Abstract
The efficacy of rituximab treatment in multiple sclerosis has renewed interest in the role of B cells in CNS autoimmunity. Here we show that B cells are the predominant MHC class II+ subset in the naïve CNS in mice, and they constitutively express pro-inflammatory cytokines. Incidence of experimental autoimmune encephalomyelitis (EAE) induced by adoptive transfer was significantly reduced in C3HeB/Fej μMT (B cell-deficient) mice, suggesting an important role for CNS B cells in initiating inflammatory responses. Initial T cell infiltration of the CNS occurred normally in μMT mice; however, lack of production of T cell cytokines and other immune mediators indicated impaired T cell reactivation. Subsequent recruitment of immune cells from the periphery driven by this initial T cell reactivation did not occur in μMT mice. B cells required exogenous IL-1β to reactivate Th17 but not Th1 cells in vitro. Similarly, reactivation of Th1 cells infiltrating the CNS was selectively impaired compared to Th17 cells in μMT mice, causing an increased Th17:Th1 ratio in the CNS at EAE onset and enhanced brain inflammation. These studies reveal an important role for B cells within the CNS in reactivating T cells and influencing the clinical manifestation of disease.
Introduction
Multiple sclerosis (MS) is an inflammatory, demyelinating disease of the central nervous system (CNS). Lesions comprised of immune cell infiltrates, plaques of demyelination, and axonal damage are hallmarks of MS, although substantial variation is seen among patients in both disease course and pathological features (1). Most patients with MS exhibit lesions primarily in the brain; only a small subset of patients exhibit lesions in the spinal cord without substantial involvement of the brain (2). The mechanisms that govern the pathological spectrum in MS, including localization of inflammatory lesions within the CNS, are poorly understood. Experimental autoimmune encephalomyelitis (EAE) is an animal model that has been widely used to study the pathogenesis of MS. EAE is induced by activation of T cells specific for myelin proteins such as myelin oligodendrocyte glycoprotein (MOG), either by immunization with myelin antigens in adjuvant (active induction) or by adoptive transfer of myelin-specific CD4+ T cells (passive induction) (3). Once the activated myelin-specific T cells cross the blood brain barrier and infiltrate the CNS, they are reactivated upon encounter with local antigen presenting cells (APCs) presenting endogenous myelin antigen. T cell reactivation triggers an inflammatory cascade that ultimately results in demyelination and axonal loss (3).
In contrast to MS, parenchymal lesions are localized predominantly in the spinal cord in most EAE models. However, we developed a new EAE model in C3HeB/Fej mice in which significant inflammation and tissue damage occurs in the brain as well as the spinal cord (4). In contrast to the C57BL/6 EAE model, this model has the unique advantage that mechanisms responsible for regulating inflammation in the brain versus the spinal cord can be studied. We found that the ratio of myelin-specific T cells secreting IL-17 (Th17) to T cells secreting IFN-γ (Th1) infiltrating the CNS is a critical factor in determining whether inflammation is induced in the brain. T cells that are primed by immunization with MOG exhibit a higher Th17:Th1 ratio in C3HeB/Fej mice compared to other strains, and this higher ratio generates the conditions necessary to induce brain inflammation. In contrast, spinal cord inflammation occurred over a wide range of Th17:Th1 ratios, demonstrating that inflammatory responses triggered by T cell reactivation are regulated differently in the brain compared to the spinal cord.
While much attention has focused on the role of T cells in EAE, the observation that treatment with a B cell-depleting mAb targeting CD20 (rituximab) can reduce the number of lesions and relapses in relapsing-remitting MS patients has renewed interest in the role of B cells in the pathogenesis of MS (5, 6). The mechanisms by which B cell depletion ameliorates the clinical signs of MS are not understood. Early studies in patients with MS demonstrated a role for B cells in the production of antibodies that bind myelin components, promoting inflammation and demyelination (7). However, treatment with rituximab does not deplete antibody-secreting plasma cells, suggesting that other B cell functions such as antigen presentation or cytokine production may be responsible for their pathogenic effects in MS. In support of a pathogenic role for B cells in promoting T cell activity, B cell depletion in MS patients following rituximab treatment resulted in a decrease in absolute number of T cells in the cerebrospinal fluid (8), as well as decreased cytokine-producing and proliferating T cells among PBMCs (9).
Studies in EAE suggested that B cells exert distinct pathogenic or regulatory functions depending on the stage of disease pathogenesis. In some studies that actively induced EAE in B cell-deficient (μMT) mice, a regulatory role for B cells was observed that was associated with B cell-dependent production of IL-10 (10, 11). More recently, EAE has been studied using anti-CD20 mAb to deplete B cells. In one study, B cell depletion prior to active EAE induction with MOG35-55 peptide exacerbated disease, which was associated with loss of an IL-10-secreting regulatory B cell subset (12). In contrast, B cell depletion after EAE onset decreased disease severity and reduced the proliferation of naïve T cells, suggesting that B cell-mediated activation of T cells may be more influential than regulatory activity later in disease (12). Another study found that immunization with recombinant MOG protein (rMOG) versus MOG peptide influenced the outcome of B cell depletion. Depletion of B cells either before or after active induction of EAE with MOG peptide resulted in more severe EAE (13). However, when EAE was induced by immunization with rMOG, B cell depletion before or after induction of EAE resulted in reduced severity of disease. This study suggested that B cells may promote EAE induction by acting as APCs that process and present MOG protein, as peripheral B cells exhibited evidence of activation upon immunization with MOG protein but not peptide (13).
Other studies that focused on T cell responses to myelin protein rather than synthetic peptide also implicated a pathogenic role for B cells as APCs. In a model that combined a MOG-specific transgenic TCR with a transgenic heavy chain from a MOG-specific B cell receptor, the increased frequency of MOG-specific B cells promoted activation and cytokine-production by the transgenic T cells in vivo, which increased the incidence of spontaneous EAE compared to mice expressing only the transgenic TCR (14, 15). In another study, mice expressing a different transgenic MOG-specific TCR developed spontaneous EAE that was accompanied by production of anti-MOG-specific antibodies by endogenous B cells (16). Importantly, anti-CD20-mediated depletion of B cells in this model largely eliminated the incidence of spontaneous EAE, suggesting that T cell interactions with B cells played a crucial role in initiating the spontaneous disease. Cytokines secreted by B cells may play an important role in shaping the outcome of T/B cell interactions that occur during CNS autoimmune disease. Secretion of IL-10 by naïve B cells and TNFα, lymphotoxin, and IL-6 by memory B cells has been implicated in the regulation of MS and EAE (9, 11, 17, 18). Production of these and other cytokines during antigen presentation to T cells could influence the effector function of the activated T cells. However, it is not known whether B cell production of these cytokines is important in the periphery and/or the CNS.
The studies described above suggest that B cells can play different roles at different times during the pathogenesis of CNS autoimmunity. However, these studies did not determine whether the B cell activities that influence CNS autoimmunity occur in the periphery or within the CNS. Here we addressed the role of B cells within the CNS during the earliest phase of T cell entry when reactivation of T cells is required. Surprisingly, we found that B cells represent the majority of MHC class II+ cells in the naïve CNS, and that they constitutively express pro-inflammatory cytokines. In the absence of B cells, adoptively transferred T cells still infiltrate the CNS, but in contrast to wild-type (WT) mice, do not trigger production of immune mediators necessary for recruitment of cells from the periphery. We also found that B cells preferentially reactivate IFN-γ-compared to IL-17-producing T cells. Similarly, the absence of B cells in μMT mice resulted in impaired reactivation of Th1 compared to Th17 cells in the CNS. This caused an increase in the Th17:Th1 ratio of T cells in the brain and altered the clinical manifestation of disease. Together, our data identify novel mechanisms by which B cells not only contribute to the initiation of inflammatory responses in the CNS but may also influence the localization of lesions within the brain and spinal cord.
Materials and Methods
Mice
C3HeB/Fej, C3.SW-H-2b/SnJ (C3H.SW), B6.129S2-Igh-6tm1Cgn/J and B10.PL mice were purchased from The Jackson Laboratory and maintained in a specific pathogen free facility at the University of Washington. To generate μMT mice, the B6.129S2-Igh-6tm1Cgn/J strain was backcrossed to C3HeB/Fej and C3H.SW for 12 generations. The Institutional Animal Care and Use Committee at the University of Washington approved all procedures.
rMOG protein and peptides
Rat rMOG protein (1–125) was produced in Escherichia coli and purified as previously described (19). MOG peptides 79–90 (GKVALRIQNVRF) and 97–114 (TCFFRDHSYQEEAAVELK) were synthesized by GenScript.
Active EAE Induction
Active EAE was induced by immunizing 8–12 week old mice subcutaneously with 100 μg of rMOG emulsified in CFA containing 1 mg/ml of heat-killed mycobacteria (Sigma), accompanied by two injections of 200 ng pertussis toxin (List Biological Laboratories), as previously described (20). Animals were observed daily for clinical signs. We scored the severity of EAE as follows: grade 1, paralyzed tail, hindlimb clasping, hyperactivity; grade 2, head tilt, hindlimb weakness; grade 3, one paralyzed leg, mild body leaning; grade 4, two paralyzed legs, moderate body leaning; grade 5, forelimb weakness, severe body leaning; grade 6, hunched, breathing difficulty, body rolling; grade 7, moribund. Atypical EAE was determined by the presence of one or more of the following symptom(s): hyperactivity, head tilt, body leaning and rolling.
Passive EAE induction/adoptive transfers
Cells were isolated from spleen and lymph nodes of WT mice 7 days after rMOG immunization and cultured at 1 × 107 cells per ml for 3 days with MOG97-114 (10 μM). To generate cells for transfer with a Th17:Th1 ratio of 1:1, we included 10 ng/ml rIL-23 (R&D) in the culture. To skew cells toward a Th1 phenotype (Th17:Th1 ratio ~1:8), we included 10 ng/mL IL-12 (eBioscience). To skew cells toward a Th17 phenotype (Th17:Th1 ratio ~3:1), we included 10 ng/mL IL-23 (R&D) and 10 μg/mL anti-IFN-γ (XMG1.2, eBioscience). Viable cells were isolated from a Lympholyte gradient (Cedarlane) and intraperitoneally injected (2 × 107 cells per mouse) into mice that were sublethally irradiated (250 rads) on day -1. In some experiments, we purified the CD4+ T cells using a CD4+ T cell isolation kit and an AutoMACS separator (Miltenyi) and injected 5 × 106 CD4+ T cells intraperitoneally into non-irradiated mice. The severity of EAE was scored as described above.
