In response to data indicating that persons over age 65 account for almost half of all days of care in short stay hospitals (Graves & Kozak, 1999), constitute the majority of residents of nursing homes (Strahan, 1997), and account for over 75% of required formal home-based care supports (Levit et al., 1997; Office, 1996), the National Institute on Aging and the National Institute for Nursing Research funded an initiative to test the effectiveness of cognitive interventions in maintaining cognitive health and functional independence in older adults. This initiative was based on evidence that the cognitive performance of older adults can be improved through systematic training focused on cognitive skills (Baltes, Kuhl, Gutzmann, & Sowarka, 1995; Caprio-Prevette & Fry, 1996; Hayslip, Maloy, & Kohl, 1995; Kramer, Larish, & Strayer, 2002; Mohs et al., 1998; Neely & Backman, 1995; Noice, Noice, & Staines, 2004; Oswald, Rupprecht, Gunzelmann, & Tritt, 1996) paired with evidence of the importance of cognitive functioning for performing activities of daily living (Allaire & Marsiske, 1999; Backman & Hill, 1996; Burdick et al., 2005; Cahn-Weiner, Malloy, Boyle, Marran, & Salloway, 2000; Owsley, Sloane, McGwin, & Ball, 2002) and maintaining health related quality of life among older adults (Hultsch, Hammer, & Small, 1993; Swan, Carmelli, & LaRue, 1995; Wolinsky & Johnson, 1991). At that time, essentially no research had been conducted demonstrating training transfer to real-world functional outcomes in later adulthood. The Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) trial addressed this gap.
The goal of ACTIVE was to test the effectiveness of three cognitive interventions (memory, reasoning, and visual speed of processing) in maintaining cognitive health and functional independence in older adults. The targeted abilities-- memory, reasoning, and speed of processing—were selected based on evidence that they exhibit relatively early age-related decline, beginning on average in the mid-sixties (Schaie, 1996), that interventions have been shown to be effective in training these abilities (K Ball, 1997; K. Ball & Owsley, 2000; Kliegl, Smith, & Baltes, 1990; Lachman, Weaver, Bandura, Elliott, & Lewkowicz, 1992; McDougal, 1999; Mohs et al., 1998; Oswald et al., 1996; Rasmusson, Rebok, Bylsma, & Brandt, 1999; Rebok & Balcerak, 1989; S. Willis, 1990; S. Willis, Cornelius, Blow, & Baltes, 1983; S. Willis & Nesselroade, 1990; S. Willis & Schaie, 1986, 1994), and that performance on these abilities is associated with performance of cognitively demanding instrumental activities of daily living, critical for independent living (K. Ball & Owsley, 2000; K. Ball, Owsley, Sloane, Roenker, & Bruni, 1993; Diehl, Willis, & Schaie, 1995; S. L. Willis, 1996; S. L. Willis, Jay, Diehl, & Marsiske, 1992).
ACTIVE began in September, 1996 at six field centers: the University of Alabama at Birmingham, the Boston Hebrew Rehabilitation Center for Aged (now Hebrew Senior Life), the Indiana University School of Medicine, the Johns Hopkins University, the Pennsylvania State University, and Wayne State University, with a data coordinating center at the New England Research Institutes.
The conceptual model that informed the design of ACTIVE (Figure 1) was based on prior evidence showing that cognitive training would be domain specific. That is, each intervention was expected to result in specific improvement on measures of the trained ability relative to the other interventions and control group. For example, training in memory was expected to improve memory function (the proximal outcome) but was not expected to improve reasoning or speed of processing skills. On the other hand, intervention effects were expected to show some level of general transfer to daily function (the primary outcome) based on the critical assumption that declines in cognitive function lead to declines in activities in daily living. In other words, improvement in cognitive ability should result in maintenance of functional independence. In turn, maintained functional independence could result in a positive cascade of effects including improvements in quality of life, mobility, and health service utilization.
Figure 1.
Hypothesized mode of effects in ACTIVE trial. Influence of intervention on primary and secondary outcomes is mediated through trained abilities. Bold lines represent specific effects of training. Dashed lines represent non-specific effects of training on related abilities, e.g., through social contact or general cognitive arousal.
