Abstract
Background
Pelvic floor symptoms are common and are negatively associated with sexual function which, in turn, is an important aspect of quality of life. The majority of older women with pelvic floor symptoms are treated in general practice but evidence from studies in general practice on the sexual functioning of these women is scarce.
Aim
This study examined predictors of sexual inactivity in older women with pelvic floor symptoms in general practice and of sexual functioning in those women who are sexually active.
Design and setting
Cross-sectional study in women (aged ≥55 years) from 20 general practices who screened positive on a pelvic floor symptom questionnaire.
Method
Logistic and linear regression analyses were used to determine predictors of sexual inactivity and sexual functioning (PISQ-12) by assessing their association with patient characteristics, symptoms (PFDI-20) and degree of prolapse (POP-Q).
Results
A total of 639 women were included (sexually active n = 393, sexually inactive n = 246). Predictors of sexual inactivity were increasing age (odds ratio [OR] = 1.13; 95% confidence interval [CI] = 1.10 to 1.17) and lower education (OR = 2.31; 95% CI = 1.50 to 3.54; Nagelkerke R2 = 0.208). In sexually active women, sexual functioning was associated with pelvic floor symptom distress (P<0.001) and pelvic floor surgery (P = 0.018; R2 = 0.138).
Conclusion
In older women with pelvic floor symptoms, increasing age and lower educational level are predictors of sexual inactivity. Many of these older women are sexually active and pelvic floor symptom distress is negatively associated with sexual functioning. These results may encourage GPs to ask about sexual problems in women with pelvic floor symptoms.
Keywords: general practice, pelvic floor disorders, pelvic organ prolapse, postmenopause, psychological, sexual activity, sexual dysfunctions
INTRODUCTION
Pelvic floor disorders (such as pelvic organ prolapse and dysfunction of micturition and defaecation) are common in elderly women: about 37% of older women (aged 60–79 years) suffer from symptoms caused by one of these disorders.1 Pelvic floor symptoms include vaginal bulging, pelvic pressure or heaviness, pelvic pain, and urinary or faecal incontinence or obstruction.2
The presence of a prolapse is thought to have a negative impact on sexual function.3–5 Women with an advanced prolapse feel less feminine and less physically and sexually attractive compared to women with normal pelvic support.6 More than one-third of sexually active women with an advanced prolapse indicate that their sexuality is affected by prolapse symptoms,7 and experiencing more pelvic floor symptoms is associated with poorer sexual function.8,9 Embarrassment or discomfort, resulting from prolapse or urinary incontinence, can result in sexual inactivity.7 However, the evidence on the relationship between pelvic floor symptoms and sexual function remains conflicting.10–13
Research in general practice is scarce, and it is difficult to extrapolate evidence from research performed in urogynaecology clinics3,7–9 to general practice, since women presenting at these clinics may be a selection of women with more severe symptoms. The present study was performed to gather information on pelvic floor symptoms and sexual functioning of older women in general practice, both of which can affect quality of life.14,15 This information is relevant to GPs, since the vast majority of pelvic floor symptoms are treated in general practice. The aim of this study was to investigate whether pelvic floor symptoms, pelvic organ prolapse, and other patient characteristics are predictors of sexual inactivity in all women, and of sexual functioning in those women who are sexually active. This study was performed in older women with pelvic floor symptoms in general practice.
METHOD
Study design and participants
This cross-sectional study used data from two randomised controlled trials on the effects and cost effectiveness of conservative treatments for pelvic organ prolapse in older women (the POPPS project).16 Data were collected during the baseline assessment prior to randomisation and concerned women both with and without a pelvic organ prolapse.
From October 2009 to December 2012, all women (aged ≥55 years), registered in 20 general practices in the northern part of the Netherlands, were sent a screening questionnaire on pelvic floor symptoms (urinary incontinence, vaginal bulging, pelvic heaviness and/or pressure, or vaginal splinting to start or complete micturition or defecation), unless they met the exclusion criteria. The exclusion criteria were current prolapse treatment or treatment in the past year, current treatment for another urogynaecological disorder, malignancy of pelvic organs, impaired mobility, severe and/ or terminal illness, cognitive impairment, and insufficient command of the Dutch language.