Isolation of CNS mononuclear cells
Mononuclear cells were isolated from the CNS after cardiac perfusion with PBS as previously described (21). Briefly, brain and spinal cord were dissociated through sterile stainless steel mesh and centrifuged at 4°C for 10 min at 3000 rpm. Cell pellets were resuspended in 30% Percoll, overlaid onto 70% Percoll, and centrifuged without brake at 25°C for 20 min at 2600 rpm. Cells were collected from the 30%–70% Percoll interface.
Flow cytometry
Cells were incubated with Fc block (clone 2.4G2; eBioscience) in 5% normal mouse serum for 15 min at room temperature, washed and stained with mAbs for 30 min at 4°C. mAbs for CD45 (30-F11), CD19 (1D3), F4/80 (BM8), CD11b (M1/70), and CD11c (N418) were from eBioscience. mAbs for MHC class II (I-Ak; 11-5.2), CD4 (RM4-5), Thy1.1 (OX7), CD79b (HM79b), CD138 (281-2), CD80 (16-10A1), CD86 (GL1), and CD43 (S7) were from BD Biosciences. Intracellular cytokine staining for IL-17 and IFN-γ was performed according to manufacturer’s directions using mAbs and staining kits from BD Biosciences. BrdU and AnnexinV staining kits were purchased from BD Biosciences.
In vivo T cell recruitment assay
Thy1.1+ T cells from MOG-immunized donors were activated in vitro for three days with MOG97-114 and transferred into wild-type Thy1.2 recipients. Either 3 mg/kg FTY720 or vehicle (5% DMSO) was injected intraperitoneally daily beginning on day 4 post-transfer. CNS mononuclear cells were isolated from mice on day 7 for analysis.
ELISPOT assays
Numbers of antigen-specific cytokine-producing cells were determined by culturing cells overnight with and without antigen in duplicate wells of 96-well ELISPOT plates (Millipore). ELISPOT assays were carried out according to BD Biosciences protocols and analyzed on an ImmunoSpot Analyzer (CTL). IFN-γ–specific mAb pairs, IL-17–specific (TC11-18H10) and biotinylated IL-17–specific (TC11-8H4.1) mAbs were from BD Biosciences. For detection of IL-17 and IFN-γ producing cells in the CNS of mice with EAE, total mononuclear cells isolated separately from the brains and spinal cords of perfused mice (typically 1–10 × 105 cells per well) were plated with or without MOG97-114 (10 μM).
Th1 and Th17 reactivation assays
Th1 and Th17 cells were generated by culturing cells isolated from spleen and lymph nodes of WT mice immunized 7 days earlier with rMOG at 1 × 107 cells per ml for 3 days with MOG97-114 (10 μM). The cultures included IL-12 (10 ng/mL, eBioscience) to generate Th1-skewed cells and IL-23 (10 ng/ml, R&D) and anti-IFN-γ (10 μg/ml, XMG1.2, eBioscience) to generate Th17-skewed cells. Cells were split after 3 days and maintained in culture with 10 U/ml IL-2 without MOG97-114 for an additional 4 days. CD4+ T cells from these cultures as well as naïve splenic B cells (CD19+CD43−) or non-B cells (CD43+, CD11c+ and CD11b+) were sorted on a FACS Aria cell sorter (BD) and the CD4+ T cells (5 × 104 cells per well) were co-cultured overnight with either B cells, non-B cells, or bulk splenocytes (each at 5 × 105 cells per well) with or without 25 μg/ml rMOG. Where indicated as “Activated”, the B or non-B cell APCs were stimulated with 10 μg/mL anti-CD40 (R&D) and 20 μg/ml LPS for 4 hours prior to culture. IL-23 (10 ng/ml, R&D), IL-6 (20 ng/ml, eBioscience), and IL-1β (10 ng/ml, eBioscience) were included as indicated. The number of antigen-specific T cells producing IL-17 or IFN-γ was detected by ELISPOT as described. The percent reactivation was calculated by normalizing the number of antigen-specific spots produced by either Th1 or Th17 cells in response to the indicated APCs to the number of antigen-specific IFN-γ or IL-17 spots obtained by co-culture of either Th1 or Th17 cells with bulk naïve splenocytes (100%). In ELISPOT experiments using CNS cells as APCs, bulk CNS cells were pooled from 6–8 perfused, naïve WT or μMT mice and stained for MHC class II, CD19, CD11b, and CD11c to calculate the number of B cells and non-B cell APCs in the bulk population. The number of CNS cells plated in each ELISPOT well was normalized to the number of non-B cell APCs such that wells containing either WT or μMT CNS APCs had the same number of non-B APCs (approximately 7000 per well), but wells with WT CNS APCs also contained approximately 2 × 104 B cells. Th1 and Th17 cells were generated and sorted as described above and co-cultured overnight with the APCs and 25 μg/ml rMOG or media alone to determine numbers of antigen-specific IFN-γ or IL-17 spots.
RT-qPCR of gene expression
For analysis of gene expression in tissue, total RNA was isolated using the RNeasyLipid Tissue Midi kit (Qiagen) from brains of recipients of MOG-specific T cells (Th17:Th1 ~1.1) on day 5 post-transfer or after onset of EAE, or from healthy control mice on day 5 post-transfer of 5 × 106 activated polyclonal T cells. The activated polyclonal T cells were generated by stimulating naïve CD4+ T cells with anti-CD3/anti-CD28 Dynabeads according to Invitrogen protocols. For analysis of gene expression in CNS cells, RNA was isolated from unstimulated cells sorted from the CNS of perfused naïve wild-type mice or from mice at onset of EAE induced by adoptive transfer of CD4+ MOG-specific T cells (Th17:Th1 ~1:1) into non-irradiated recipients. Mononuclear cells were pooled from the CNS of 3–5 mice, and approximately 1 × 105 B cells (CD45hiCD19hiCD11b−), plasmablasts (CD45intCD19int CD11b−), CD45hiCD11b+/CD11c+ cells, and microglia (CD45intCD11b+) were sorted using a FACS Aria cell sorter (BD). B cells were also sorted simultaneously from the spleen and blood of naïve mice. Total RNA was isolated from cells directly ex vivo using the RNeasy Micro kit (Qiagen). cDNA was generated using the Superscript III first strand synthesis system (Invitrogen). Real time quantitative PCR was performed in triplicate using SYBR Green PCR master mix and an AB7300 or Viia7 (Applied Biosystems). All data were normalized to GAPDH. Data were analyzed using the comparative Ct method to obtain relative quantitation values. Fold induction for day 5 brain tissue was calculated relative to healthy brain tissue from mice that had received anti-CD3/anti-CD28 activated T cells. Mouse primer sequences are listed in Supplementary Table I.
Analysis of Th17 and Th1 cell priming in vivo
Wild-type or μMT mice were immunized with rMOG in CFA and injected with pertussis toxin. Splenocytes were isolated 7 days after immunization and plated at 1 × 106 cells per well in anti-IL-17 or anti-IFN-γ coated wells of an ELISPOT plate with or without rMOG (25 μg/ml), MOG79-90 (10 μM), or MOG97-114 (10 μM). After 16 hours culture, IL-17 and IFN-γ producing cells were detected as described above.
Perfusion control
Splenocytes isolated from naïve wild-type mice were labeled with CFSE (1 μM, Molecular Probes) and 2 × 107 cells were transferred i.v. into naïve recipients. One hour later, half of the mice were perfused, while the other half were not perfused prior to isolation of CNS mononuclear cells. Cells were analyzed by flow cytometry to compare the percentage of CFSE+ cells within a CD45+CD19+ gate in CNS cells from perfused and unperfused mice.
Statistics
Statistical analysis was performed using Prism software (GraphPad). Significance between groups was determined using either Fisher’s exact test, Chi squared test, Student’s t test, or Mann Whitney non-parametric t test, as indicated. A p value of less than 0.05 was considered significant.