DESIGN
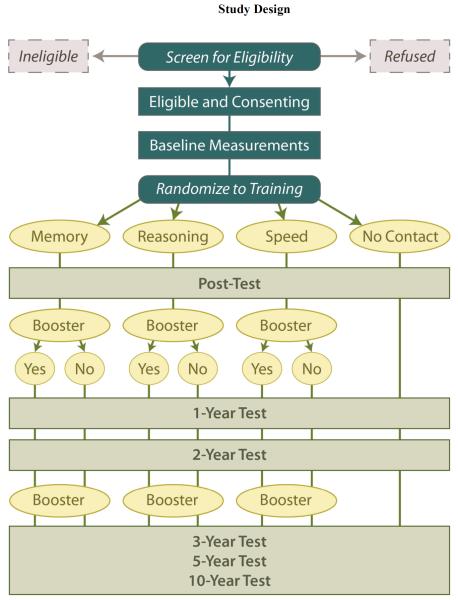
ACTIVE is a randomized, controlled, single-masked trial utilizing a four-group design (Figure 2) with three intervention arms and a no-contact control group. Details of the study design are provided in(Jobe et al., 2001). Eligibility criteria were established to ensure that the study population would be in good physical and cognitive health at the time of enrollment, yet at risk for cognitive and functional decline. Prior longitudinal studies have demonstrated that significant age-related decline occurs in the mid-sixties for cognitive abilities, the targets of training (Schaie, 2005). In contrast, significant age-related decline on daily function has been shown to occur later than for the targeted mental abilities, occurring for IADLs in the mid seventies to early eighties. Thus, intervention on the cognitive abilities was timed to occur at the normative onset of age-related decline in these abilities, but prior to expected normative decline in the functional outcomes.
Figure 2.
Participants
Recruitment occurred from March 1998 through October 1999 in six metropolitan areas using a variety of sampling strategies. Community-dwelling adults aged 65 years and older were eligible. Persons were excluded if they had significant cognitive dysfunction (score < 23 on the Mini-mental State Examination, MMSE (Folstein, Folstein, & McHugh, 1975)); functional impairment (dependency or regular assistance in ADL on Minimum Data Set Home Care (J. N. Morris et al., 1997)); self-reported diagnoses of Alzheimer disease, stroke within the last 12 months, or certain cancers; current chemotherapy or radiation therapy; or poor vision, hearing, or communicative ability that would have interfered with the interventions or outcome assessments. Enrollment resulted in a sample of 2,802 individuals (average age 74 years, average education 13 years, 74% white and 26% African American, and 76% women).
The ACTIVE sample was not intended to be representative of the US population. As shown in Table 1, ACTIVE participants were slightly younger than the U.S. population over age 65 and were more likely to be female and not married. As a result of the targeted efforts to recruit African Americans, they were over-represented in this sample. ACTIVE participants have slightly less health care utilization and slightly better perceived health compared to the general population of older adults in the U.S.; however, the prevalence of selected health conditions, especially those associated with lower cognitive functioning such as hypertension and diabetes (Carmelli et al., 1998), indicate that they were at risk for cognitive decline.
Table 1.
Baseline Characteristics of Participants (n=2,802)
| Sample | General Population | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||
| N | % | Mean | S.D. | Range | % | Mean | Ref. | p | |
|
|
|||||||||
| Sociodemographics | |||||||||
| Age (years) | 2802 | 73.6 | 5.9 | 65–94 | |||||
| Age 65–74 | 60.1 | 57.6 | 1 | n.s. | |||||
| Age 75–84 | 35.0 | 32.5 | |||||||
| Age 85 + | 4.9 | 9.9 | |||||||
| Gender (% female) | 2802 | 75.9 | 57.9 | 2 | *** | ||||
| Race | 2802 | 2 | *** | ||||||
| Caucasian | 73.3 | 83.5 | |||||||
| African-American | 26.0 | 8.1 | |||||||
| Other or unknown | 0.7 | ||||||||
| Education | |||||||||
| High School diploma (%) | 2800 | 88.6 | 67.0 | *** | |||||
| Caucasians | 2052 | 91.4 | 71.6 | 3 | *** | ||||
| African Americans | 728 | 80.4 | 43.7 | *** | |||||
| Marital Status (married) | 2802 | 35.9 | 56.6 | 4 | *** | ||||
| SF-36 Physical Function | 2802 | 68.8 | 24.1 | 0–100 | 62.0 | 5 | *** | ||
| Health Status: Good-Excellent | 2753 | 84.3 | 72.2 | 6 | *** | ||||
| Caucasians | 2019 | 86.7 | 74.0 | *** | |||||
| African-Americans | 714 | 77.6 | 58.4 | *** | |||||
| Chronic Diseases | |||||||||
| Hypertension | 2792 | 51.1 | 45.0 | 7 | *** | ||||
| Caucasians | 2044 | 45.1 | 44.0 | n.s. | |||||
| African Americans | 728 | 67.7 | 58.7 | *** | |||||
| Diabetes Mellitus | 2802 | 12.8 | 12.0 | 7 | n.s. | ||||
| Caucasians | 2054 | 9.9 | 10.9 | n.s. | |||||
| African Americans | 728 | 21.2 | 20.4 | n.s. | |||||
| TIA/Stroke | 2791 | 7.0 | 8.9 | 7 | *** | ||||
| Caucasians | 2043 | 7.4 | 8.6 | n.s. | |||||
| African Americans | 728 | 5.9 | 12.2 | *** | |||||
| Ischemic Heart Disease | 2792 | 11.0 | 13.9 | 8 | *** | ||||