How this fits in
The majority of older women with pelvic floor symptoms are treated in general practice. Evidence from primary care studies on sexual functioning of these women is scarce because most evidence is derived from studies in women seen in urogynaecologic clinics. Many older women with pelvic floor symptoms are sexually active and the pelvic floor symptom distress is negatively associated with sexual functioning. This may encourage GPs to ask about sexual problems in women with pelvic floor symptoms.
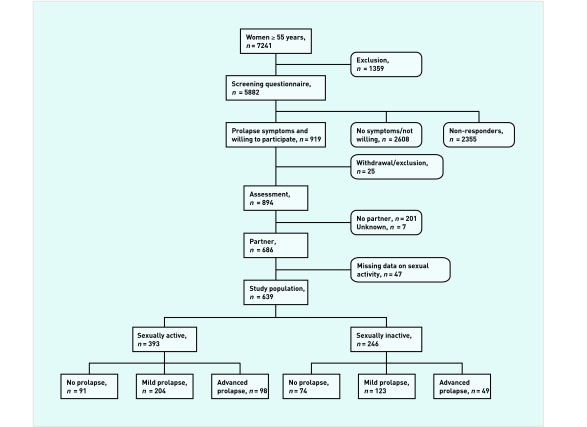
Women who reported one or more of the aforementioned symptoms and who were willing to participate were invited for a baseline assessment, at which time written informed consent was obtained. Since the absence of a partner is the most important predictor of sexual inactivity for older women,11,13 only women with a partner were included (Figure 1).
Figure 1.
Flowchart of the study population.
Main outcomes
The main outcomes of this study were predictors of sexual inactivity in all women and level of sexual functioning in those women who were sexually active.
Measurements
During the assessment, a standardised interview was performed to collect information about patient characteristics, comorbidity (weighted Charlson Comorbidity Index),17 and medical and obstetric history.
Prior to the assessment, women were asked to fill in questionnaires at home. Sexual inactivity or activity was measured with the question ‘Are you sexually active?’. Women were asked to consider their sexuality during the past 6 months. Women who reported being sexually inactive could skip the questions about sexual functioning. To measure sexual function in women who reported being sexually active, a Dutch translation of the validated and condition-specific Pelvic Organ Prolapse/Urinary Incontinence Sexual Function Questionnaire (PISQ-12) was used.18,19 This questionnaire consists of 12 questions about physical, behavioural–emotive and partner-related aspects of sexual functioning. The sum score ranges from 0–48, with a higher score indicating better sexual functioning.19
For the assessment of pelvic floor symptom distress, a Dutch translation of the validated Pelvic Floor Distress Inventory-20 (PFDI-20) was used.20,21 This questionnaire consists of 20 questions divided into three subscales to measure symptoms that are related to prolapse, bowel, or bladder. Subscale scoring is from 0–100. The total PFDI-20 score (sum of the three subscale scores) ranges from 0–300, with higher scores indicating more distress.21
Patients underwent a gynaecological examination. Prolapse was assessed using the Pelvic Organ Prolapse-Quantification system (POP-Q).22 This system distinguishes five stages of pelvic organ prolapse (0–IV). In the present study, women were divided into three categories: no prolapse, mild prolapse, or advanced prolapse. Women with the leading edge of prolapse staying above the hymenal remnants (POP-Q stage I, IIa) were considered as having a mild prolapse, and women with the leading edge at or beyond the hymenal remnants (POP-Q stage IIb, III, IV) were considered as having an advanced prolapse. Women were divided into these three categories because the hymenal ring (formerly the introitus) is a reference point for the classification of the severity of prolapse still used by many gynaecologists (Baden–Walker classification),23 and many studies looking for a relationship between symptoms and pelvic organ prolapse also use the hymen as a cut-off point.
Analysis
For calculation of the three PFDI-20 subscale scores and also for calculation of the total PISQ-12 score, a maximum of two missing items was accepted. In cases of one or two missing answers, the mean from the answered items only was used.19,21 In cases where there were more than two missing items, no total PISQ-12 or PFDI-20 subscale score could be calculated. A total PFDI-20 score was computed only if a score on all three subscales could be calculated. Data of women for whom a total PFDI-20 or PISQ-12 score could not be computed were excluded from the regression analyses. A complete case analysis was performed when there were <5% incomplete cases, otherwise multiple imputations techniques were used to allow for missing cases.