Results
B cells influence both the priming and effector stages of EAE
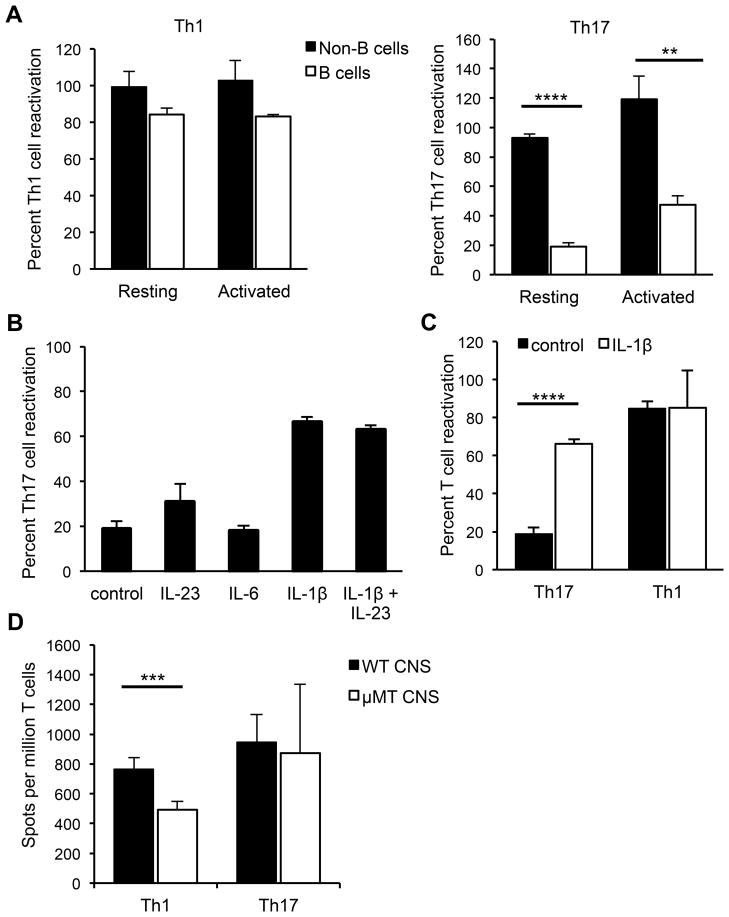
Our previous studies showed that C3HeB/Fej (H-2k) mice immunized with rMOG exhibited the spinal cord inflammation typically seen in EAE but differed from most mouse strains by also exhibiting extensive parenchymal brain inflammation. Inflammatory lesions were consistently observed in the cerebellar and periventricular white matter, brain stem, fimbria hippocampi, and cortex (4). Lesions in one or more of these areas of the brain correlate with clinical signs such as leaning, rolling, spasticity, and hyperreflexia, referred to as atypical EAE. In contrast, MHC congenic C3H.SW (H-2b) mice developed classic EAE characterized by tail and hind limb paralysis with parenchymal inflammation restricted predominantly to the spinal cord (4). The difference in inflammatory patterns was due to the preponderance of Th17 cells generated in C3HeB/Fej mice that respond to one of the epitopes in rMOG (MOG97-114) presented by I-Ak. The abundance of Th17 cells resulted in a Th17:Th1 ratio ≥ 1 among T cells infiltrating the brain, which we found was a critical factor in promoting brain inflammation. In contrast, the MOG35-55 epitope targeted in C3H.SW mice elicited a strong predominance of Th1 cells such that the Th17:Th1 ratio in the brain was < 1 and brain inflammation was not induced. We also investigated whether B cells played a role in defining the clinical phenotype of EAE in C3HeB/Fej mice; however, our results showed that C3HeB/Fej WT and μMT (B cell-deficient) mice exhibited the same atypical EAE and neuroinflammatory patterns in actively induced EAE (4). Interestingly, the incidence of EAE in μMT mice was lower compared to WT mice, suggesting that B cells may influence the events that trigger EAE. To explore this finding further, we compared the incidence of EAE in both C3HeB/Fej and C3H.SW mice on the μMT background. The incidence of EAE induced by immunization with rodent rMOG protein was significantly reduced compared to WT mice for both strains (Table I). The μMT C3HeB/Fej and C3H.SW mice that did develop EAE exhibited a similar day of onset, clinical course, and severity compared to their wild-type controls. These data suggest that B cells may be important in EAE to trigger the initial inflammatory response, but may be less essential once the disease has been initiated in these strains.
Table I.
Influence of μMT genotype on susceptibility of C3HeB/Fej and C3H.SW mice to EAE
| Induction Method | Strain | Genotype | Incidence | Mean day of onseta | Mean maximum scorea | Recovery |
|---|---|---|---|---|---|---|
| Active | C3H/Fej | WT | 88% (7/8) | 18 ± 5 | 5.0 ± 0.3 | 0/7 |
| Active | C3H/Fej | μMT | 33% (5/15)b | 21 ± 4 | 4.9 ± 0.5 | 0/5 |
| Active | C3H.SW | WT | 100% (6/6) | 14 ± 1 | 3.1 ± 0.1 | 3/6 |
| Active | C3H.SW | μMT | 50% (5/10)c | 15 ± 4 | 3.6 ± 0.2 | 3/5 |
| Passive | C3H/Fej | WT | 96% (29/30) | 6.7 ± 0.9 | 4.6 ± 1.3 | 0/29 |
| Passive | C3H/Fej | μMT | 21% (6/28)d | 6.6 ± 0.5 | 4.3 ± 1.9 | 0/6 |
Data shown (mean ± SEM) are from mice with EAE. p values indicating significant differences between WT vs μMT mice are as follows:
p=0.01,
p=0.03,
p<0.0001, Chi-squared test.
Our results suggested that B cells play a pathogenic role in EAE by promoting initiation of disease, which is consistent with a function for B cells as APCs. We analyzed whether B cells influence priming of Th1 and Th17 cells in C3HeB/Fej mice by immunizing WT and μMT mice with rodent rMOG in CFA. Splenocytes were harvested 7 days post-immunization and the responses of T cells were analyzed by ELISPOT. T cells in C3HeB/Fej mice respond to two epitopes within MOG, MOG79-90 and MOG97-114 (4). Compared to WT mice, significantly fewer T cells from μMT mice produced IL-17 in response to both MOG79-90 and MOG97-114, and significantly fewer T cells produced IFN-γ in response to MOG97-114 (Supplementary Figure 1). These data indicate that B cells in C3HeB/Fej mice facilitate effector T cell priming in vivo.
Our data on T cell priming are consistent with earlier studies that implicated a role for T/B cell interactions in the periphery in promoting EAE (14–16). However, the low incidence of EAE in μMT mice could also occur if B cells promote antigen presentation in the CNS during the initiation of EAE, which has not been addressed in previous studies. To investigate whether B cells play a role during the effector stage of disease when T cells are reactivated within the CNS, we induced EAE in C3HeB/Fej WT and μMT mice by adoptive transfer of T cells isolated from rMOG-immunized WT mice and re-stimulated in vitro with MOG97-114. In these experiments, the ratio of Th17:Th1 cells in the transferred population was approximately 1:1 (data not shown). The incidence of EAE was significantly reduced in μMT compared to WT recipients (Table I), indicating that B cells play an important role in the effector stage of disease independent of their role during T cell priming.
B cells are the predominant MHC class II+ population within the naïve CNS
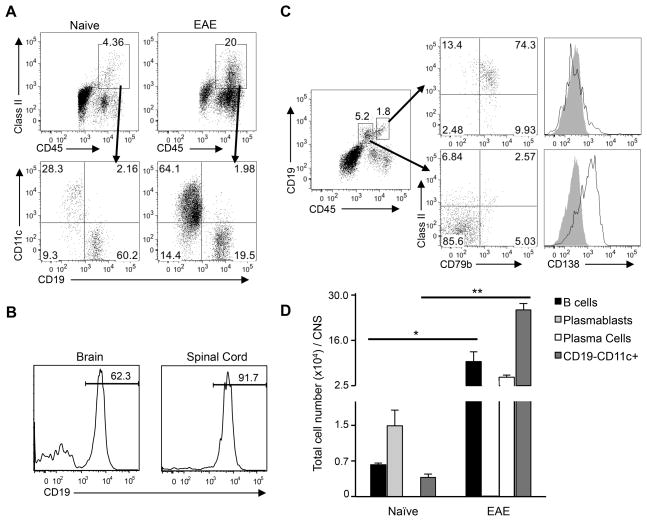
To determine whether B cells could influence the activity of CD4+ T cells that infiltrate the CNS, we first determined what percentage of MHC class II+ cells in the CNS of naïve mice are B cells. Mononuclear cells were isolated from well-perfused, naïve C3HeB/Fej mice and analyzed by flow cytometry. Surprisingly, we found that the majority of the MHC class II+ cells in the brain were CD19+ B cells (Figure 1A, left), with the remaining MHC class II+ cells consisting primarily of CD11c+ dendritic cells. Control experiments that compared the B cell population in cells isolated from the CNS of perfused versus non-perfused mice following injection of labeled B cells confirmed that blood contamination did not account for the B cells that we observed in the naïve CNS (Supplementary Figure 2A). In the spinal cord, B cells represented an even greater fraction of the MHC class II+ population, with > 90% of the MHC class II+ cells expressing CD19 (Figure 1B). Thus, while the absolute number of B cells in the naïve CNS is small, the abundance of B cells within the MHC class II+ population suggests that they could be the predominant APCs for the initial population of T cells infiltrating the non-inflamed CNS. The large influx of inflammatory cells that occurs at onset of EAE changes the composition of the MHC class II+ population, such that CD11c+ cells predominate and the percentage of CD19+ B cells is reduced. (Figure 1A, right). This suggests that B cells may be more influential as APCs during the initial reactivation of T cells required to trigger disease, while CD11c+ dendritic cells may be more influential at later stages of disease.
Figure 1. B cells comprise the majority of MHC class II+ cells in the naïve CNS.
Brain or spinal cord cells were pooled from 3–6 well-perfused C3HeB/Fej naïve mice or from mice at onset of EAE to analyze a minimum of 50,000 CD45+ events per sample by flow cytometry. (A) Representative MHC class II staining is shown for brain cells from naïve or EAE mice through a CD45+ gate (top panels). Lower panels show CD19 and CD11c staining through the MHC class II+ gate. (B) CD19 expression was analyzed on naïve CD45+MHC class II+-gated brain or spinal cord cells. (C) CD19 expression on CD45+-gated cells from brains of naïve mice reveals two distinct CD45+CD19+ populations (left). MHC class II and CD79b (middle) and CD138 (right) expression was analyzed on cells gated on each population (grey shading indicates isotype control). (D) Total number/brain (mean ± SEM) is shown for naïve and EAE mice of B cells (CD45hiCD19hi), plasmablasts (CD45intCD19int), plasma cells (CD45intCD19−) and dendritic cells (CD45hiCD11c+). (A–D) Similar results were observed in at least 3 independent experiments for both actively and passively induced EAE. *p < 0.05; **p < 0.01, Student’s t test.
Because our data indicate that CD19+ cells comprise the majority of MHC class II+ cells in the naïve CNS, we examined their phenotype in greater detail. By analyzing CD19 expression among CD45+ cells, we detected two distinct populations of CD19+ cells in the brain and spinal cord that were differentiated by levels of CD45 and CD19 expression (Figure 1C). The CD45hiCD19hi cells were MHC class II (I-Ak)+, CD79b+, CD138 (Syndecan-1)− (Figure 1C), and predominantly IgM+, CD80−, and CD43− (data not shown). This phenotype is typical of resting B cells found in secondary lymphoid tissues. In contrast, the CD45intCD19int cells expressed low-negative levels of I-Ak and were CD79b− and CD138+ (Figure 1C), a phenotype resembling short-lived, antibody-secreting plasmablasts. However, this plasmablast-like population observed in the CNS differed from plasmablasts in the spleen or blood of naïve mice by their lack of CD79b expression and low expression of MHC class II and other activation markers. To confirm that these findings were not specific to the C3Heb/Fej strain, we analyzed CNS cells in B10.PL mice. The two CD19+ populations described above were also observed in this mouse strain, indicating that the predominance of B cells among MHC class II+ cells in the CNS is not unique to a particular mouse strain (Supplementary Figure 2B).