| Caucasians | 2044 | 11.9 | 14.7 | *** | |||||
| African Americans | 728 | 8.4 | 8.2 | n.s. | |||||
| Health Service Utilization (prior 12 months) | |||||||||
| Physician visits | 2772 | 96.6 | 5.2 | 6.4 | 0–99 | 92.1 | 6.1 | 9 | *** |
| E.D. Visits | 2785 | 22.4 | 0.3 | 0.8 | 0–12 | 21.9 | 0.5 | 10 | n.s. |
| Hospitalizations | 2505 | 16.3 | 0.2 | 0.6 | 0–12 | 28.3 | 11 | *** | |
| Hospital Days (LOS) | 405 | 4.6 | 5.3 | 1–42 | 6.3 | 11 | *** | ||
p<.001
Study Procedures
Eligibility and demographic data (age, gender, race, education, and marital status) were gathered in a telephone screening. Health history (self-report of diabetes, myocardial infarction, angina, heart failure, stroke, hypertension, high cholesterol, and current alcohol use), physical status (MOS Short-form 36 (Ware & Sherbourne, 1992)), functional status (MDS, see below), mental status (MMSE (Folstein et al., 1975)) and cognitive and function measures (see below) were gathered via in-person examinations in individual and small-group formats at baseline. Eligible participants were randomly assigned to one of three intervention arms or the no-contact control group. Screening and baseline assessment took place before randomization. Due to logistical considerations related to testing and training a large sample, recruitment and all subsequent field work were conducted in six replicates of approximately eight weeks duration. Outcome assessments were conducted immediately following and 1, 2, 3, and 5 years after the intervention (Figure 2). A 10-year follow-up was recently completed.
Site staff who conducted the training interventions (trainers) and completed assessments (assessors) were trained centrally, followed by performance-based certification. Trainers for an intervention were not allowed to be cross-trained in the other interventions. Assessors were masked to participant assignments. Annual recertification was required. Annual monitoring visits were conducted by the Data Coordinating Center which included data audits and observations of trainers and assessors to check for drift.
Study procedures were approved by the institutional review boards at the collaborating institutions, and all subjects gave informed consent to participate.
Interventions
Interventions were conducted in small groups in ten 60–75 minute sessions over 5 to 6 weeks. Memory training focused on improving verbal episodic memory through instruction and practice in strategy use. Reasoning training focused on improving the ability to solve problems that contained a serial pattern. Speed training focused on visual search and the ability to process increasingly more information presented in successively shorter inspection times. In all three interventions, sessions 1–5 focused on strategy instruction and exercises to practice the strategy while sessions 6–10 provided additional practice exercises. Content for each of the 10 sessions was scripted in a trainer's manual. Booster training (four 75-minute sessions) was provided at 11 and 35 months after training to a randomly selected subset of participants in each intervention arm who completed initial training (defined as 8 of 10 sessions).
Proximal Outcomes - Measures of Cognitive Abilities
Multiple measures of basic mental ability for memory (Hopkins Verbal Learning Test total of the 3 learning trials (Brandt, 1991), Rey Auditory-Verbal Learning Test total of the 5 learning trials (Rey, 1941), and Rivermead Behavioral Memory Test immediate recall (Wilson, Cockburn, & Baddeley, 1985)), reasoning (Letter Series total correct (Thurstone & Thurstone, 1949), Letter Sets total correct (Ekstrom, French, Harman, & Derman, 1976), and Word Series total correct (Gonda & Schaie, 1985)), speed of processing (Useful Field of View (Owsley et al., 1998)), and vocabulary (Ekstrom et al., 1976) formed the proximal outcomes. Individual scales were normalized to the same metric with a z-score transformation using the control group's baseline mean and standard deviation (each participant's test score subtracted from the control group mean score at baseline and the difference divided by the control group standard deviation at baseline resulting in z-score with mean of 0 and standard deviation of 1), and subsequently combined into domain-specific composites (average of the component z-scores).