Independent t tests were used to compare continuous variables for sexually active and sexually inactive women. Variables were transformed when normal distribution could not be assumed. A Mann–Whitney U test was used if a normal distribution could not be assumed and if transformation did not result in a normal distribution. χ2 or Fisher’s exact tests were used for categorical data. All reported probability values are two-tailed; P<0.05 was considered to be statistically significant.
Logistic and linear regression analyses were performed to investigate which variables were predictors of sexual inactivity in all included women, and sexual functioning (PISQ-12 score) in those women who were sexually active, respectively. Potential predictors in both regression models were patient characteristics (Table 1), severity of pelvic floor symptoms (PFDI-20 score), and severity of prolapse (no, mild, advanced prolapse). Only determinants with a significant association at the P<0.25 level in the univariate analysis were included in the multivariable analyses. In these multivariable analyses, a best subset backward stepwise elimination procedure was manually performed using P≥0.05 as the criterion for removal from the model. Statistical analyses were performed using SPSS (version 20.0).
Table 1.
Characteristics of the study population
| Characteristics | Sexually active n = 393 (61.5%) | Sexually inactive n = 246 (38.5%) | P-value |
|---|---|---|---|
| Age, years (mean ± SD) | 62.6 ± 5.2 | 67.3 ±6.6 | <0.001a |
|
| |||
| Body mass index, kg/m2 (mean ± SD) | 26.6 ± 4.7 | 27.3 ± 5.5 | 0.13a |
|
| |||
| Parity (mean ± SD) | 2.3 ± 1.1 | 2.3 ± 1.1 | 0.99a |
|
| |||
| Education level, n (%) | <0.001b | ||
| Lower education | 136 (34.6) | 138 (56.3) | |
| Intermediate education | 124 (31.6) | 58 (23.7) | |
| Higher education | 133 (33.8) | 49 (20.0) | |
|
| |||
| Charlson Comorbidity Index, medianc (25th to 75th percentile) | 0 (0 to 0) | 0 (0 to 1) | 0.008d |
|
| |||
| Pelvic floor surgery, n (%) | 80 (20.4) | 74 (30.1) | 0.004e |
|
| |||
| Total PFDI-20 score, medianf (25th to 75th percentile) | 57.3 (33.3 to 83.3) | 59.0 (39.6 to 87.5) | 0.20a |
|
| |||
| POPDI-6 score, median (25th to 75th percentile) | 12.5 (4.2 to 25) | 12.5 (5 to 25) | 0.92b |
|
| |||
| CRADI-8 score, median (25th to 75th percentile) | 12.5 (6.3 to 25) | 15.6 (6.3 to 28.1) | 0.24b |
|
| |||
| UDI-6 score, median (25th to 75th percentile) | 25 (16.7 to 41.7) | 29.6 (16.7 to 45.8) | 0.070b |
|
| |||
| PISQ-12 score, mediang (25th to 75th percentile) | 37 (33 to 39) | n/a | n/a |
|
| |||
| Prolapse stage, n (%)h | 0.10b | ||
| No prolapse | 91 (23.2) | 74 (30.1) | |
| Mild prolapse | 204 (51.9) | 123 (50.0) | |
| Advanced prolapse | 98 (24.9) | 49 (19.9) | |
CRADI-8 = ColoRectal–Anal Distress Inventory-8, range 0–100. PFDI-20 = Pelvic Floor Distress Inventory-20, range 0–300. PISQ-12 = Pelvic Organ Prolapse/Urinary Incontinence Sexual Function Questionnaire-12, range 0–48; POPDI-6 = Pelvic Organ Prolapse Distress Inventory-6, range 0–100. POP-Q = Pelvic Organ Prolapse-Quantification. SD = standard deviation. UDI-6 = Urinary Distress Inventory-6, range 0–100.
Independent t-test.
χ2 test.
Missing items for Charlson Comorbidity Index: sexually active women (n = 1), sexually inactive women (n = 1).
