI] [PubMed] [Google Scholar]
- 7.Rudiger A, Singer M. Mechanisms of sepsis-induced cardiac dysfunction. Critical Care Medicine. 2007;35(6):1599–1608. doi: 10.1097/01.CCM.0000266683.64081.02. [DOI] [PubMed] [Google Scholar]
- 8.Wu CK, Lee JK, Chiang FT, Yang CH, Huang SW, Hwang JJ, Lin JL, Tseng CD, Chen JJ, Tsai CT. Plasma levels of tumor necrosis factor-α and interleukin-6 are associated with diastolic heart failure through downregulation of sarcoplasmic reticulum Ca2+ ATPase. Critical Care Medicine. 2011;39(5):984–992. doi: 10.1097/CCM.0b013e31820a91b9. [DOI] [PubMed] [Google Scholar]
- 9.Brix-Christensen V, Andersen SK, Andersen R, Mengel A, Dyhr T, Andersen NT, Larsson A, Schmitz O, Ørskov H, Tønnesen E. Acute hyperinsulinemia restrains endotoxin-induced systemic inflammatory response: an experimental study in a porcine model. Anesthesiology. 2004;100(4):861–870. doi: 10.1097/00000542-200404000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Dandona P, Aljada A, Mohanty P, Ghanim H, Hamouda W, Assian E, Ahmad S. Insulin inhibits intranuclear nuclear factor kappaB and stimulates IkappaB in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? Journal of Clinical Endocrinology and Metabolism. 2001;86(7):3257–3265. doi: 10.1210/jcem.86.7.7623. [DOI] [PubMed] [Google Scholar]
- 11.Leffler M, Hrach T, Stuerzl M, Horch RE, Herndon DN, Jeschke MG. Insulin attenuates apoptosis and exerts anti-inflammatory effects in endotoxemic human macrophages. Journal of Surgical Research. 2007;143(2):398–406. doi: 10.1016/j.jss.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 12.Jeschke MG, Klein D, Bolder U, Einspanier R. Insulin attenuates the systemic inflammatory response in endotoxemic rats. Endocrinology. 2004;145(9):4084–4093. doi: 10.1210/en.2004-0592. [DOI] [PubMed] [Google Scholar]
- 13.Jeschke MG, Klein D, Herndon DN. Insulin treatment improves the systemic inflammatory reaction to severe trauma. Annals of Surgery. 2004;239(4):553–560. doi: 10.1097/01.sla.0000118569.10289.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forni M, Mazzola S, Ribeiro LA, Pirrone F, Zannoni A, Bernardini C, Bacci ML, Albertini M. Expression of endothelin-1 system in a pig model of endotoxic shock. Regulatory Peptides. 2005;131(1–3):89–96. doi: 10.1016/j.regpep.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Holger JS, Dries DJ, Barringer KW, Peake BJ, Flottemesch TJ, Marini JJ. Cardiovascular and metabolic effects of high-dose insulin in a porcine septic shock model. Academic Emergency Medicine. 2010;17(4):429–435. doi: 10.1111/j.1553-2712.2010.00695.x. [DOI] [PubMed] [Google Scholar]
- 16.Brierley J, Carcillo JA, Choong K, Cornell T, Decaen A, Deymann A, Doctor A, Davis A, Duff J, Dugas MA, Duncan A, Evans B, Feldman J, Felmet K, Fisher G, Frankel L, Jeffries H, Greenwald B, Gutierrez J, Hall M, Han YY, Hanson J, Hazelzet J, Hernan L, Kiff J, Kissoon N, Kon A, Irazuzta J, Lin J, Lorts A, Mariscalco M, Mehta R, Nadel S, Nguyen T, Nicholson C, Peters M, Okhuysen-Cawley R, Poulton T, Relves M, Rodriguez A, Rozenfeld R, Schnitzler E, Shanley T, Kache S, Skippen P, Torres A, von Dessauer B, Weingarten J, Yeh T, Zaritsky A, Stojadinovic B, Zimmerman J, Zuckerberg A. Clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock: 2007 update from the American College of Critical Care Medicine. Critical Care Medicine. 2009;37(2):666–688. doi: 10.1097/CCM.0b013e31819323c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pathan N, Franklin JL, Eleftherohorinou H, Wright VJ, Hemingway CA, Waddell SJ, Griffiths M, Dennis JL, Relman DA, Harding SE, Levin M. Myocardial depressant effects of interleukin 6 in meningococcal sepsis are regulated by p38 mitogen-activated protein kinase. Critical Care Medicine. 2011;39(7):1692–1711. doi: 10.1097/CCM.0b013e3182186d27. [DOI] [PubMed] [Google Scholar]