Analyses of the absolute number of cells in the different cell subsets in the CNS of naïve mice revealed that both the plasmablast-like cells and MHC class II+ B cells outnumbered the CD11c+ cells (Figure 1D). In contrast, the number of CD11c+ dendritic cells in mice with EAE increased significantly accounting for their predominance in the MHC class II+ population. However, the absolute number of CD45hiCD19hi B cells also increased significantly in mice with EAE, suggesting that B cells may not only be the predominant APC at the initiation of disease, they could also play a substantial role after onset of EAE. Interestingly, the absolute number of CD45intCD19int plasmablast-like cells was dramatically reduced in mice with EAE, indicating that cells with this phenotype are not maintained in the inflammatory milieu in the CNS during EAE. However, a plasma cell population (CD45hiCD19−CD138+I-Ak−CD86+) was detected in mice with EAE that was comparable in number to the resting B cells (Figure 1D).
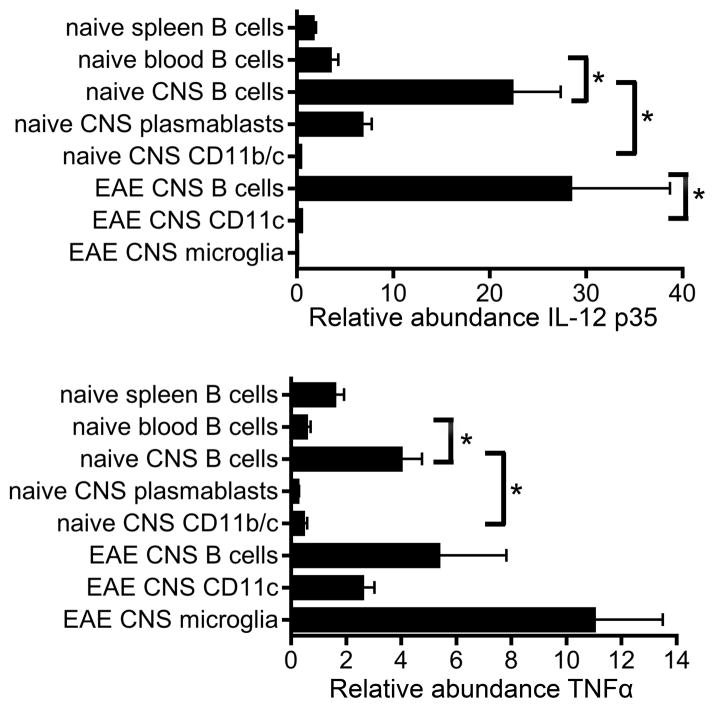
To determine whether CNS B cells influence the local environment in the naïve or inflamed CNS via cytokine production, we sorted B cells, plasmablasts, CD45hiCD11b/c+ cells, and microglia (CD45intCD11bint) from the CNS of naïve and EAE mice and measured cytokine mRNA levels directly ex vivo (without stimulation). Interestingly, IL-12 p35 was expressed by CNS B cells from both naïve and EAE mice at significantly higher levels than by CD11b/c+ cells (Figure 2). Naïve CNS plasmablasts also expressed IL-12 p35. TNFα mRNA expression was also detected in CNS B cells from both naïve and EAE mice at higher levels than CD11b/c+ cells, although more TNFα was produced by microglia than B cells during EAE. CNS B cells in naïve mice constitutively expressed significantly higher levels of IL-12 p35 and TNFα than B cells in the blood, indicating that CNS B cells have a functionally distinct phenotype compared to B cells in the periphery. IL-10, IL-1β, GM-CSF, IL-6, and IL-23 p19 transcripts were not detected in B cells sorted from the CNS of either naïve or EAE mice (data not shown). Additionally, IL-12 p35 and TNFα transcripts were not detected in CD45− cells from the CNS of naïve mice, indicating that astrocytes do not produce these cytokines in healthy mice. Thus, CNS B cells and plasmablasts are the major source of IL-12 p35 and TNFα within the non-inflamed CNS during the initial T cell infiltration.
Figure 2. CNS B cells express pro-inflammatory cytokines.
Relative abundance of IL-12p35 or TNFα transcripts was analyzed by RT-qPCR directly ex vivo for the indicated cell types sorted from naïve mice or mice with passive EAE. Each subset was analyzed in 3–4 independent samples generated by pooling cells from 3–5 mice prior to sorting. Relative transcript values were normalized to GAPDH. *p < 0.05, Student’s t test.
CNS B cells promote T cell recruitment to the CNS
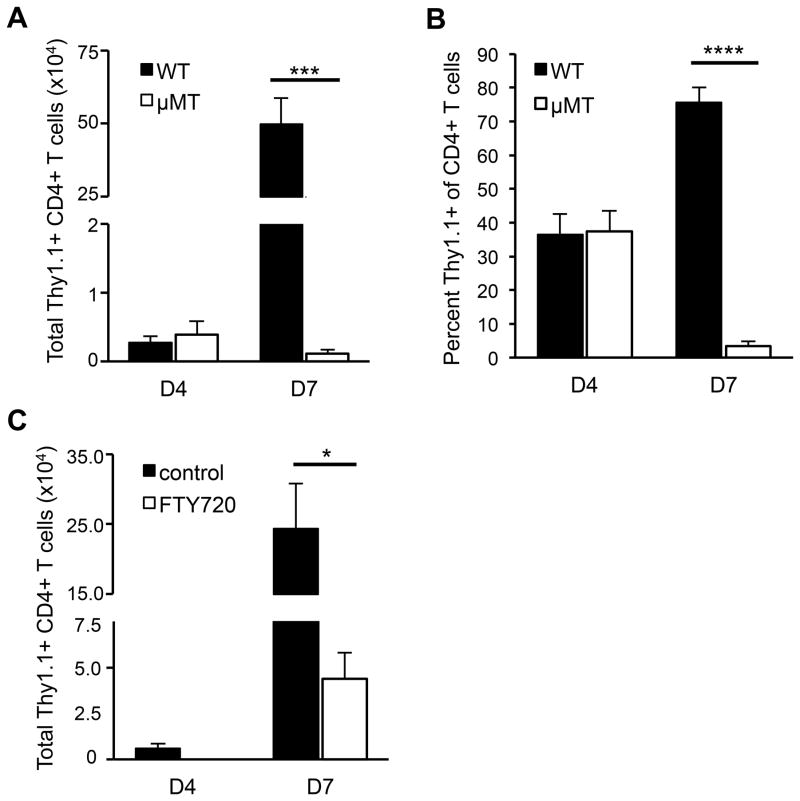
Because the majority of the MHC class II+ cells in the naïve CNS are B cells, we hypothesized that they may influence the earliest events in EAE induction that occur directly after T cells infiltrate the CNS. To test this, we transferred activated T cells isolated from rMOG-immunized mice into WT and μMT recipients and compared the numbers of donor T cells in the CNS on day 4 (pre-clinical) and day 7 (average day of EAE onset in WT mice). Comparable numbers of donor T cells were found in the CNS of both WT and μMT recipients on day 4 post-transfer (Figure 3A). At this time point, the number of endogenous host T cells in the CNS of both types of recipients was not increased beyond that seen in the CNS of naïve mice (data not shown). Donor T cells (5–10 × 105) were also found in the spleen on day 4 post-transfer in both WT and μMT recipients (data not shown). By day 7 post-transfer, the donor T cell number increased one hundred-fold relative to day 4 in the CNS of WT recipients (Figure 3A), and the mice exhibited clinical signs of EAE. In contrast, donor T cell numbers decreased on day 7 in the CNS of μMT recipients relative to day 4 and the mice exhibited no clinical signs. Additionally, while the number of host T cells increased slightly in the WT CNS, there was no increase in the number of host T cells in the CNS of μMT recipients on day 7 (data not shown). These data indicate that the initial donor T cell infiltration to the CNS is not impaired in μMT mice. However, in the absence of B cells, infiltrating T cells fail to initiate events that lead to an increase in T cell number and ultimately to EAE.
Figure 3. MOG-specific T cells fail to accumulate in the CNS in the absence of B cells.
Thy1.1+ T cells from MOG-immunized donors were activated in vitro with MOG97-114 and transferred into Thy1.2 WT or μMT recipients. CNS mononuclear cells were isolated on days 4 and 7 post-transfer and analyzed for (A) the total number of donor CD4+ Thy1.1+ T cells and (B) percent of Thy1.1+ donor cells among total CD4+ T cells (means ± SEM, n ≥ 5 mice per group). (C) FTY720 or vehicle was injected into WT mice daily starting on day 4 post-transfer of MOG-specific T cells. CNS cells were isolated from recipients prior to treatment on day 4 post-transfer and on day 7 from both treated and control recipients. The total number of CD4+ Thy1.1+ donor T cells in the CNS was determined by flow cytometry (means ± SEM, n ≥ 4 mice per group). (AC) Significant differences between means are indicated; *p < 0.05; ***p < 0.001; ****p < 0.0001, Student’s t test. Results are representative of at least 3 independent experiments.
The increase in T cell number in the CNS during the preclinical stage of EAE is usually attributed to a combination of proliferation of the initial infiltrating T cells and recruitment of a second wave of T cells from the periphery. To determine if proliferation of T cells that initially infiltrate the CNS is impaired in μMT mice, we analyzed BrdU incorporation in donor CD4+ T cells in the CNS of WT and μMT recipients between day 4 and 5 post-transfer. BrdU incorporation in donor T cells within the CNS was comparable between WT and μMT recipients during this time, indicating that B cells do not influence proliferation of the initial infiltrating T cells (Supplementary Figure 3A). The percent of BrdU+ T cells in the CNS of both WT and μMT recipients was comparable to levels of T cell proliferation within the CNS observed in other studies (22, 23), and was slightly but not significantly increased in the CNS compared to blood and spleen. Thus, most of the donor T cell proliferation observed in the periphery and CNS likely reflects the in vitro stimulation prior to T cell transfer rather than encounter with antigen in vivo. The similar extent of BrdU incorporation in donor T cells in the CNS of WT and μMT recipients between days 4 and 5 post-transfer suggests that proliferation does not account for the increase in donor T cells in the CNS of WT mice observed at day 7 post-transfer. We also investigated whether B cells influenced donor CD4+ T cell survival by analyzing AnnexinV+ donor T cells in the CNS of WT and μMT recipients on day 5 post-transfer. The percent of AnnexinV+ donor T cells was similar in WT and μMT recipients, indicating that T cell survival in the CNS is not significantly enhanced by the presence of B cells (Supplementary Figure 3B).
