Abstract
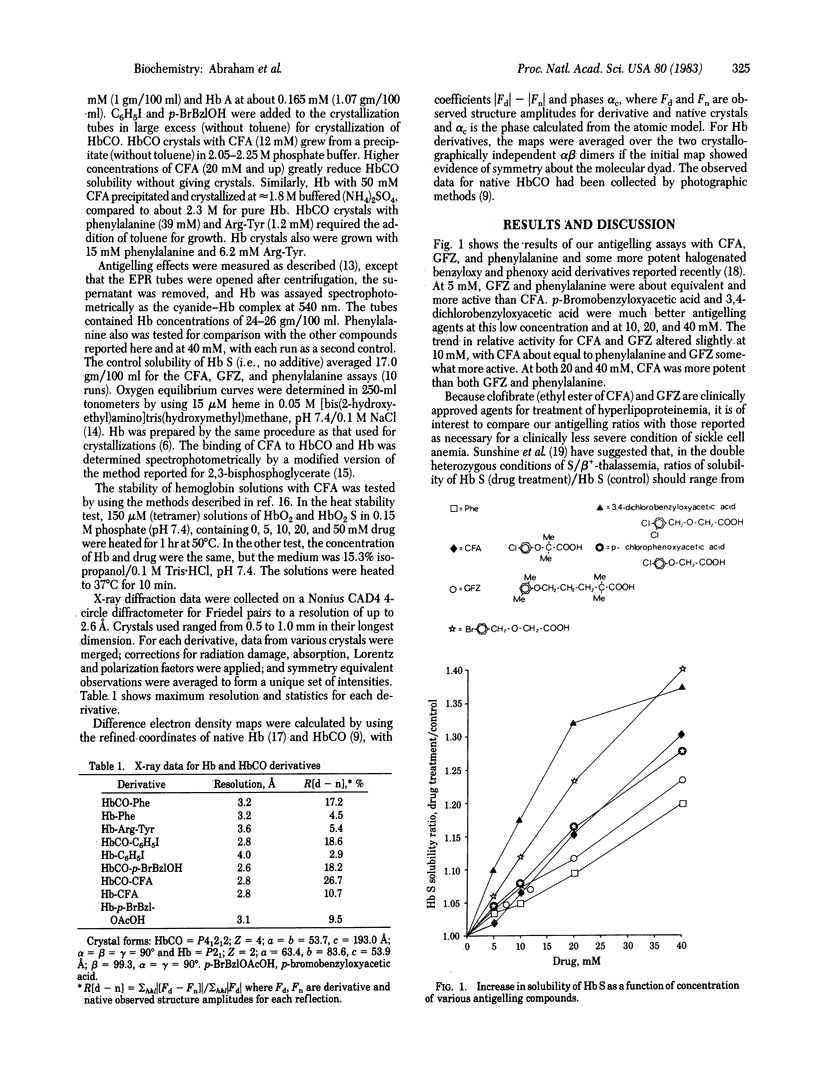
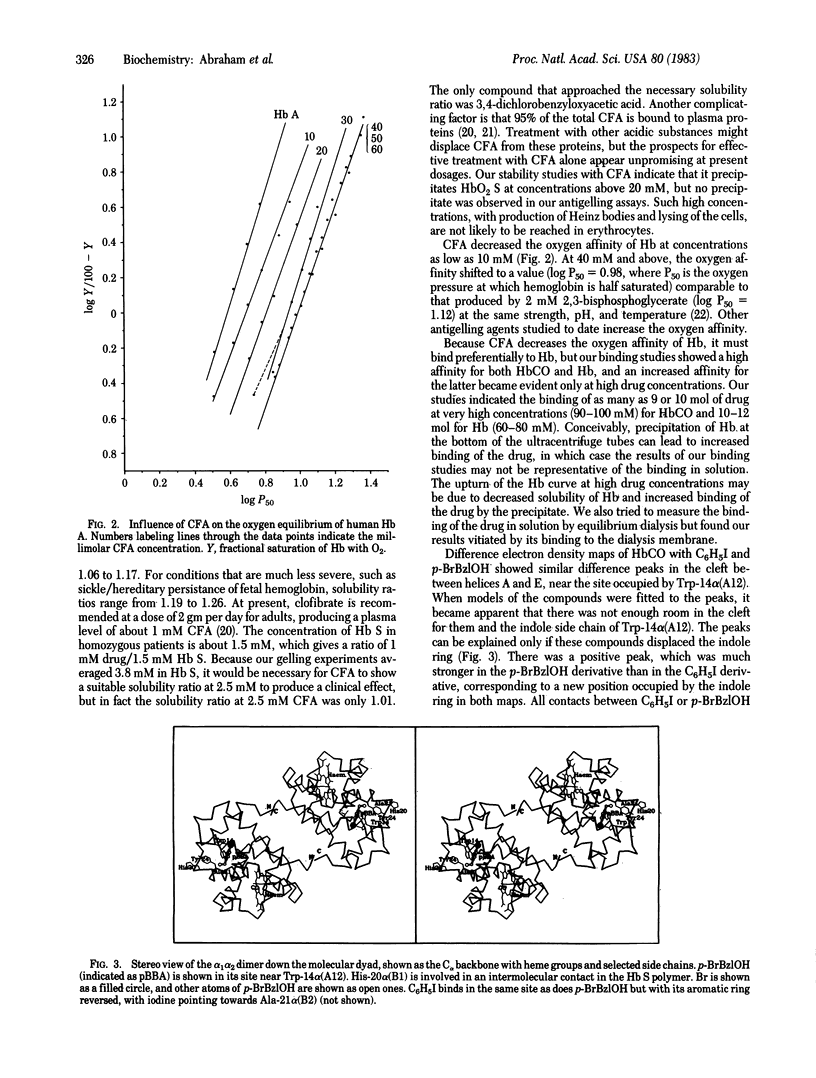
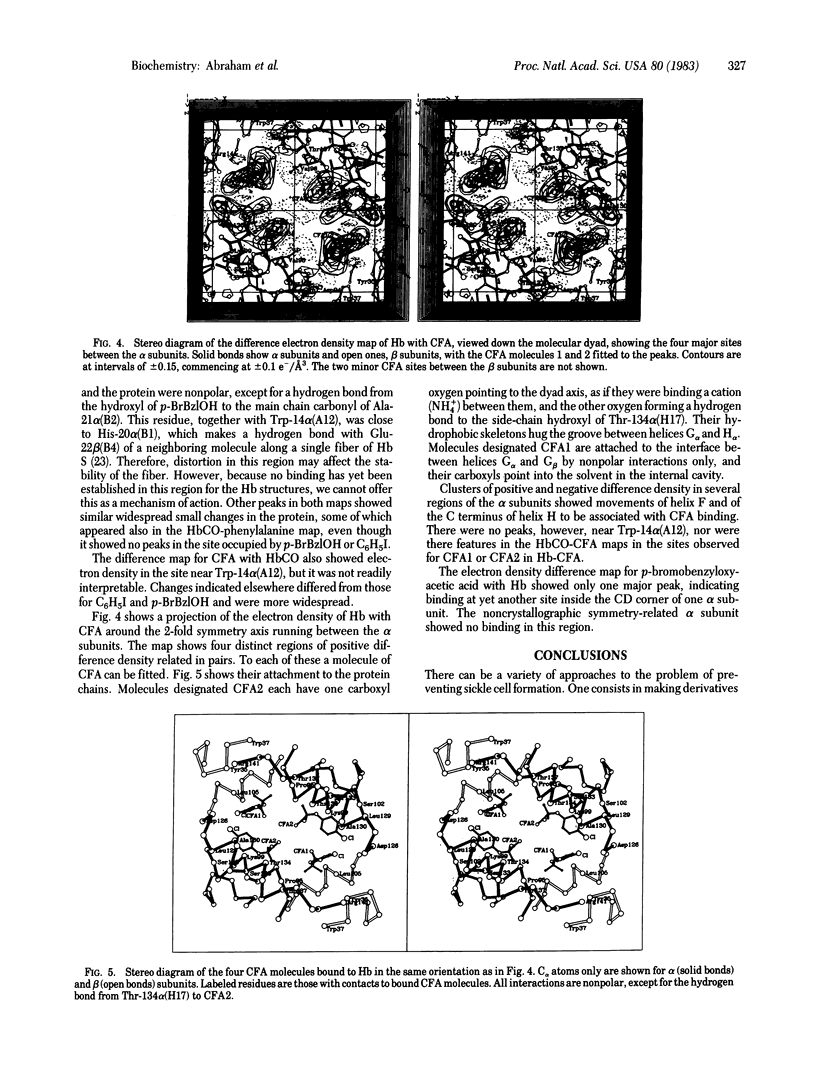
Several aromatic compounds have been found to inhibit the gelling of sickle cell hemoglobin. We have tried to correlate the antigelling activity of such compounds with the stereo-chemistry of their binding sites in the hemoglobin molecule. This approach led to the discovery that two known antilipoproteinemia drugs, clofibrate and gemfibrozil, have antigelling activity. X-ray analysis showed that three pairs of molecules of clofibric acid, the active metabolite of clofibrate, bound to the walls of the internal cavity of deoxyhemoglobin A; only one pair bound to a quite different site, between helices A, E, and H of the alpha chains of carbon monoxide hemoglobin A. Unlike other antigelling agents, clofibric acid and related compounds decrease rather than increase the oxygen affinity of hemoglobin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham D. J., Mehanna A. S., Williams F. L. Design, synthesis, and testing of potential antisickling agents. 1. Halogenated benzyloxy and phenoxy acids. J Med Chem. 1982 Sep;25(9):1015–1017. doi: 10.1021/jm00351a002. [DOI] [PubMed] [Google Scholar]
- Baldwin J. M. The structure of human carbonmonoxy haemoglobin at 2.7 A resolution. J Mol Biol. 1980 Jan 15;136(2):103–128. doi: 10.1016/0022-2836(80)90308-3. [DOI] [PubMed] [Google Scholar]
- Behe M. J., Englander W. S. Quantitative assessment of the noncovalent inhibition of sickle hemoglobin gelation by phenyl derivatives and other known agents. Biochemistry. 1979 Sep 18;18(19):4196–4201. doi: 10.1021/bi00586a025. [DOI] [PubMed] [Google Scholar]