Mann–Whitney U test.
Fisher’s exact test.
Missing items for PFDI-20 score: sexually active women (n = 9), sexually inactive women (n = 15).
Missing items for PISQ-12 score: sexually active women (n = 7).
Prolapse stage: no prolapse, mild prolapse (POP-Q stage I, IIa), advanced prolapse (POP-Q stage IIb, III, IV). n/a = not applicable.
RESULTS
An assessment was performed in 894 women: 686 with a partner and 201 without a partner. Data on partners were missing for seven women (Figure 1). Women with a partner (n = 686) were included in the present study. Of these latter women, 393 were sexually active, 246 were sexually inactive, and sexual activity was unknown in 47. These 47 women were excluded from further analyses, leaving a total study population of 639 women. A PFDI-20 score could not be calculated for 24 women (9 sexually active, 15 sexually inactive) and a Charlson comorbidity index for another two women (1 sexually active, 1 sexually inactive; 26 out of 639 = 4.1% incomplete cases in the total study population). Within the group of sexually active women, another seven women did not complete the PISQ- 12 (17 out of 393 = 4.3% incomplete cases within the group of sexually active women).
The characteristics of all women in the study population are presented in Table 1.
Table 2 shows the results of the univariate and multivariable logistic regression analyses. The final multivariable logistic regression model included age (odds ratio [OR] = 1.13; 95% confidence interval [CI] = 1.10 to 1.17) and education (OR = 2.31; 95% CI = 1.50 to 3.54). The explained variance (Nagelkerke R2) of this model was 0.208. These results indicate that increasing age and lower educational level are independent predictors of sexual inactivity.
Table 2.
Association between patient characteristics, pelvic floor symptoms (PFDI-20), degree of prolapse, and sexual inactivity (n = 639): results of logistic regression analyses
| Variables | Univariate logistic regression | Multivariable logistic regressiona | ||
|---|---|---|---|---|
|
|
|
|||
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| PFDI-20, per point (0 to 300)b,c | 1.00 (0.99 to 1.01) | 0.17 | ||
|
| ||||
| Prolapseb | 0.10 | |||
| Mild (versus no prolapse) | 0.74 (0.51 to 1.08) | 0.12 | ||
| Advanced (versus no prolapse) | 0.62 (0.39 to 0.97) | 0.038 | ||
|
| ||||
| Age, per yearb | 1.14 (1.11 to 1.18) | <0.001 | 1.13 (1.10 to 1.17) | <0.001 |
|
| ||||
| Body mass indexb | 1.03 (0.99 to 1.06) | 0.11 | ||
|
| ||||
| Parity | 1.00 (0.87 to 1.15) | 0.99 | ||
|
| ||||
| Education levelb | <0.001 | |||
| Lower (versus higher) | 2.75 (1.84 to 4.13) | <0.001 | 2.31 (1.50 to 3.54) | <0.001 |
| Intermediate (versus higher) | 1.27 (0.81 to 1.20) | 0.30 | ||
|
| ||||
| Pelvic floor surgeryb | 1.68 (1.17 to 2.43) | 0.005 | ||
|
| ||||
| Charlson Comorbidity Indexb,d | 1.41 (1.13 to 1.76) | 0.003 | ||
OR = odds ratio. PFDI-20 = Pelvic Floor Distress Inventory-20, range 0–300.
Final multivariable logistic regression model, criterion for removal P≥0.05, Nagelkerke R2 = 0.208.
Items selected with P<0.250.
24 missing items for PFDI-20 score.
2 missing items for Charlson Comorbidity Index.
Table 3 presents the results of the univariate and multivariable linear regression analyses within the group of sexually active women. The final multivariable linear regression model included PFDI-20 score (P<0.001) and pelvic floor surgery (P = 0.018). The explained variance of this model was 0.138. This indicates that more pelvic floor symptom distress, indicated by a higher PFDI-20 score, and having had pelvic floor surgery in the past are independent predictors of poor sexual functioning (lower PISQ-12 score).
Table 3.