Primary Outcomes - Measures of Daily Function
Daily functional was measured with an instrument based on the Minimum Data Set for Home Care (MDS) (J. N. Morris et al., 1997) which taps instrumental and basic activities of daily living (ADL). The instrumental activities covered by the MDS include 19 daily tasks spanning meal preparation, housework, finances, health care, telephone, shopping, and travel over the past seven days. The basic activities covered by the MDS include need for assistance in dressing, personal hygiene, and bathing. The Performance subscale assesses the degree of independent completion of tasks. The Difficulty subscale assesses the perceived degree of difficulty in completing these subtasks. The MDS has high correlations with Barthel measure of basic ADL (r = .74) and the Lawton measure of instrumental ADL (r = .81) (Landi et al., 2000). Outcome measures based on the MDS scores have been shown to be valid(J.N. Morris, Carpenter, Berg, & Jones, 2000) and have demonstrated utility for quality monitoring in home care settings (Hirdes et al., 2004).
Performance-based measures of daily functioning included: Everyday Problems Test (EPT) (ability to utilize information from 14 daily tasks (S. L. Willis et al., 1998); Observed Tasks of Daily Living (OTDL) (Diehl et al., 2005) (ability to perform daily actions like searching medication label for side effects, making change, using a telephone); Complex Reaction Time (CRT, a computer-administered test of reaction time to traffic signs (Roenker, Cissell, Ball, Wadley, & Edwards, 2003); and Timed IADL (TIADL, measures time to complete five daily tasks like finding a number in telephone book, finding items on a simulated grocery shelf) (Owsley, McGwin, Sloane, Stalvey, & Wells, 2001). The EPT and OTDL were combined to form an Everyday Problem Solving Composite. The CRT and TIADL were combined to form an Everyday Speed Composite.
Secondary Outcomes
If the cognitive interventions transferred to daily function, it was hypothesized that training would have farther reaching effects on health-related quality of life, everyday mobility, and health service utilization. Health-related quality of life was measured with the MOS SF-36 (Ware & Sherbourne, 1992). Everyday mobility included self-reported falls, a measure of life space and abstracted archival driving record information on crashes (Fitti & Kovar, 1987; Myers, Juster, & Suzman, 1997; Stalvey, Owsley, Sloane, & Ball, 1999; Ware & Sherbourne, 1992). Utilization of health, nursing home and home health services was captured by self-report and Medicare claims data.
SUMMARY OF MAJOR FINDINGS-TO-DATE
ACTIVE data through five years are archived at National Archive of Computerized Data on Aging (NACDA, http://www.icpsr.umich.edu/icpsrweb/NACDA/).
Training Effects on Cognitive Abilities
Each intervention produced an immediate improvement in the cognitive ability trained (K. Ball et al., 2002) that was durable through five years of follow-up (S. L. Willis et al., 2006). Training produced ability-specific effects. For example, Reasoning training did not result in improvement in memory or speed of processing indicating that training effects were not explained by social contact. The largest improvements were seen for Speed of Processing intervention followed by the Reasoning and Memory. Each type of training produced its largest effect immediately after the intervention and with some dissipation over time; however, training gains remained statistically and practically significant at the 5 year follow-up (S. L. Willis et al., 2006). Booster training for Reasoning and Speed training groups produced significantly better performance (above the basic or initial training effect) on their targeted cognitive abilities (S. L. Willis et al., 2006).
A subgroup analysis using an algorithm-based definition of mild cognitive impairment (MCI) was done (Unverzagt et al., 2007). At 2 years, a total of 193 subjects were defined as MCI using this criterion and results indicated that MCI participants failed to benefit from Memory training but did show significant training response to Reasoning and Speed interventions; thus, MCI status mediates response to ACTIVE.
Training Effects on Daily Functioning
At five years, subjects in all three intervention groups reported significantly less difficulty than did participants in the control group in performing instrumental activities of daily living. Since functional decline has been shown to occur first for instrumental tasks, the hypothesis that training benefits would first be detected for these more cognitively challenging everyday tasks was supported. For the total sample in each treatment group, the performance-based measures of Everyday Problem Solving or Everyday Speed did not show this general benefit of training.
The performance-based functional measures, however, did show the hypothesized targeted transfer of training effects at five years for participants receiving booster training. Improved performance on the Everyday Problem Solving composite was found for the boosted Reasoning training group. Likewise, improved performance on the Everyday Speed composite was shown for the boosted Speed training group. Early effects of booster training on performance-based functional measures was also found at the initial booster at 1 year. The boosted Speed training group at 1 year showed an effect for Everyday Speed and the boosted Reasoning group showed an effect for IADL Difficulty.
Training effects on Quality of Life and Driving
The impact of ACTIVE training on health-related quality of life (QOL) was investigated using the SF-36 (Wolinsky et al., 2006). Clinically relevant QOL decline was defined as a drop of 0.5 standard deviations or more from baseline on 3 or more SF-36 scales. At five years, 47.3% of the sample had experienced clinically relevant drops on 3 or more SF-36 scales and logistic regression indicated that participants in each of the interventions were significantly less likely than controls to have QOL decline.
