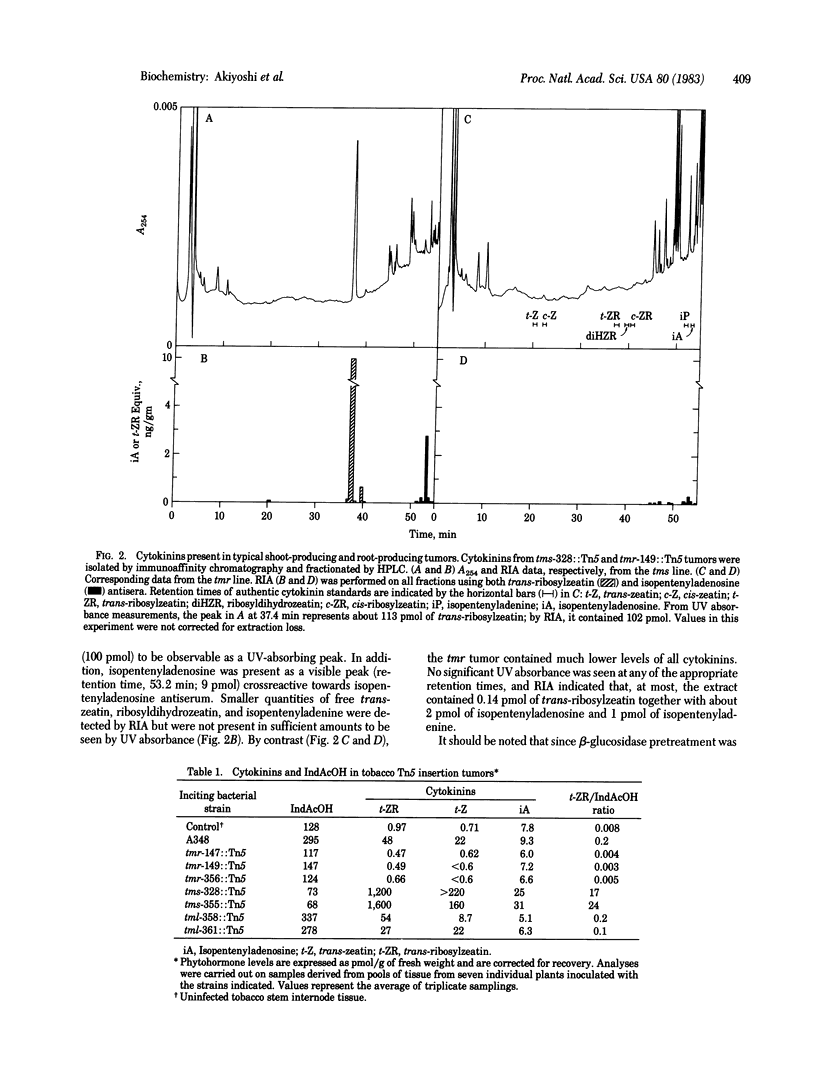
Abstract
Insertion of the transposon Tn5 into the T-region of the octopine Ti plasmid of Agrobacterium tumefaciens gives rise to crown gall tumors having altered morphology. Three loci within the T-DNA that control tumor morphology have been detected [Garfinkel, D. J., Simpson, R. B., Ream, L. W., White, F. F., Gordon, M. P. & Nester, E. W. (1981) Cell 27, 143-153]. They influence tumor size (tml), production of roots (tmr), or production of shoots (tms). Cytokinin and auxin levels in such mutant tumors were examined by HPLC/radioimmunoassay and HPLC/fluorescence assay, respectively. Free indoleacetic acid levels (in pmol/g) were: uninfected tobacco stem tissues, 128; wild-type A348 tumors, 295; tml mutant tumors, 307; tmr mutant tumors, 129; and tms mutant tumors, 70. Average trans-ribosylzeatin levels were correspondingly: 0.97, 48, 40, 0.54, and 1,400 pmol/g. trans-Ribosylzeatin/indoleacetic acid ratios were as high as 24 in shoot-producing tumors and as low as 0.003 in root-producing tumors. The evidence strongly suggests that tumor phytohormone levels are determined by genes in the T-DNA.