Association between patient characteristics, pelvic floor symptoms (PFDI-20), degree of prolapse, and sexual function in sexually active women (PISQ-12) (n = 387)a: results of linear regression analyses
| Variables | Univariate logistic regression | Multivariable logistic regressionb | ||
|---|---|---|---|---|
|
|
|
|||
| B (95% CI) | P-value | B (95% CI) | P-value | |
| PFDI-20, per point (0 to 300)c,d | −0.05 (−0.07 to −0.04) | <0.001 | −0.051 (−0.07 to −0.04) | <0.001 |
|
| ||||
| Prolapse | ||||
| Mild (versus no prolapse) | −0.19 (−1.56 to 1.18) | 0.79 | ||
| Advanced (versus no prolapse) | 0.35 (−1.15 to 1.86) | 0.64 | ||
|
| ||||
| Age, per yearc | −0.06 (−0.17 to 0.04) | 0.23 | ||
|
| ||||
| Body mass index | −0.03 (−0.15 to 0.08) | 0.60 | ||
|
| ||||
| Parity | 0.12 (−0.35 to 0.59) | 0.62 | ||
|
| ||||
| Education | ||||
| Lower (versus higher) | 0.076 (−1.18 to 1.33) | 0.91 | ||
| Intermediate (versus higher)c | −0.81 (−2.11 to 0.50) | 0.22 | ||
|
| ||||
| Pelvic floor surgeryc | −2.27 (−3.60 to −0.95) | 0.001 | −1.517 (−2.77 to − 0.26) | 0.018 |
|
| ||||
| Charlson Comorbidity Indexe | −0.20 (−1.07 to 0.68) | 0.66 | ||
B = unstandardised B coefficient. CI = confidence interval. PFDI-20 = Pelvic Floor Distress Inventory-20. PISQ-12 = Pelvic Organ Prolapse/Urinary Incontinence Sexual Function Questionnaire-12. R2 = explained variance.
6 out of 393 missing values for PISQ-12, resulting in n = 387 eligible women.
Final multivariable linear regression model, criterion for removal P≥0.05, R2 = 0.138.
Items selected with P<0.250.
9 missing items for PFDI-20 score.
1 missing item for Charlson Comorbidity Index.
DISCUSSION
Summary
More than 60% of women in the study population were sexually active. In the total population of older women with pelvic floor symptoms, it was found that increasing age and lower educational level were predictors of sexual inactivity, whereas pelvic floor symptoms and prolapse were not. This indicates that sexually inactive women do not abstain from sexual intercourse because of pelvic floor symptoms or prolapse. In the sexually active women, poorer sexual functioning was associated with more symptom distress and with pelvic floor surgery, but not with severity of prolapse. Sexual inactivity and dysfunctioning are multifactorial problems. It must be emphasised that part of the variance of these problems is explained by the study models but also that a considerable part of the variance is explained by variables that were not investigated.
Strengths and limitations
One strength of the study is the primary care setting; this allows inferences to be made about women visiting a GP, which cannot be done with the results from studies done in urogynaecology clinics. Another strength is that the study included both sexually active and sexually inactive women with a partner. The absence of a partner is the main cause of sexual inactivity among older women.11,13 Therefore, this selection allows examination of whether it is likely that these sexually inactive women abstain from sexual intercourse because of pelvic floor symptoms or prolapse. Finally, a condition-specific questionnaire for sexual functioning was used; this type of questionnaire is more sensitive than general questionnaires for detecting differences in sexual functioning that are due to pelvic floor symptoms.24
A limitation of the study is that women were screened for pelvic floor symptoms that were possibly related to a urogenital prolapse, including faecal obstruction for which women have to use vaginal splinting. However, the study did not screen for faecal incontinence. About 6% of women (aged ≥40 years) suffer from faecal incontinence without any other pelvic floor symptoms.25 This means that women with faecal incontinence may be under-represented in the study population. All women included in this study were screened for pelvic floor symptoms and were consequently recruited for a trial in which conservative treatments for pelvic organ prolapse are investigated. Therefore, care must be taken in generalising the results of this study to all women (≥55 years) who visit their GP for pelvic floor problems. However, the study considers that most women who consult their GP for pelvic floor symptoms do this because they want to be treated. For this reason, they believe that the study selection is comparable to women (≥55 years) who visit their GP for pelvic floor problems. Finally, the cross-sectional design does not allow causal inferences to be made. This means that predictors of poor sexual functioning that were found in this study may be useful for the recognition of sexual problems but the effect of interventions on sexual functioning has to be explored.