- Dean J., Schechter A. N. Sickle-cell anemia: molecular and cellular bases of therapeutic approaches (third of three parts). N Engl J Med. 1978 Oct 19;299(16):863–870. doi: 10.1056/NEJM197810192991605. [DOI] [PubMed] [Google Scholar]
- Faed E. M., McQueen E. G. Serum clofibrate concentrations in patients on continuous medication. Pharmacology. 1974;12(3):144–151. doi: 10.1159/000136532. [DOI] [PubMed] [Google Scholar]
- Farnell K. J., McMeekin T. L. The effect of organic compounds on the crystal habit and crystallization of normal and sickle cell hemoglobin in phosphate buffer. Arch Biochem Biophys. 1973 Oct;158(2):702–710. doi: 10.1016/0003-9861(73)90564-x. [DOI] [PubMed] [Google Scholar]
- Fermi G. Three-dimensional fourier synthesis of human deoxyhaemoglobin at 2-5 A resolution: refinement of the atomic model. J Mol Biol. 1975 Sep 15;97(2):237–256. doi: 10.1016/s0022-2836(75)80037-4. [DOI] [PubMed] [Google Scholar]
- Frier J. A., Perutz M. F. Structure of human foetal deoxyhaemoglobin. J Mol Biol. 1977 May 5;112(1):97–112. doi: 10.1016/s0022-2836(77)80158-7. [DOI] [PubMed] [Google Scholar]
- Garby L., Gerber G., De Verdier C. H. Binding of 2,3-diphosphoglycerate and adenosine triphosphate to human haemoglobin A. Eur J Biochem. 1969 Aug;10(1):110–115. doi: 10.1111/j.1432-1033.1969.tb00662.x. [DOI] [PubMed] [Google Scholar]
- Gorecki M., Acquaye C. T., Wilchek M., Votano J. R., Rich A. Antisickling activity of amino acid benzyl esters. Proc Natl Acad Sci U S A. 1980 Jan;77(1):181–185. doi: 10.1073/pnas.77.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofrichter J., Ross P. D., Eaton W. A. Supersaturation in sickle cell hemoglobin solutions. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3035–3039. doi: 10.1073/pnas.73.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai K. Hemoglobin Chesapeake (92 alpha, arginine--leucine). Precise measurements and analyses of oxygen equilibrium. J Biol Chem. 1974 Dec 10;249(23):7607–7612. [PubMed] [Google Scholar]