Older drivers who completed cognitive speed of processing training were 40% less likely to cease driving over the subsequent three years (p = .048) (Edwards, Delahunt, & Mahncke, 2009). Speed-of-processing and Reasoning training resulted in a 50% lower rate (per person-mile) of at-fault motor vehicle collisions lower rates than for controls over the subsequent approximately 6-year period (K. Ball, Edwards, Ross, & McGwin, 2010). There was no significant difference observed for the Memory group.
PAPERS IN THIS SUPPLEMENT
In this Supplement, we report both baseline and longitudinal data from the ACTIVE Study. Cognitive training has been shown to improve both cognitive and everyday abilities in older adults, however, little is known concerning the amount of training needed, or the characteristics of those who benefit. These analyses examined the longitudinal impact of dosage (number of training sessions) on the improvement and maintenance of cognitive and everyday function. Three papers address this issue for each of the cognitive training interventions. Using latent growth models, each analysis focuses on participants in the respective training groups to examine the impact of initial and booster training on the maintenance of cognitive and everyday function. As reported previously (S. L. Willis et al., 2006), effects of each training intervention were maintained through five years. However, for memory training, Rebok and co-authors report that neither booster training nor adherence to training significantly influenced this effect. In contrast, Ball and her co-authors al report that the effects of initial speed of processing training effects were amplified by booster sessions. This analysis showed that a single booster session counteracted about five months of age-related processing speed decline. Willis and Caskie report similar findings for the Reasoning intervention, including positive effects for the third annual booster and adherence to training.
The paper by Jones and colleagues aimed to better understand the effects of the ACTIVE training interventions. In particular, they addressed an interesting observation by Salthouse (Salthouse, 2006) that trained subjects had an accelerated rate of decline in cognitive change over time compared to non-trained subjects. They used growth curve models to decompose this change and found that the appearance of accelerated change in cognition reported by Salthouse is the result of age-related decline coupled with loss of training gains. For example, Speed training resulted in very large gains in processing speed. However, these gains were lost quickly and therefore appeared to be greater age-related decline, suggesting that the intervention did more harm than good. However, all trained subjects performed better than non-trained subjects at five years, with performance differences equivalent to about 2, 5, and 7 years of aging for Memory, Reasoning and Speed training, respectively. Reasoning training was the one intervention to attenuate the pace of normal cognitive decline.
To date, most investigations of the cognitive interventions had focused on the effects of training on the composite measures in each cognitive domain. Sisco and colleagues extend this work by investigating how Memory training improved specific aspects of memory function as well as the durability of that effect. The Memory intervention included mnemonic and structure strategy training, the latter shown to result in improved memory of everyday life information. Their work extended prior analyses of training effects by examining the effects of structure strategy training on prose recall. Their results show that training improved verbatim recall but not the hypothesized paraphrase recall, possibly related to emphasis on mnemonic strategies in the initial sessions. However, durability of this effect was limited to post-initial and booster training only, indicating that intermittent training is necessary to maintain effect on memory performance and potential transfer to daily function.
In addition to the effects of the cognitive training interventions, the ACTIVE study offers broad-based opportunities to investigate cognitive and daily function in a large and diverse population of older adults. The proportion of African-America participants (26%) is considerable, and the size of the control group (n=698) constitutes a large natural longitudinal sample in its own right. While the sample is positively selected (because of study inclusion criteria), it produces a kind of “natural experiment” that permits comparison of race-group trajectories in cognition when African American and White groups are demographically similar. The papers by Marsiske and colleagues and by Yam and Marsiske illustrate such an opportunity. In the first of these papers, Marsiske and colleagues use the control sample to explore 5-year change across multiple cognitive abilities. They report a small effect of race, specifically being African American, on level of cognitive performance after controlling age, gender, education, and health. An important finding is that race was not associated with rates of change in cogntiove performance over 5 years. Similar to findings regarding those with low education, African Americans seem to enter late life at a cognitive disadvantage, but they do not experience heightened rates of decline.
Yam and Marsiske use baseline data for the no-contact control group to identify predictors of IADL performance over 5 years. They distinguish between basic mental abilities (memory, reasoning, processing speed) and `everyday' cognitive skills, defined as the application of these basic abilities in real world contexts. Results of the multilevel analyses across 5 years show that, in addition to physical function, this higher order of cognitive skills appears to be a more proximal predictor of everyday IADL function. These findings identify another potential target of interventions to promote daily functions in older age.