Comparison with existing literature
In the study population of women aged ≥55 years, it was found that increasing age was a predictor of sexual inactivity,26 but not of poor sexual functioning. This is in agreement with the study of Handa et al,8 who also found that increasing age was not a predictor of poor sexual functioning. Studies that also included younger women found that a decrease in sexual functioning was mainly attributable to increasing age.3,10–13 This indicates that age may be a predictor of poor sexual functioning in premenopausal women but not in older women.
The study also found that distress as a result of pelvic floor symptoms was a predictor of poor sexual functioning, whereas a more severe prolapse was not; this is in agreement with Handa et al and Lowenstein et al, who concluded that worse sexual function is associated with more severe pelvic floor symptoms and not with prolapse stage.8,9
In the study population, having had pelvic floor surgery was related to poor sexual functioning. From previous studies, it is known that improvement or deterioration of sexual function after surgery seems to depend on the surgical technique used.27 However, there is no information available about the surgical procedure that was used in women who underwent pelvic floor surgery and therefore it is hard to explain the association between pelvic floor surgery and sexual functioning. Although the current evidence on the influence of pelvic floor surgery on sexual function is still inconclusive,28–31 pelvic floor surgery results in anatomic and functional changes in the pelvic floor area that potentially influence sexuality.
The study also found that lower educational level is a predictor of sexual inactivity in older women, but evidence on this topic is also conflicting. Some researchers have concluded that a lower educational level is associated with sexual inactivity, while others concluded that it was not.32,33
Implications for research and practice
The results of this study are useful for GPs, as they may encourage them to ask about sexual problems in women who present with pelvic floor symptoms. Actively asking about sexual problems may decrease the threshold for women experiencing these problems to discuss them with their GP.34 Another implication of the study findings is related to the indications for pelvic floor surgery. The decision to perform pelvic floor surgery should be based on the burden of symptoms, not on the severity of prolapse. That is why it is important to carefully take a medical history, in which asking about sexual problems should be an integral part.
Further research should focus on other predictive factors for sexual functioning of older women, such as physical and psychological wellbeing, comorbidity, medication, social functioning, and partner-related factors. Such a study should preferably also include women without pelvic floor symptoms, in order to be able to compare sexual functioning in women with these symptoms to that of women who are symptom free. It is also recommended to include women without a partner, to investigate whether pelvic floor symptoms inhibit women from entering new sexual relationships. Furthermore, it would be useful to know if improvement of pelvic floor symptoms leads to an improvement of sexual function, since this would offer valuable information to GPs for the treatment of women with sexual problems and pelvic floor symptoms. Therefore, future studies should focus on the effects of treatment of pelvic floor symptoms on sexual functioning, preferably, in a randomised controlled trial to allow causal inferences.
Acknowledgments
The authors thank all the women and GPs who participated in this study.
Funding
The Netherlands Organization for Health Research and Development (ZonMw) funded this study (4201.1001).
Ethical approval
This study was approved by the Medical Ethics Committee of the University Medical Centre Groningen, the Netherlands (METc2009.215).
Competing interests
The authors have stated that there are none.