Because proliferation and apoptosis of donor T cells in the CNS were comparable in WT and μMT mice, we hypothesized that the increase in donor T cells in the CNS of WT mice on day 7 post-transfer resulted from recruitment of additional donor T cells from the periphery. This notion is supported by the observation that donor T cells represented approximately 35% of the total CD4+ CNS T cells on day 4 but represented 75% on day 7 post-transfer in WT recipients (Figure 3B). To test this hypothesis, we administered FTY720, a sphingosine-1-phosphate receptor modulator that causes T cell retention in secondary lymphoid tissues (24), daily beginning on day 4 to WT recipients of MOG-specific T cells, as only these recipients exhibited an increase in T cell number between days 4 and 7. The donor CD4+ T cell number in the CNS was analyzed on day 4 and day 7. The donor T cells in the CNS of vehicle-treated WT mice increased significantly between day 4 and day 7 (Figure 3C). In contrast, there were significantly fewer donor T cells on day 7 in mice treated with FTY720 compared to vehicle-treated mice (Figure 3C). Thus, recruitment of additional donor T cells from the periphery, and not enhanced proliferation or survival of the original infiltrating T cells, accounts for the preclinical increase in CNS donor T cells. These data suggest that the recruitment of a second wave of T cells is impaired in the absence of CNS B cells.
B cells promote production of inflammatory mediators triggered by infiltrating T cells
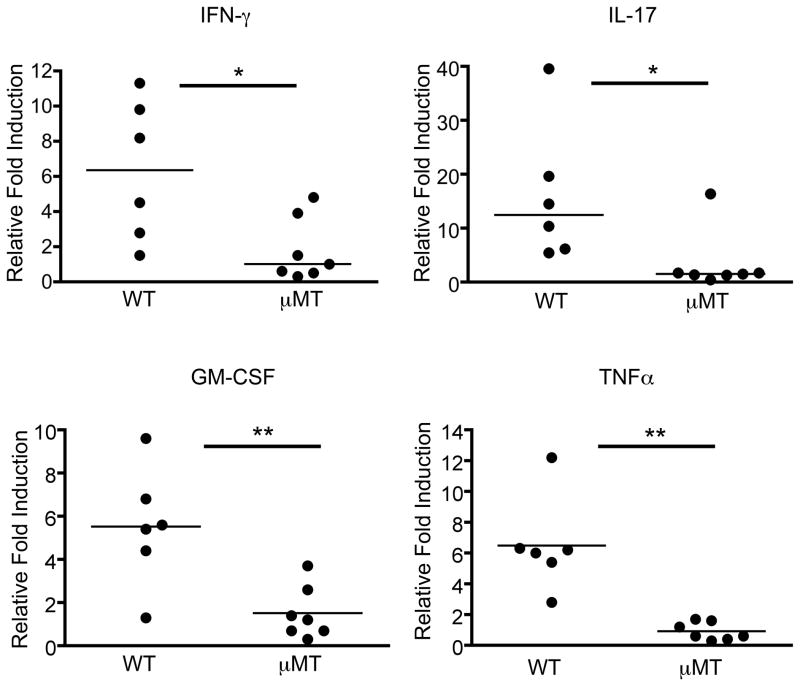
We hypothesized that the failure to recruit peripheral T cells during preclinical EAE in B cell-deficient recipients is due to impaired reactivation of the initial T cells that infiltrated the CNS. Reactivation of T cells within the CNS is believed to induce production of multiple cytokines, including IL-17, IFN-γ, GM-CSF, and TNFα (25). These cytokines act on various cell types within the CNS, including astrocytes and endothelial cells of the blood brain barrier, to elicit expression of adhesion molecules, integrin ligands, and chemokines that are important for recruiting cells from the periphery (26). Our hypothesis predicts that expression of these gene products involved in facilitating T cell recruitment would be impaired in μMT mice. To test this prediction, we first confirmed that genes reported to be induced in other EAE models (25) were also induced in C3HeB/Fej mice during EAE. Gene expression was compared by RT-PCR between brains of WT mice with EAE induced by adoptive transfer of MOG-specific T cells and brains of WT mice that received polyclonal CD4+ T cells activated in vitro with anti-CD3 and anti-CD28 (healthy controls). Of the genes induced in the brains of mice with clinical EAE, we then determined which were induced in preclinical WT C3HeB/Fej mice (5 days post-transfer of MOG-specific CD4+ T cells) compared to healthy controls (Supplementary Table I). Using these genes as indicators of T cell reactivation, we compared their expression in the brains of WT and μMT mice 5 days after transfer of CD4+ MOG-specific T cells. To ensure that tissue was harvested from mice at the same preclinical stage, half of each brain was used for RT-PCR analyses, and cells were isolated from the other half of each brain to determine donor T cell numbers by flow cytometry. Only brains from WT and μMT recipients with comparable numbers of donor CD4+ T cells were used for gene expression analyses. Strikingly, the expression of all selected genes was significantly reduced in the brains of μMT recipients compared to WT recipients (Figure 4 and Table II). Impaired gene expression was observed for pro-inflammatory T cell- and non-T cell-derived cytokines, cell surface molecules involved in adhesion to and transmigration across the blood brain barrier, and chemokines involved in peripheral T cell recruitment. Expression of a few genes was induced in μMT mice relative to healthy controls, but their level of expression was always significantly less than that seen in WT recipients (Table II). The greater variability in gene expression in WT mice likely reflects differences in the extent of T cell reactivation at this preclinical time point. These observations suggest that although equal numbers of donor T cells are initially present in the μMT CNS, their reactivation is significantly impaired in the CNS in the absence of B cells, resulting in an inability to recruit additional T cells from the periphery.
Figure 4. T cell infiltration into the CNS fails to trigger cytokine production in B cell-deficient mice.
Activated Thy1.1+ T cells from MOG-immunized donors were transferred into WT or μMT Thy1.2 recipients. Five days post-transfer, the brains were harvested and cut in half at the sagittal midline. Cells from one half of the brain were analyzed by flow cytometry to determine the number of donor (Thy1.1+) T cells. RNA was harvested for analysis by real-time qPCR from the remaining half of the brains that exhibited comparable numbers of infiltrating donor T cells. The fold induction relative to healthy brain tissue of the indicated cytokines is shown. Each data point represents a single mouse. Samples were obtained in at least 3 independent adoptive transfer experiments. Significant differences between WT and μMT recipients are indicated; *p < 0.05; **p < 0.01, Mann-Whitney non-parametric t test.
Table II.
mRNA fold induction of immune mediators in the brains of mice with preclinical EAE relative to healthy tissue
| Gene | Wildtypea | μMTa | p valueb |
|---|---|---|---|
| CXCL1 | 17.5 ± 3.7 | 1.9 ± 0.4 | 0.001 |
| CXCL2 | 3.8 ± 0.9 | 0.9 ± 0.2 | 0.004 |
| CXCL9 | 17.2 ± 2.9 | 5.5 ± 1.8 | 0.01 |
| CXCL10 | 126.4 ± 27.2 | 12.3 ± 3.3 | 0.001 |
| CCL2 | 47.4 ± 12.8 | 5.9 ± 2.1 | 0.008 |
| CCL4 | 2.8 ± 0.4 | 0.8 ± 0.1 | 0.001 |
| CCL7 | 19.2 ± 4.8 | 1.9 ± 0.4 | 0.002 |
| CCL20 | 3.8 ± 0.9 | 1.1 ± 0.3 | 0.01 |
| IL-1β | 2.1 ± 0.4 | 0.3 ± 0.06 | 0.002 |
| IL-6 | 13.1 ± 3.3 | 1.7 ± 0.4 | 0.004 |
| E-selectin | 22.4 ± 7.8 | 5.3 ± 2.4 | 0.01 |
| P-selectin | 49.4 ± 14 | 11.6 ± 4.1 | 0.01 |
| ICAM | 3.9 ± 0.5 | 0.9 ± 0.1 | 0.001 |
mRNA fold induction (mean ± SEM) determined from brain tissue of at least 6 mice harvested 5 days post-transfer.
Significant differences between WT and μMT recipients are indicated with the corresponding p values, Mann-Whitney non-parametric t test.
Peripheral B cells reactivate Th1 but not Th17 cells in vitro
B cells could influence reactivation of T cells infiltrating the non-inflamed CNS by functioning as APCs. To test whether resting B cells can process and present MOG protein to CD4+ MOG-specific Th1 and Th17 cells, we sorted resting B cells (CD19+CD43−) from spleens of naïve C3HeB/Fej mice and cultured them with rMOG and MOG-specific CD4+ T cells skewed toward a Th1 or Th17 phenotype that had been sorted to remove contaminating APCs. The non-B cell fraction sorted from the splenocytes (containing dendritic cells and macrophages) as well as unsorted splenocytes were used as control APCs in separate co-cultures with the MOG-specific Th1 and Th17 cells. MOG-specific stimulation of T cell secretion of IL-17 and IFN-γ by the different types of APCs was quantified by ELISPOT. The fraction of cells containing dendritic cells and macrophages efficiently reactivated T cells that had been skewed to either a Th1 or Th17 phenotype. In contrast, resting B cells reactivated the Th1-skewed but not the Th17-skewed cells (Figure 5A). To determine if activated B cells could reactivate Th17 cells, naïve splenic B cells were stimulated with LPS and anti-CD40 prior to culturing with the T cells. A small increase in IL-17-producing T cells was observed when activated versus resting B cells were used as APCs; however, the extent of Th17 cell reactivation was still significantly reduced compared to the reactivation observed when non-B cells were used as APCs (Figure 5A). The addition of IL-23 or IL-6 to the co-cultures containing resting B cells had no effect on their ability to reactivate Th17 cells (Figure 5B). In contrast, addition of exogenous IL-1β strongly enhanced the ability of resting B cells to reactivate MOG-specific Th17 cells, while reactivation of Th1 cells was unaffected by addition of IL-1β (Figure 5B and 5C), reflecting the ability of IL-1β to promote IL-17 production in T cells (27). Thus, resting B cells do not appear competent to reactivate Th17 cells due to a lack of production of IL-1β.