- Noguchi C. T., Schechter A. N. Effects of amino acids on gelation kinetics and solubility of sickle hemoglobin. Biochem Biophys Res Commun. 1977 Jan 24;74(2):637–642. doi: 10.1016/0006-291x(77)90350-3. [DOI] [PubMed] [Google Scholar]
- Novak R. F., Dershwitz M., Novak F. C. Characterization of the interaction of the aromatic hydrocarbons benzene and toluene with human hemoglobin. Mol Pharmacol. 1979 Nov;16(3):1046–1058. [PubMed] [Google Scholar]
- Ross P. D., Subramanian S. Inhibition of sickle cell hemoglobin gelation by some aromatic compounds. Biochem Biophys Res Commun. 1977 Aug 22;77(4):1217–1223. doi: 10.1016/s0006-291x(77)80109-5. [DOI] [PubMed] [Google Scholar]
- Schoenborn B. P. Dichloromethane as an antisickling agent in sickle cell hemoglobin. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4195–4199. doi: 10.1073/pnas.73.11.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunshine H. R., Hofrichter J., Eaton W. A. Requirement for therapeutic inhibition of sickle haemoglobin gelation. Nature. 1978 Sep 21;275(5677):238–240. doi: 10.1038/275238a0. [DOI] [PubMed] [Google Scholar]
- THROP J. M. Experimental evaluation of an orally active combination of androsterone with ethyl chlorophenoxy-isobutyrate. Lancet. 1962 Jun 23;1(7243):1323–1326. doi: 10.1016/s0140-6736(62)92423-6. [DOI] [PubMed] [Google Scholar]
- Wishner B. C., Ward K. B., Lattman E. E., Love W. E. Crystal structure of sickle-cell deoxyhemoglobin at 5 A resolution. J Mol Biol. 1975 Oct 15;98(1):179–194. doi: 10.1016/s0022-2836(75)80108-2. [DOI] [PubMed] [Google Scholar]