Discuss this article
Contribute and read comments about this article: www.bjgp.org/letters
REFERENCES
- 1.Nygaard I, Barber MD, Burgio KL, et al. Prevalence of symptomatic pelvic floor disorders in US women. JAMA. 2008;300(11):1311–1316. doi: 10.1001/jama.300.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jelovsek JE, Maher C, Barber MD. Pelvic organ prolapse. Lancet. 2007;369(9566):1027–1038. doi: 10.1016/S0140-6736(07)60462-0. [DOI] [PubMed] [Google Scholar]
- 3.Athanasiou S, Grigoriadis T, Chalabalaki A, et al. Pelvic organ prolapse contributes to sexual dysfunction: a cross-sectional study. Acta Obstet Gynecol Scand. 2012;91(6):704–709. doi: 10.1111/j.1600-0412.2012.01396.x. [DOI] [PubMed] [Google Scholar]
- 4.Novi JM, Jeronis S, Morgan MA, Arya LA. Sexual function in women with pelvic organ prolapse compared to women without pelvic organ prolapse. J Urol. 2005;173(5):1669–1672. doi: 10.1097/01.ju.0000154618.40300.c8. [DOI] [PubMed] [Google Scholar]
- 5.Tok EC, Yasa O, Ertunc D, et al. The effect of pelvic organ prolapse on sexual function in a general cohort of women. J Sex Med. 2010;7(12):3957–3962. doi: 10.1111/j.1743-6109.2010.01940.x. [DOI] [PubMed] [Google Scholar]
- 6.Jelovsek JE, Barber MD. Women seeking treatment for advanced pelvic organ prolapse have decreased body image and quality of life. Am J Obstet Gynecol. 2006;194(5):1455–1461. doi: 10.1016/j.ajog.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 7.Barber MD, Visco AG, Wyman JF, et al. Continence Program for Women Research Group Sexual function in women with urinary incontinence and pelvic organ prolapse. Obstet Gynecol. 2002;99(2):281–289. doi: 10.1016/s0029-7844(01)01727-6. [DOI] [PubMed] [Google Scholar]
- 8.Handa VL, Cundiff G, Chang HH, Helzlsouer KJ. Female sexual function and pelvic floor disorders. Obstet Gynecol. 2008;111(5):1045–1052. doi: 10.1097/AOG.0b013e31816bbe85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lowenstein L, Gamble T, Sanses TV, et al. Sexual function is related to body image perception in women with pelvic organ prolapse. J Sex Med. 2009;6(8):2286–2291. doi: 10.1111/j.1743-6109.2009.01329.x. [DOI] [PubMed] [Google Scholar]
- 10.Weber AM, Walters MD, Schover LR, Mitchinson A. Sexual function in women with uterovaginal prolapse and urinary incontinence. Obstet Gynecol. 1995;85(4):483–487. doi: 10.1016/0029-7844(94)00434-F. [DOI] [PubMed] [Google Scholar]
- 11.Rogers GR, Villarreal A, Kammerer-Doak D, Qualls C. Sexual function in women with and without urinary incontinence and/or pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12(6):361–365. doi: 10.1007/s001920170012. [DOI] [PubMed] [Google Scholar]
- 12.Lukacz ES, Whitcomb EL, Lawrence JM, et al. Are sexual activity and satisfaction affected by pelvic floor disorders? Analysis of a community-based survey. Am J Obstet Gynecol. 2007;197(1):88.e1–88.e6. doi: 10.1016/j.ajog.2007.02.053. [DOI] [PubMed] [Google Scholar]
- 13.Fashokun TB, Harvie HS, Schimpf MO, et al. Sexual activity and function in women with and without pelvic floor disorders. Int Urogynecol J. 2013;24(1):91–97. doi: 10.1007/s00192-012-1848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birkhauser MH. Quality of life and sexuality issues in aging women. Climacteric. 2009;12(suppl 1):52–57. doi: 10.1080/13697130903013163. [DOI] [PubMed] [Google Scholar]
- 15.Fritel X, Varnoux N, Zins M, et al. Symptomatic pelvic organ prolapse at midlife, quality of life, and risk factors. Obstet Gynecol. 2009;113(3):609–616. doi: 10.1097/AOG.0b013e3181985312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiegersma M, Panman CM, Kollen BJ, et al. Pelvic floor muscle training versus watchful waiting or pessary treatment for pelvic organ prolapse (POPPS): Design and participant baseline characteristics of two parallel pragmatic randomized controlled trials in primary care. Maturitas. 2014;77(2):168–173. doi: 10.1016/j.maturitas.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 17.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 18.Rogers RG, Kammerer-Doak D, Villarreal A, et al. A new instrument to measure sexual function in women with urinary incontinence or pelvic organ prolapse. Am J Obstet Gynecol. 2001;184(4):552–558. doi: 10.1067/mob.2001.111100. [DOI] [PubMed] [Google Scholar]