Figure 5. Resting B cells reactivate Th1 but not Th17 cells in vitro.
(A) Splenocytes from naïve mice were FACS-sorted into B cell (CD19+CD43−) or non-B cell (CD19−CD43+) fractions and used as APCs in ELISPOT assays with sorted CD4+ MOG-specific Th1 or Th17 cells to detect IL-17- or IFN-γ-producing cells responding to MOG97-114. Th1 or Th17 cells were co-cultured overnight with B or non-B cells, with or without rMOG. The APCs were used either directly (resting) or were stimulated with LPS and anti-CD40 (activated) prior to culture with T cells. (B) Th17 cells were co-cultured with splenic B cells and rMOG either alone, with IL-23, IL-6, IL-1β or IL-23 and IL-1β. (C) Th17 or Th1 cells were co-cultured with resting spleen B cells with or without IL-1β. Control indicates the absence of added cytokine. (A–C) In each experiment, data are normalized to the number of antigen-specific spots obtained by co-culture of either Th1 or Th17 cells with bulk naïve splenocytes (100%). Data (means ± SEM) are pooled from at least 3 independent experiments. (D) Sorted MOG-specific Th1 or Th17 cells were co-cultured with or without rMOG, with bulk CNS cells from naïve WT or μMT mice that were normalized to ensure equal numbers of non-B APCs in all wells, as described in Materials and Methods. Numbers of antigen-specific spots per million T cells are shown. Data (means ± SEM) are pooled from at least 4 independent experiments. **p < 0.01; ***p < 0.001; ****p < 0.0001, Student’s t test.
CNS B cells promote reactivation of Th1 cells in vitro
Due to the small number of B cells in the naive CNS, purifying B cells from the CNS and utilizing them in vitro as APCs was not feasible. Therefore, we used an alternative approach to determine if CNS B cells preferentially reactivated Th1 compared to Th17 cells, as we had observed for splenic B cells. Bulk CNS cells were isolated from perfused, naïve WT or μMT mice and used as APCs in ELISPOT assays to compare reactivation of Th1 and Th17 cells. The CNS cells were stained for MHC class II, CD19, CD11b, and CD11c and plated such that all wells contained the same number of non-B cell APCs, but CNS cells from WT mice also contained B cells. The presence of B cells in the WT CNS APCs significantly enhanced reactivation of MOG-specific Th1 cells, but not Th17 cells, over levels achieved with μMT CNS APCs (Figure 5D). These data suggest that naïve CNS B cells are similar to peripheral B cells in their preferential ability to reactivate Th1 but not Th17 cells.
B cells affect both the Th17:Th1 ratio in the CNS and the manifestation of EAE
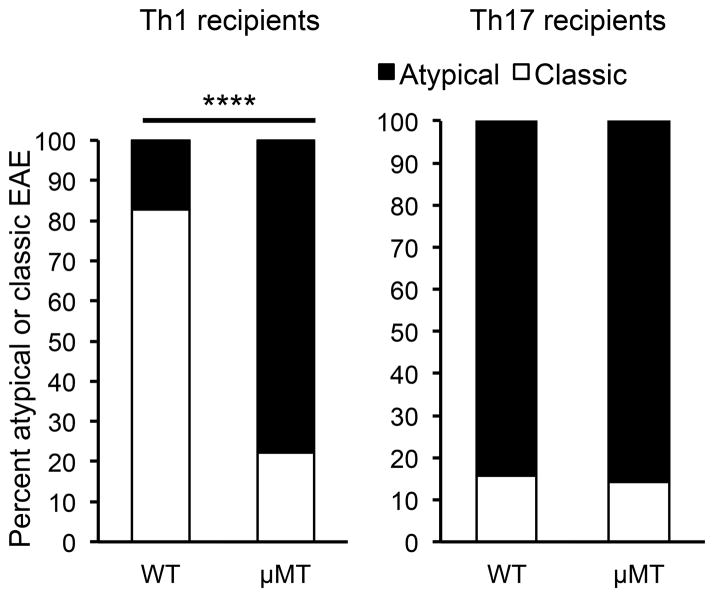
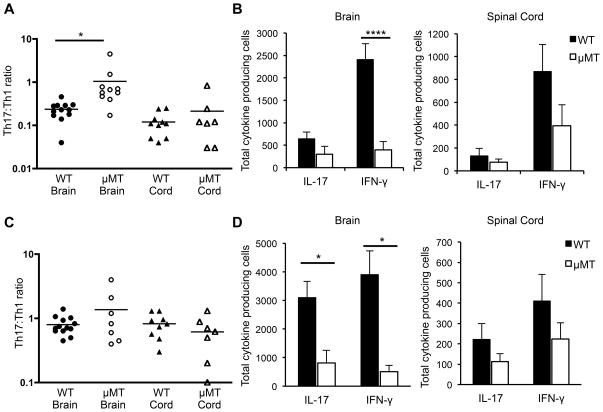
Upon observing the preferential reactivation of Th1 cells by CNS B cells in vitro, we hypothesized that the lack of B cells in μMT mice in vivo should differentially affect reactivation in the CNS of adoptively transferred Th1 compared to Th17 cells. If Th1 cells are reactivated less efficiently in the absence of B cells (while Th17 reactivation remains relatively unaffected), then the Th17:Th1 ratio of T cells within the CNS would increase, and this in turn should increase the susceptibility to brain inflammation. This hypothesis could not be tested in actively induced EAE in C3HeB/Fej mice because WT C3HeB/Fej mice already generate a high Th17:Th1 ratio that results in atypical disease. We therefore induced EAE by transfer of Th1-skewed cells that induces primarily classic EAE in C3HeB/Fej WT mice (4). MOG-specific T cells that were skewed to a Th17:Th1 ratio of either ~1:8 (Th1-skewed) or ~3:1 (Th17-skewed) were transferred into C3HeB/Fej WT and μMT mice. The incidence of disease was significantly reduced in μMT mice after transfer of either Th1- or Th17-skewed cells (reduction from 94% to 37% for Th1 cell transfers and 97% to 36% for Th17 cell transfers, p<0.001 for both Th1 and Th17, Fisher’s exact test). This reduced incidence in μMT mice is similar to that observed when the transferred T cells exhibited a Th17:Th1 ratio of 1:1 (Table I), and likely reflects the loss of the majority of the MHC class II+ APCs in the CNS. The day of onset (day 6–7) and severity (score 4–5) were not significantly different between WT and μMT recipients of either Th1- or Th17-skewed cells, and were also similar to the results shown in Table I. Importantly, there were differences in the clinical manifestation of EAE in the WT and μMT recipients of Th1-skewed cells. As we previously observed, Th1-skewed cells induced predominantly classic EAE and Th17-skewed cells induced predominantly atypical EAE in WT recipients (4). In contrast, in the μMT recipients that did develop EAE, both Th1- and Th17-skewed cells induced predominantly atypical EAE, with the majority of mice exhibiting ataxia, leaning, and rolling in addition to spinal cord signs (Figure 6). As predicted, this shift from classic to atypical EAE when Th1-skewed cells were transferred into μMT instead of WT mice correlated with a significant increase in the Th17:Th1 ratio of cells in the brains of μMT compared to WT recipients at EAE onset (Figure 7A). Consistent with our in vitro observation that B cells preferentially reactivate Th1 compared to Th17 cells, there were significantly fewer IFN-γ-producing T cells detected in the brains of μMT compared to WT mice that received Th1-skewed cells (Figure 7B). The number of IL-17-producing T cells was also decreased in μMT compared to WT mice, but the difference was not significant. These data suggest that B cells preferentially promote reactivation of Th1 cells in the CNS.
Figure 6. EAE shifts from classic to atypical disease in B cell-deficient recipients of Th1 cells.
EAE was induced by adoptive transfer of Th1- or Th17-skewed cells into C3HeB/Fej WT or μMT recipients. The percentages of classic and atypical EAE observed in recipient mice that developed EAE are shown (n=7–24 per group). A significant difference between WT and μMT recipients of Th1 cells was observed; ****p < 0.0001, Fisher’s exact test.
Figure 7. B cell-deficient recipients that developed EAE exhibit higher Th17:Th1 ratios in the brain compared to wild-type recipients.
EAE was induced by adoptive transfer of Th1- or Th17-skewed cells into C3HeB/Fej WT or μMT recipients. At EAE onset, CNS cells were isolated from the brain and spinal cord and plated in ELISPOT assays to detect IL-17- or IFN-γ-producing cells. Numbers of antigen-specific spots were determined by comparing spots in wells with and without MOG97-114. (A and B) Mice received Th1-skewed cells. (C and D) Mice received Th17-skewed cells. (A and C) The Th17:Th1 ratio is shown, calculated from the number of IL-17/IFN-γ producing cells in each culture. Each data point represents a single mouse. Data are pooled from at least 3 independent experiments. *p < 0.05; ***p < 0.001; ****p < 0.0001, Student’s t test.
In mice that received Th17-skewed cells, the Th17:Th1 ratio in the brains of μMT recipients was not significantly different compared to WT recipients (Figure 7C). The absolute numbers of both IFN-γ and IL-17-producing T cells were significantly decreased in the brains of these μMT recipients compared to WT (Figure 7D), indicating that the absence of B cells has a global effect on antigen presentation in the CNS. However, the fold decrease in IFN-γ producing cells was significantly greater than the fold decrease in IL-17-producing cells in the brains of μMT relative to WT recipients (7.6 ± 1.6 vs 3.7 ± 0.7; p=0.04, Student’s t test). Together these data suggest that B cells promote reactivation of T cells producing IFN-γ more strongly than T cells producing IL-17 within the CNS.
Discussion
Defining the mechanisms by which B cells influence CNS autoimmunity is of great interest in light of the efficacy of rituximab-mediated B cell depletion in MS patients (5, 6). Because rituximab does not deplete plasma cells (5), its therapeutic benefit suggests that B cells play a pathogenic role independent of antibody production in MS. Rituximab depletes B cells from both the periphery and the CSF (8), and it is not clear whether B cells exert a pathogenic effect in one or both compartments. Our studies demonstrate that B cells play an important role in the pathogenesis of EAE by promoting the reactivation of T cells infiltrating the CNS that is required to trigger inflammation. The presence of B cells in the CNS also results in preferential reactivation of Th1 cells, and this differential reactivation of Th1 versus Th17 cells can influence the regional localization of inflammation. Together, these findings reveal an important role for CNS B cells in promoting initial inflammatory responses and shaping neuroinflammatory patterns.