The paper by Rexroth and colleagues is another example of the utility of the ACTIVE data beyond intervention effects. They investigated the relationship of demographic factors and health conditions, alone and in combination, on the composite measures of memory, reasoning, and processing speed in these healthy community-dwelling older adults. They hypothesized that each cognitive domain would be affected by age and education and that the effect of demographics on cognition would be attenuated by chronic health conditions and discrete illnesses. They report that younger age, more education and white race are related to better cognitive function in all three domains after adjusting for gender, chronic health conditions, and discrete illnesses. These findings are consistent with and support results of prior studies, particularly in less diverse or younger populations.
Lohman and colleagues considered depressive symptoms in relation to baseline memory ability and responsiveness to Memory training. They report that the more depressive symptoms a subject had, the lower their memory ability at baseline. However, elevated depressive symptoms did not attenuate the effects of Memory training on memory ability. This is an important finding supporting the robustness of the ACTIVE Memory training program.
Two papers report about driving status. O'Connor and colleagues investigated health and physical performance as mediators of the association between driving cessation and mortality. Mortality risk was 1.7 times higher for non-drivers than for drivers, and this risk was mediated by physical performance and social, physical, and general health. Choi and colleagues looked at whether driving cessation as well as the effect of cognitive training on driving cessation differed by gender and race. Driving has long been associated with functional independence, and most prior studies report that older women and ethnic minorities are less likely to drive. Their results were consistent. However, the effects of the cognitive interventions on driving cessation over five years did not differ by gender or race. In conjunction with prior data showing that Speed training delays driving cessation among subjects with pre-existing deficits in processing speed (Edwards et al., 2009) and that both Speed and Reasoning training reduce the number of motor vehicle collisions (K. Ball et al., 2010), these findings support the robustness of these ACTIVE training interventions in maintaining driving and functional mobility.
DISCUSSION
The ACTIVE study is the first large-scale, randomized trial to test the long-term outcomes of cognitive training effects on prevention of decline in daily function. Results support the effectiveness of cognitive intervention in maintaining cognitive health over the long-term and indicate modest but detectable far transfer to instrumental activities of daily living, health-related quality of life, and driving outcomes. The critical importance of ACTIVE and similar preventive cognitive interventions is that they may preserve the cognitive resources shown to be effective both in maintaining functional competence and in coping with functional impairments. Given the lagged relationship between cognitive decline and functional deficits, however, we expected a delay in the observed effects of cognitive interventions on functional outcomes and planned long-term follow-up of participants. The results at 5 years provide supportive evidence for that decision.
There are many strengths of the including a large, diverse sample that was reasonably cognitively well-functioning at baseline, design of interventions that could be delivered in a multisite format, comprehensive cognitive and functional assessments, rigorous certification and ongoing quality assurance methods for both trainers and assessors, and long follow-up interval. One important limitation relates to the representativeness of the ACTIVE sample. The sample was composed of community volunteers and the final sample was advantaged relative to the general population in terms of age, education, and MMSE; therefore, the results of ACTIVE should be interpreted cautiously as they may apply to the general population. Also, the design of the booster training made it difficult to examine dose effects. We did see significant attrition over the follow-up interval (retention at the 5-year assessment was 67%). Participants who were older, had more health problems and lower cognitive function were more likely to drop out. However, through 5 years, there has not been differential attrition by condition. Therefore, the attrition does not affect the between-group comparisons of intervention effects.
The critical importance of ACTIVE and similar preventive cognitive interventions is that they may preserve the cognitive resources shown to be effective both in maintaining functional competence and in coping with functional impairments. Being the first study to demonstrate the long-term potency of cognitive training and far transfer to daily function, ACTIVE will hopefully stimulate new programs of research on cognitive and behavioral interventions in older adults. Ongoing research with the ACTIVE sample is focused on establishing the limits and determinants of transfer of the ACTIVE cognitive training programs to cognitive, functional, and other outcomes like health care utilization.
Acknowledgments
ACTIVE is supported by grants from the National Institute on Aging and the National Institute of Nursing Research to Hebrew Senior Life (U01NR04507), Indiana University School of Medicine (U01NR04508), Johns Hopkins University (U01AG14260), New England Research Institutes (U01AG14282), Pennsylvania State University (U01AG14263), University of Alabama at Birmingham (U01AG14289), University of Florida (U01AG14276).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research, National Institute on Aging, or the National Institutes of Health. Representatives of the funding agency have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. Dr. Unverzagt has received research support from Posit Science, Inc., in the form of site licenses for cognitive training programs for different research projects.