- 18.Fallach R, Shainberg A, Avlas O, Fainblut M, Chepurko Y, Porat E, Hochhauser E. Cardiomyocyte Toll-like receptor 4 is involved in heart dysfunction following septic shock or myocardial ischemia. Journal of Molecular and Cellular Cardiology. 2010;48(6):1236–1244. doi: 10.1016/j.yjmcc.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 19.Zhu H, Shan L, Schiller PW, Mai A, Peng T. Histone deacetylase-3 activation promotes tumor necrosis factor-alpha (TNF-alpha) expression in cardiomyocytes during lipopolysaccharide stimulation. Journal of Biological Chemistry. 2010;285(13):9429–9436. doi: 10.1074/jbc.M109.071274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dhainaut JF, Huyghebaert MF, Monsallier JF, Lefevre G, Dall’Ava-Santucci J, Brunet F, Villemant D, Carli A, Raichvarg D. Coronary hemodynamics and myocardial metabolism of lactate, free fatty acids, glucose, and ketones in patients with septic shock. Circulation. 1987;75(3):533–541. doi: 10.1161/01.cir.75.3.533. [DOI] [PubMed] [Google Scholar]
- 21.Parrillo JE, Burch C, Shelhamer JH, Parker MM, Natanson C, Schuette W. A circulating myocardial depressant substance in humans with septic shock. Septic shock patients with a reduced ejection fraction have a circulating factor that depresses in vitro myocardial cell performance. The Journal of Clinical Investigation. 1985;76(4):1539–1553. doi: 10.1172/JCI112135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abi-Gerges N, Tavernier B, Mebazaa A, Faivre V, Paqueron X, Payen D, Fischmeister R, Méry PF. Sequential changes in autonomic regulation of cardiac myocytes after in vivo endotoxin injection in rat. American Journal of Respiratory and Critical Care Medicine. 1999;160(4):1196–1204. doi: 10.1164/ajrccm.160.4.9808149. [DOI] [PubMed] [Google Scholar]
- 23.Zhong J, Hwang TC, Adams HR, Rubin LJ. Reduced L-type calcium current in ventricular myocytes from endotoxemic guinea pigs. American Journal of Physiology. 1997;273(5 Pt 2):H2312–2324. doi: 10.1152/ajpheart.1997.273.5.H2312. [DOI] [PubMed] [Google Scholar]
- 24.Liu RH, Hotchkiss JH. Potential genotoxicity of chronically elevated nitric oxide: a review. Mutation Research. 1995;339(2):73–89. doi: 10.1016/0165-1110(95)90004-7. [DOI] [PubMed] [Google Scholar]
- 25.Aulbach F, Simm A, Maier S, Langenfeld H, Walter U, Kersting U, Kirstein M. Insulin stimulates the L-type Ca2+ current in rat cardiac myocytes. Cardiovascular Research. 1999;42(1):113–120. doi: 10.1016/s0008-6363(98)00307-1. [DOI] [PubMed] [Google Scholar]
- 26.von Lewinski D, Bruns S, Walther S, Kögler H, Pieske B. Insulin causes [Ca2+]i-dependent and [Ca2+]i-independent positive inotropic effects in failing human myocardium. Circulation. 2005;111(20):2588–2595. doi: 10.1161/CIRCULATIONAHA.104.497461. [DOI] [PubMed] [Google Scholar]
- 27.Sethi R, Barwinsky J, Beamish RE, Dhalla NS. Mechanism of the positive inotropic action of insulin. Journal of Applied Cardiology. 1991;6:199–208. [Google Scholar]
- 28.Hsu CH, Wei J, Chen YC, Yang SP, Tsai CS, Lin CI. Cellular mechanisms responsible for the inotropic action of insulin on failing human myocardium. The Journal of Heart and Lung Transplantation. 2006;25(9):1126–1134. doi: 10.1016/j.healun.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Viardot A, Grey ST, Mackay F, Chisholm D. Potential antiinflammatory role of insulin via the preferential polarization of effector T cells toward a T helper 2 phenotype. Endocrinology. 2007;148(1):346–353. doi: 10.1210/en.2006-0686. [DOI] [PubMed] [Google Scholar]
- 30.Zanotti-Cavazzoni SL, Hollenberg SM. Cardiac dysfunction in severe sepsis and septic shock. Current Opinion in Critical Care. 2009;15(5):392–397. doi: 10.1097/MCC.0b013e3283307a4e. [DOI] [PubMed] [Google Scholar]
- 31.Aljada A, Saadeh R, Assian E, Ghanim H, Dandona P. Insulin inhibits the expression of intercellular adhesion molecule-1 by human aortic endothelial cells through stimulation of nitric oxide. Journal of Clinical Endocrinology and Metabolism. 2000;85(7):2572–2575. doi: 10.1210/jcem.85.7.6677. [DOI] [PubMed] [Google Scholar]
- 32.Pinsky MR. Hemodynamic monitoring over the past 10 years. Critical Care. 2006;10(1):117. doi: 10.1186/cc3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hilton AK. Echocardiography is the best cardiovascular ‘monitor’ in septic shock. Critical Care and Resuscitation. 2006;8(3):247–251. [PubMed] [Google Scholar]