B cells appear to contribute to multiple steps in the pathogenesis of CNS autoimmunity, accounting for reports of both pathogenic and regulatory B cell activities in EAE. Most studies have analyzed the effects of eliminating B cells in models of active EAE induction using μMT mice. Therefore, results from these studies reflect the cumulative effects of peripheral B cells on T cell priming, the contribution of regulatory B cells and the activity of B cells in the CNS. The impact of these different B cell functions may vary in different systems, depending on the strength and nature of the effector T cell response and the ability of regulatory B cells to modulate the response. B cells appeared to enhance pathogenicity in EAE models employing certain strain and antigen combinations (28–30), but exerted a regulatory role or had no impact in other models (10, 30, 31). Both pathogenic and regulatory effects in EAE have also been observed when anti-CD20 was used to deplete B cells (12, 13). Anti-CD20 depletion can be used to determine the effects of depleting B cells either before or after disease onset; however, treatment before initiation of disease is unlikely to deplete B cells within the CNS, as antibodies do not efficiently cross the intact blood-brain barrier. Thus, this strategy does not permit assessment of the role of CNS B cells during initiation of disease.
In our studies, we used an adoptive transfer model in C3HeB/Fej μMT mice to analyze the contribution of B cells residing within the CNS to the pathogenesis of EAE. Similar to our results in active EAE induction, a significant decrease in incidence rather than severity was observed in C3HeB/Fej μMT mice following adoptive transfer of MOG-specific T cells. This decrease in incidence of EAE demonstrated that B cells can increase susceptibility to disease by acting at a point subsequent to T cell priming but prior to onset of clinical signs. Importantly, the initial T cell infiltration of the CNS was not impaired in μMT mice. Thus, regardless of whether B cells in peripheral lymph nodes play a role in either the priming of myelin-specific T cells or in promoting expression of tissue-homing cell-surface molecules on activated T cells that enter peripheral lymph nodes (32), our adoptive transfer model allowed us to avoid effects on T cell activation in the periphery due to the altered lymphoid architecture seen in μMT mice (33), and instead investigate how B cells influence the activity of T cells once they enter the CNS.
Our results indicate that the inflammatory cascade triggered by early T cell reactivation in the CNS that generates immune mediators necessary to recruit additional T cells from the periphery was severely impaired in the absence of B cells, thus preventing initiation of EAE. The impaired reactivation of the myelin-specific T cells suggests that B cells play a critical role as APCs within the CNS during the early stages of EAE induction. While other studies have suggested that B cells may act as APCs in the CNS at a later stage after disease onset (12, 13), the role of B cells in the CNS during initial T cell reactivation has not been previously studied. The fact that B cells comprise the predominant MHC class II+ population in the naïve CNS supports this early role for CNS B cells as APCs. It is not yet known if B cell receptor specificity for myelin antigen is required for their APC function, or if the CNS B cells acquire myelin antigen via a non-B cell receptor-dependent mechanism as previously described for peripheral B cells (34).
Recent work has shown that C57BL/6 mice with MHC class II expression restricted to CD19+ B cells do not develop EAE, suggesting that B cells are not sufficient as the sole APCs in initiating disease (35). Early studies indicated that dendritic cells were sufficient as APCs to initiate T cell responses (36), however, more recent studies indicate that a requirement for dendritic cells in EAE is controversial (37–39). Our studies demonstrate that B cells are not essential to initiate EAE as some μMT mice still succumb to EAE. However, our data show that B cells play an important role in promoting the reactivation of CNS-infiltrating, myelin-specific T cells. The population of B cells in the naïve CNS significantly expands the pool of APCs that infiltrating T cells would encounter and thus would increase the likelihood of reactivation events during the initiation of EAE.
B cells in the naïve CNS differed functionally from B cells in the spleen and blood with respect to constitutive cytokine production, potentially reflecting the unique microenvironment of the CNS. Similarly, the plasmablasts that we identified in the naïve CNS also differed from plasmablasts in the periphery in that CNS plasmablasts did not express activation markers or MHC class II. The origin of these plasmablasts is unknown; they may either differentiate within the CNS from the B cell population into phenotypically distinct plasmablasts, or infiltrate the CNS as plasmablasts and undergo a phenotypic change within the microenvironment of the CNS. It is not clear whether these plasmablasts significantly influence immune responses in the CNS as their cytokine production is much lower than that of CNS resting B cells and their lack of MHC class II expression indicates that they do not participate in T cell reactivation.
In the naïve CNS, B cells expressed significantly higher levels of IL-12 p35 and TNFα compared to CD11b/c+ cells, and both of these cytokines could influence the responses of T cells that initially infiltrate the CNS. T cell proliferation and IFN-γ production are enhanced by B cell-derived TNFα in vitro (9). In addition to promoting Th1 cell differentiation, IL-12 produced by B cells in vitro has been shown to stimulate IFN-γ production from effector Th1 cells (40, 41). While p35 is most widely recognized as a subunit of IL-12, and increased expression of IL-12 by B cells in WT mice is consistent with preferential reactivation of Th1 cells in the CNS of WT compared to B cell-deficient mice, p35 is also a subunit of IL-35. Thus, it is possible that the increased expression of p35 could enhance production of IL-35, a cytokine that may have regulatory functions (42). We did not detect IL-6, IL-1β, IL-23 p19 or IL-10 mRNA in B cells analyzed directly ex vivo from the CNS. The lack of IL-10 expression suggests that CNS B cells may not play a strong regulatory role during disease initiation in our model. IL-6 production by B cells was previously shown to influence the severity, but not the incidence of EAE, suggesting that B cells expressing this cytokine may play a role later in disease (18).
Constitutive production of IL-12 p35 and TNFα by CNS B cells suggested that they may promote reactivation of Th1 cells more efficiently than Th17 cells. Our observations that splenic B cells required exogenous IL-1β to reactivate Th17 but not Th1 cells, and that IL-1β is not expressed in the naïve CNS and is only marginally increased in the preclinical stage of EAE, also raised the possibility that B cells in the CNS may preferentially reactivate Th1 cells. Our in vitro data using CNS cells as APCs confirmed a deficiency in the ability of CNS cells from μMT mice to reactivate Th1 cells compared to CNS cells from WT mice. Consistent with this in vitro data, ex vivo analysis showed that the Th17:Th1 ratio in the brain increased following transfer of Th1-skewed cells into μMT C3HeB/Fej mice due to a greater loss of T cells producing IFN-γ compared to IL-17. In this model, the increase in the Th17:Th1 ratio in μMT mice caused a shift from the classic EAE seen in WT mice to atypical EAE, confirming that CNS B cells can play a critical role in determining the localization of inflammation in the CNS by preferentially reactivating Th1 cells during the initial infiltration. However, the clinical impact of this preferential reactivation will depend on the relative abundance of Th1 and Th17 cells that initially infiltrate the CNS. If the percentage of Th17 cells in the population is very small, as is the case in C3H.SW mice, even eliminating the preferential reactivation of Th1 cells by B cells in the CNS may not be sufficient to increase the Th17:Th1 ratio enough to permit brain inflammation. Additionally, the influence of B cells on the localization of CNS lesions may be greatest at the initiation of disease or of a relapse when the CNS is relatively non-inflamed, as the increase in IL-1β during peak inflammatory conditions should allow B cells to become proficient in activating Th17 as well as Th1 cells.
In conclusion, our studies indicate an important role for B cells as APCs in the CNS during the initial reactivation of myelin-specific T cells in EAE. Their differential ability to reactivate Th1 versus Th17 cells in a non-inflammatory milieu may influence the initial localization of lesions in the CNS. A similar role for human CNS B cells as APCs could be clinically relevant during newly occurring MS relapses in which the CNS milieu may be similar to a healthy CNS. Our studies suggest that the effectiveness of rituximab treatment may in part reflect an overall decrease of APCs in the CNS by eliminating B cells, and that this therapy may be especially effective in patients in which Th1 cells are more prominent in the infiltrating population.
Supplementary Material
Acknowledgments
We thank Neal Mausolf for animal husbandry and technical assistance and Sarah Simmons for critical reading of the manuscript.
This work was supported in part by PHS NRSA 2T32 GM007270 from NIGMS to E.R.P. and by NIH grant R37 AI107494-01 to J.M.G.