REFERENCES
- Allaire JC, Marsiske M. Everyday cognition: age and intellectual ability correlates. Psychol Aging. 1999;14(4):627–644. doi: 10.1037//0882-7974.14.4.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman L, Hill RD. Cognitive performance and everyday functioning: Patterns in normal aging and dementia. In: Woods RT, editor. Handbook of the Clinical Psychology of Ageing. John Wiley & Sons, Ltd.; West Sussex: 1996. pp. P73–92. [Google Scholar]
- Ball K. Enhancing mobility in the elderly: Attentional interventions for driving. In: Dollinger C, DiLalla L, editors. Assessment and Intervention Issues across the Lifespan. Lawrence Erlbaum Associates; Mahwah, NJ: 1997. [Google Scholar]
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M, et al. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288(18):2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Edwards JD, Ross LA, McGwin G., Jr. Cognitive training decreases motor vehicle collision involvement of older drivers. J Am Geriatr Soc. 2010;58(11):2107–2113. doi: 10.1111/j.1532-5415.2010.03138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K, Owsley C. Increasing mobility and reducing accidents in older drivers. In: Schaie K, editor. Societal Impacts on Mobility in the Elderly. Springer; New York: 2000. [Google Scholar]
- Ball K, Owsley C, Sloane ME, Roenker DL, Bruni JR. Visual attention problems as a predictor of vehicle crashes in older drivers. Invest Ophthalmol Vis Sci. 1993;34(11):3110–3123. [PubMed] [Google Scholar]
- Baltes MM, Kuhl KP, Gutzmann H, Sowarka D. Potential of cognitive plasticity as a diagnostic instrument: a cross-validation and extension. Psychol Aging. 1995;10(2):167–172. doi: 10.1037//0882-7974.10.2.167. [DOI] [PubMed] [Google Scholar]
- Brandt J. The Hopkins Verbal Learning Test: development of a new memory test with six equivalent forms. The Clinical Neuropsychologist. 1991;5(2):125–142. [Google Scholar]
- Burdick DJ, Rosenblatt A, Samus QM, Steele C, Baker A, Harper M, et al. Predictors of functional impairment in residents of assisted-living facilities: the Maryland Assisted Living study. J Gerontol A Biol Sci Med Sci. 2005;60(2):258–264. doi: 10.1093/gerona/60.2.258. [DOI] [PubMed] [Google Scholar]
- Cahn-Weiner DA, Malloy PF, Boyle PA, Marran M, Salloway S. Prediction of functional status from neuropsychological tests in community-dwelling elderly individuals. Clin Neuropsychol. 2000;14(2):187–195. doi: 10.1076/1385-4046(200005)14:2;1-Z;FT187. [DOI] [PubMed] [Google Scholar]
- Caprio-Prevette MD, Fry PS. Memory enhancement program for community-based older adults: development and evaluation. Exp Aging Res. 1996;22(3):281–303. doi: 10.1080/03610739608254012. [DOI] [PubMed] [Google Scholar]
- Carmelli D, Swan GE, Reed T, Miller B, Wolf PA, Jarvik GP, et al. Midlife cardiovascular risk factors, ApoE, and cognitive decline in elderly male twins. Neurology. 1998;50(6):1580–1585. doi: 10.1212/wnl.50.6.1580. [DOI] [PubMed] [Google Scholar]
- Diehl M, Marsiske M, Horgas AL, Rosenberg A, Saczynski JS, Willis SL. The revised Observed Tasks of Daily Living: A performance-based assessment of everyday problem solving in older adults. J Appl Gerontol. 2005;24(3):211–230. doi: 10.1177/0733464804273772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl M, Willis SL, Schaie KW. Everyday problem solving in older adults: observational assessment and cognitive correlates. Psychol Aging. 1995;10(3):478–491. doi: 10.1037//0882-7974.10.3.478. [DOI] [PubMed] [Google Scholar]
- Edwards JD, Delahunt PB, Mahncke HW. Cognitive speed of processing training delays driving cessation. J Gerontol A Biol Sci Med Sci. 2009;64(12):1262–1267. doi: 10.1093/gerona/glp131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstrom R, French J, Harman H, Derman D. Kit of factor-referenced cognitive tests (Rev. ed.) Educational Testing Service; Princeton, NJ: 1976. [Google Scholar]
- Fitti JE, Kovar MG. The supplement on aging to the 1984 National Health Interview Survey. Vital Health Stat. 1987;1(21):1–115. [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gonda J, Schaie K. Schaie-Thurstone Mental Abilities Test: Word Series Test. Consulting Psychologists Press; Palo Alto, CA: 1985. [Google Scholar]