- 19.Rogers RG, Coates KW, Kammerer-Doak D, et al. A short form of the Pelvic Organ Prolapse/Urinary Incontinence Sexual Questionnaire (PISQ-12) Int Urogynecol J Pelvic Floor Dysfunct. 2003;14(3):164–168. doi: 10.1007/s00192-003-1063-2. discussion 168. [DOI] [PubMed] [Google Scholar]
- 20.Barber MD, Kuchibhatla MN, Pieper CF, Bump RC. Psychometric evaluation of 2 comprehensive condition-specific quality of life instruments for women with pelvic floor disorders. Am J Obstet Gynecol. 2001;185(6):1388–1395. doi: 10.1067/mob.2001.118659. [DOI] [PubMed] [Google Scholar]
- 21.Barber MD, Walters MD, Bump RC. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7) Am J Obstet Gynecol. 2005;193(1):103–113. doi: 10.1016/j.ajog.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 22.Bump RC, Mattiasson A, Bo K, et al. The standardization of terminology of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996;175(1):10–17. doi: 10.1016/s0002-9378(96)70243-0. [DOI] [PubMed] [Google Scholar]
- 23.Baden WF, Walker TA. Genesis of the vaginal profile: a correlated classification of vaginal relaxation. Clin Obstet Gynecol. 1972;15(4):1048–1054. doi: 10.1097/00003081-197212000-00020. [DOI] [PubMed] [Google Scholar]
- 24.Omotosho TB, Rogers RG. Shortcomings/strengths of specific sexual function questionnaires currently used in urogynecology: a literature review. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(suppl 1):S51–S56. doi: 10.1007/s00192-009-0829-6. [DOI] [PubMed] [Google Scholar]
- 25.Rortveit G, Subak LL, Thom DH, et al. Urinary incontinence, fecal incontinence and pelvic organ prolapse in a population-based, racially diverse cohort: prevalence and risk factors. Female Pelvic Med Reconstr Surg. 2010;16(5):278–283. doi: 10.1097/SPV.0b013e3181ed3e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindau ST, Schumm LP, Laumann EO, et al. A study of sexuality and health among older adults in the United States. N Engl J Med. 2007;357(8):762–774. doi: 10.1056/NEJMoa067423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ratner ES, Erekson EA, Minkin MJ, Foran-Tuller KA. Sexual satisfaction in the elderly female population: A special focus on women with gynecologic pathology. Maturitas. 2011;70(3):210–215. doi: 10.1016/j.maturitas.2011.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kammerer-Doak D. Assessment of sexual function in women with pelvic floor dysfunction. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(suppl 1):S45–S50. doi: 10.1007/s00192-009-0832-y. [DOI] [PubMed] [Google Scholar]
- 29.Tunuguntla HS, Gousse AE. Female sexual dysfunction following vaginal surgery: a review. J Urol. 2006;175(2):439–446. doi: 10.1016/S0022-5347(05)00168-0. [DOI] [PubMed] [Google Scholar]
- 30.Thakar R. Review of current status of female sexual dysfunction evaluation in urogynecology. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20(suppl 1):S27–S31. doi: 10.1007/s00192-009-0830-0. [DOI] [PubMed] [Google Scholar]
- 31.Dietz V, Maher C. Pelvic organ prolapse and sexual function. Int Urogynecol J. 2013;24(11):1853–1857. doi: 10.1007/s00192-013-2176-x. [DOI] [PubMed] [Google Scholar]
- 32.Palacios-Cena D, Carrasco-Garrido P, Hernandez-Barrera V, et al. Sexual behaviors among older adults in Spain: results from a population-based national sexual health survey. J Sex Med. 2012;9(1):121–129. doi: 10.1111/j.1743-6109.2011.02511.x. [DOI] [PubMed] [Google Scholar]
- 33.Addis IB, Van Den Eeden SK, Wassel-Fyr CL, et al. Sexual activity and function in middle-aged and older women. Obstet Gynecol. 2006;107(4):755–764. doi: 10.1097/01.AOG.0000202398.27428.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gott M, Hinchliff S. Barriers to seeking treatment for sexual problems in primary care: a qualitative study with older people. Fam Pract. 2003;20(6):690–695. doi: 10.1093/fampra/cmg612. [DOI] [PubMed] [Google Scholar]