Keywords: Agrobacterium tumefaciens, immunoaffinity chromatography, Tn5 mutagenesis, tumor morphology
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amasino R. M., Miller C. O. Hormonal control of tobacco crown gall tumor morphology. Plant Physiol. 1982 Feb;69(2):389–392. doi: 10.1104/pp.69.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A. C. A Physiological Basis for Autonomous Growth of the Crown-Gall Tumor Cell. Proc Natl Acad Sci U S A. 1958 Apr;44(4):344–349. doi: 10.1073/pnas.44.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einset J. W. Cytokinins in tobacco crown gall tumors. Biochem Biophys Res Commun. 1980 Mar 28;93(2):510–515. doi: 10.1016/0006-291x(80)91106-7. [DOI] [PubMed] [Google Scholar]
- Garfinkel D. J., Nester E. W. Agrobacterium tumefaciens mutants affected in crown gall tumorigenesis and octopine catabolism. J Bacteriol. 1980 Nov;144(2):732–743. doi: 10.1128/jb.144.2.732-743.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel D. J., Simpson R. B., Ream L. W., White F. F., Gordon M. P., Nester E. W. Genetic analysis of crown gall: fine structure map of the T-DNA by site-directed mutagenesis. Cell. 1981 Nov;27(1 Pt 2):143–153. doi: 10.1016/0092-8674(81)90368-8. [DOI] [PubMed] [Google Scholar]
- Gelvin S. B., Thomashow M. F., McPherson J. C., Gordon M. P., Nester E. W. Sizes and map positions of several plasmid-DNA-encoded transcripts in octopine-type crown gall tumors. Proc Natl Acad Sci U S A. 1982 Jan;79(1):76–80. doi: 10.1073/pnas.79.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosuge T., Heskett M. G., Wilson E. E. Microbial synthesis and degradation of indole-3-acetic acid. I. The conversion of L-tryptophan to indole-3-acetamide by an enzyme system from Pseudomonas savastanoi. J Biol Chem. 1966 Aug 25;241(16):3738–3744. [PubMed] [Google Scholar]
- Leemans J., Deblaere R., Willmitzer L., De Greve H., Hernalsteens J. P., Van Montagu M., Schell J. Genetic Identification of functions of TL-DNA transcripts in octopine crown galls. EMBO J. 1982;1(1):147–152. doi: 10.1002/j.1460-2075.1982.tb01138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E. W., Kosuge T. Plasmids specifying plant hyperplasias. Annu Rev Microbiol. 1981;35:531–565. doi: 10.1146/annurev.mi.35.100181.002531. [DOI] [PubMed] [Google Scholar]
- Ooms G., Hooykaas P. J., Moolenaar G., Schilperoort R. A. Grown gall plant tumors of abnormal morphology, induced by Agrobacterium tumefaciens carrying mutated octopine Ti plasmids; analysis of T-DNA functions. Gene. 1981 Jun-Jul;14(1-2):33–50. doi: 10.1016/0378-1119(81)90146-3. [DOI] [PubMed] [Google Scholar]
- SKOOG F., MILLER C. O. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol. 1957;11:118–130. [PubMed] [Google Scholar]
- Thomashow M. F., Nutter R., Montoya A. L., Gordon M. P., Nester E. W. Integration and organization of Ti plasmid sequences in crown gall tumors. Cell. 1980 Mar;19(3):729–739. doi: 10.1016/s0092-8674(80)80049-3. [DOI] [PubMed] [Google Scholar]
- Thomashow M. F., Nutter R., Postle K., Chilton M. D., Blattner F. R., Powell A., Gordon M. P., Nester E. W. Recombination between higher plant DNA and the Ti plasmid of Agrobacterium tumefaciens. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6448–6452. doi: 10.1073/pnas.77.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willmitzer L., Simons G., Schell J. The TL-DNA in octopine crown-gall tumours codes for seven well-defined polyadenylated transcripts. EMBO J. 1982;1(1):139–146. doi: 10.1002/j.1460-2075.1982.tb01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]