Abbreviations used in this article
- EAE
experimental autoimmune encephalomyelitis
- MOG
myelin oligodendrocyte glycoprotein
- MS
multiple sclerosis
- WT
wild-type
Footnotes
The authors declare no competing financial interests.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 2.Nociti V, Cianfoni A, Mirabella M, Caggiula M, Frisullo G, Patanella AK, Sancricca C, Angelucci F, Tonali PA, Batocchi AP. Clinical characteristics, course and prognosis of spinal multiple sclerosis. Spinal Cord. 2005;43:731–734. doi: 10.1038/sj.sc.3101798. [DOI] [PubMed] [Google Scholar]
- 3.Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stromnes IM, Cerretti LM, Liggitt D, Harris RA, Goverman JM. Differential regulation of central nervous system autoimmunity by T(H)1 and T(H)17 cells. Nat Med. 2008;14:337–342. doi: 10.1038/nm1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, Langer-Gould A, Smith CH. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 6.Bar-Or A, Calabresi PA, Arnold D, Markowitz C, Shafer S, Kasper LH, Waubant E, Gazda S, Fox RJ, Panzara M, Sarkar N, Agarwal S, Smith CH. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol. 2008;63:395–400. doi: 10.1002/ana.21363. [DOI] [PubMed] [Google Scholar]
- 7.Weber MS, Hemmer B, Cepok S. The role of antibodies in multiple sclerosis. Biochim Biophys Acta. 2011;1812:239–245. doi: 10.1016/j.bbadis.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 8.Cross AH, Stark JL, Lauber J, Ramsbottom MJ, Lyons JA. Rituximab reduces B cells and T cells in cerebrospinal fluid of multiple sclerosis patients. J Neuroimmunol. 2006;180:63–70. doi: 10.1016/j.jneuroim.2006.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bar-Or A, Fawaz L, Fan B, Darlington PJ, Rieger A, Ghorayeb C, Calabresi PA, Waubant E, Hauser SL, Zhang J, Smith CH. Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Ann Neurol. 2010;67:452–461. doi: 10.1002/ana.21939. [DOI] [PubMed] [Google Scholar]
- 10.Wolf SD, Dittel BN, Hardardottir F, Janeway CA., Jr Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996;184:2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- 12.Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weber MS, Prod’homme T, Patarroyo JC, Molnarfi N, Karnezis T, Lehmann-Horn K, Danilenko DM, Eastham-Anderson J, Slavin AJ, Linington C, Bernard CC, Martin F, Zamvil SS. B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity. Ann Neurol. 2010;68:369–383. doi: 10.1002/ana.22081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bettelli E, Baeten D, Jager A, Sobel RA, Kuchroo VK. Myelin oligodendrocyte glycoprotein-specific T and B cells cooperate to induce a Devic-like disease in mice. J Clin Invest. 2006;116:2393–2402. doi: 10.1172/JCI28334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krishnamoorthy G, Lassmann H, Wekerle H, Holz A. Spontaneous opticospinal encephalomyelitis in a double-transgenic mouse model of autoimmune T cell/B cell cooperation. J Clin Invest. 2006;116:2385–2392. doi: 10.1172/JCI28330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pollinger B, Krishnamoorthy G, Berer K, Lassmann H, Bosl MR, Dunn R, Domingues HS, Holz A, Kurschus FC, Wekerle H. Spontaneous relapsing-remitting EAE in the SJL/J mouse: MOG-reactive transgenic T cells recruit endogenous MOG-specific B cells. J Exp Med. 2009;206:1303–1316. doi: 10.1084/jem.20090299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H, Kim HJ, Bar-Or A. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol. 2007;178:6092–6099. doi: 10.4049/jimmunol.178.10.6092. [DOI] [PubMed] [Google Scholar]
- 18.Barr TA, Shen P, Brown S, Lampropoulou V, Roch T, Lawrie S, Fan B, O’Connor RA, Anderton SM, Bar-Or A, Fillatreau S, Gray D. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med. 2012;209:1001–1010. doi: 10.1084/jem.20111675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdul-Majid KB, Jirholt J, Stadelmann C, Stefferl A, Kjellen P, Wallstrom E, Holmdahl R, Lassmann H, Olsson T, Harris RA. Screening of several H-2 congenic mouse strains identified H-2(q) mice as highly susceptible to MOG-induced EAE with minimal adjuvant requirement. J Neuroimmunol. 2000;111:23–33. doi: 10.1016/s0165-5728(00)00360-x. [DOI] [PubMed] [Google Scholar]
- 20.Stromnes IM, Goverman JM. Active induction of experimental allergic encephalomyelitis. Nature Protocols. 2006;1:1810–1819. doi: 10.1038/nprot.2006.285. [DOI] [PubMed] [Google Scholar]
- 21.Brabb T, von Dassow P, Ordonez N, Schnabel B, Duke B, Goverman J. In situ tolerance within the central nervous system as a mechanism for preventing autoimmunity. Journal of Experimental Medicine. 2000;192:871–880. doi: 10.1084/jem.192.6.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ohmori K, Hong Y, Fujiwara M, Matsumoto Y. In situ demonstration of proliferating cells in the rat central nervous system during experimental autoimmune encephalomyelitis. Evidence suggesting that most infiltrating T cells do not proliferate in the target organ. Lab Invest. 1992;66:54–62. [PubMed] [Google Scholar]
- 23.O’Connor RA, Malpass KH, Anderton SM. The inflamed central nervous system drives the activation and rapid proliferation of Foxp3+ regulatory T cells. J Immunol. 2007;179:958–966. doi: 10.4049/jimmunol.179.2.958. [DOI] [PubMed] [Google Scholar]
- 24.Webb M, Tham CS, Lin FF, Lariosa-Willingham K, Yu N, Hale J, Mandala S, Chun J, Rao TS. Sphingosine 1-phosphate receptor agonists attenuate relapsing-remitting experimental autoimmune encephalitis in SJL mice. J Neuroimmunol. 2004;153:108–121. doi: 10.1016/j.jneuroim.2004.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Prendergast CT, Anderton SM. Immune cell entry to central nervous system--current understanding and prospective therapeutic targets. Endocrine, metabolic & immune disorders drug targets. 2009;9:315–327. doi: 10.2174/187153009789839219. [DOI] [PubMed] [Google Scholar]
- 26.Engelhardt B, Ransohoff RM. The ins and outs of T-lymphocyte trafficking to the CNS: anatomical sites and molecular mechanisms. Trends Immunol. 2005;26:485–495. doi: 10.1016/j.it.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Mills KH. Induction, function and regulation of IL-17-producing T cells. Eur J Immunol. 2008;38:2636–2649. doi: 10.1002/eji.200838535. [DOI] [PubMed] [Google Scholar]
- 28.Svensson L, Abdul-Majid KB, Bauer J, Lassmann H, Harris RA, Holmdahl R. A comparative analysis of B cell-mediated myelin oligodendrocyte glycoprotein-experimental autoimmune encephalomyelitis pathogenesis in B cell-deficient mice reveals an effect on demyelination. Eur J Immunol. 2002;32:1939–1946. doi: 10.1002/1521-4141(200207)32:7<1939::AID-IMMU1939>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 29.Lyons JA, San M, Happ MP, Cross AH. B cells are critical to induction of experimental allergic encephalomyelitis by protein but not by a short encephalitogenic peptide. European Journal of Immunology. 1999;29:3432–3439. doi: 10.1002/(SICI)1521-4141(199911)29:11<3432::AID-IMMU3432>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 30.Oliver AR, Lyon GM, Ruddle NH. Rat and human myelin oligodendrocyte glycoproteins induce experimental autoimmune encephalomyelitis by different mechanisms in C57BL/6 mice. J Immunol. 2003;171:462–468. doi: 10.4049/jimmunol.171.1.462. [DOI] [PubMed] [Google Scholar]
- 31.Hjelmstrom P, Juedes AE, Fjell J, Ruddle NH. B-cell-deficient mice develop experimental allergic encephalomyelitis with demyelination after myelin oligodendrocyte glycoprotein sensitization. J Immunol. 1998;161:4480–4483. [PubMed] [Google Scholar]
- 32.Flugel A, Berkowicz T, Ritter T, Labeur M, Jenne DE, Li Z, Ellwart JW, Willem M, Lassmann H, Wekerle H. Migratory activity and functional changes of green fluorescent effector cells before and during experimental autoimmune encephalomyelitis. Immunity. 2001;14:547–560. doi: 10.1016/s1074-7613(01)00143-1. [DOI] [PubMed] [Google Scholar]
- 33.Rivera A, Chen CC, Ron N, Dougherty JP, Ron Y. Role of B cells as antigen-presenting cells in vivo revisited: antigen-specific B cells are essential for T cell expansion in lymph nodes and for systemic T cell responses to low antigen concentrations. Int Immunol. 2001;13:1583–1593. doi: 10.1093/intimm/13.12.1583. [DOI] [PubMed] [Google Scholar]
- 34.Seamons A, Perchellet A, Goverman J. Endogenous myelin basic protein is presented in the periphery by both dendritic cells and resting B cells with different functional consequences. J Immunol. 2006;177:2097–2106. doi: 10.4049/jimmunol.177.4.2097. [DOI] [PubMed] [Google Scholar]
- 35.Archambault AS, Carrero JA, Barnett LG, McGee NG, Sim J, Wright JO, Raabe T, Chen P, Ding H, Allenspach EJ, Dragatsis I, Laufer TM, Wu GF. Cutting Edge: Conditional MHC Class II Expression Reveals a Limited Role for B Cell Antigen Presentation in Primary and Secondary CD4 T Cell Responses. J Immunol. 2013;191:545–550. doi: 10.4049/jimmunol.1201598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greter M, Heppner FL, Lemos MP, Odermatt BM, Goebels N, Laufer T, Noelle RJ, Becher B. Dendritic cells permit immune invasion of the CNS in an animal model of multiple sclerosis. Nat Med. 2005;11:328–334. doi: 10.1038/nm1197. [DOI] [PubMed] [Google Scholar]
- 37.Wu GF, Shindler KS, Allenspach EJ, Stephen TL, Thomas HL, Mikesell RJ, Cross AH, Laufer TM. Limited sufficiency of antigen presentation by dendritic cells in models of central nervous system autoimmunity. J Autoimmun. 2011;36:56–64. doi: 10.1016/j.jaut.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yogev N, Frommer F, Lukas D, Kautz-Neu K, Karram K, Ielo D, von Stebut E, Probst HC, van den Broek M, Riethmacher D, Birnberg T, Blank T, Reizis B, Korn T, Wiendl H, Jung S, Prinz M, Kurschus FC, Waisman A. Dendritic cells ameliorate autoimmunity in the CNS by controlling the homeostasis of PD-1 receptor(+) regulatory T cells. Immunity. 2012;37:264–275. doi: 10.1016/j.immuni.2012.05.025. [DOI] [PubMed] [Google Scholar]
- 39.Isaksson M, Lundgren BA, Ahlgren KM, Kampe O, Lobell A. Conditional DC depletion does not affect priming of encephalitogenic Th cells in EAE. Eur J Immunol. 2012;42:2555–2563. doi: 10.1002/eji.201142239. [DOI] [PubMed] [Google Scholar]
- 40.Schultze JL, Michalak S, Lowne J, Wong A, Gilleece MH, Gribben JG, Nadler LM. Human non-germinal center B cell interleukin (IL)-12 production is primarily regulated by T cell signals CD40 ligand, interferon gamma, and IL-10: role of B cells in the maintenance of T cell responses. J Exp Med. 1999;189:1–12. doi: 10.1084/jem.189.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gagro A, Servis D, Cepika AM, Toellner KM, Grafton G, Taylor DR, Branica S, Gordon J. Type I cytokine profiles of human naive and memory B lymphocytes: a potential for memory cells to impact polarization. Immunology. 2006;118:66–77. doi: 10.1111/j.1365-2567.2006.02342.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, Rehg JE, Jones ML, Ni HT, Artis D, Turk MJ, Vignali DA. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.