- Graves EJ, Kozak LJ. National hospital discharge survey: annual summary, 1996. Vital Health Stat. 1999;13(140):i–iv. 1–46. [PubMed] [Google Scholar]
- Hayslip B, Jr., Maloy RM, Kohl R. Long-term efficacy of fluid ability interventions with older adults. J Gerontol B Psychol Sci Soc Sci. 1995;50(3):P141–149. doi: 10.1093/geronb/50b.3.p141. [DOI] [PubMed] [Google Scholar]
- Hirdes JP, Fries BE, Morris JN, Ikegami N, Zimmerman D, Dalby DM, et al. Home care quality indicators (HCQIs) based on the MDS-HC. Gerontologist. 2004;44(5):665–679. doi: 10.1093/geront/44.5.665. [DOI] [PubMed] [Google Scholar]
- Hultsch DF, Hammer M, Small BJ. Age differences in cognitive performance in later life: relationships to self-reported health and activity life style. J Gerontol. 1993;48(1):P1–11. doi: 10.1093/geronj/48.1.p1. [DOI] [PubMed] [Google Scholar]
- Jobe JB, Smith DM, Ball K, Tennstedt SL, Marsiske M, Willis SL, et al. ACTIVE: a cognitive intervention trial to promote independence in older adults. Control Clin Trials. 2001;22(4):453–479. doi: 10.1016/s0197-2456(01)00139-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliegl R, Smith J, Baltes P. On the locus and process of magnification of age differences during mnemonic training. Dev Psychol. 1990;26(6):894–904. [Google Scholar]
- Kramer AF, Larish JF, Strayer DL. Training for attentional control in dual task settings: A comparison of young and old adults. J Exp Psychol Appl. 2002;1(1):P50–76. [Google Scholar]
- Lachman ME, Weaver SL, Bandura M, Elliott E, Lewkowicz CJ. Improving memory and control beliefs through cognitive restructuring and self-generated strategies. J Gerontol. 1992;47(5):P293–299. doi: 10.1093/geronj/47.5.p293. [DOI] [PubMed] [Google Scholar]
- Landi F, Tua E, Onder G, Carrara B, Sgadari A, Rinaldi C, et al. Minimum data set for home care: a valid instrument to assess frail older people living in the community. Med Care. 2000;38(12):1184–1190. doi: 10.1097/00005650-200012000-00005. [DOI] [PubMed] [Google Scholar]
- Levit KR, Lazenby HC, Braden BR, Cowan CA, Sensenig AL, McDonnell PA, et al. National health expenditures, 1996. Health Care Financ Rev. 1997;19(1):161–200. [PMC free article] [PubMed] [Google Scholar]
- McDougal G. Cognitive interventions among older adults. In: Fitzpatrick J, editor. Annual Review of Nursing Research. Springer Publishing; New York: 1999. [PMC free article] [PubMed] [Google Scholar]
- Mohs RC, Ashman TA, Jantzen K, Albert M, Brandt J, Gordon B, et al. A study of the efficacy of a comprehensive memory enhancement program in healthy elderly persons. Psychiatry Res. 1998;77(3):183–195. doi: 10.1016/s0165-1781(98)00003-1. [DOI] [PubMed] [Google Scholar]
- Morris JN, Carpenter I, Berg K, Jones RN. Outcome measures for use with home care clients. Can J Aging -Revue Canadienne Du Vieillissement. 2000;19:87–105. [Google Scholar]
- Morris JN, Fries BE, Steel K, Ikegami N, Bernabei R, Carpenter GI, et al. Comprehensive clinical assessment in community setting: applicability of the MDS-HC. J Am Geriatr Soc. 1997;45(8):1017–1024. doi: 10.1111/j.1532-5415.1997.tb02975.x. [DOI] [PubMed] [Google Scholar]
- Myers GC, Juster FT, Suzman RM. Asset and Health Dynamics Among the Oldest Old (AHEAD): initial results from the longitudinal study. Introduction. J Gerontol B Psychol Sci Soc Sci. 1997;52(Spec No):v–viii. [PubMed] [Google Scholar]
- Neely AS, Backman L. Effects of multifactorial memory training in old age: generalizability across tasks and individuals. J Gerontol B Psychol Sci Soc Sci. 1995;50(3):P134–140. doi: 10.1093/geronb/50b.3.p134. [DOI] [PubMed] [Google Scholar]
- Noice H, Noice T, Staines G. A short-term intervention to enhance cognitive and affective functioning in older adults. J Aging Health. 2004;16(4):562–585. doi: 10.1177/0898264304265819. [DOI] [PubMed] [Google Scholar]
- Office, G. A. Medicare: Home Health Utilization Expands While Program Controls Deteriorate. Paper presented at the Report to the Chairman, Special Committee on Aging; U.S. Senate: GAO/HEHS; 1996. pp. 96–16. [Google Scholar]
- Oswald WD, Rupprecht R, Gunzelmann T, Tritt K. The SIMA-project: effects of 1 year cognitive and psychomotor training on cognitive abilities of the elderly. Behav Brain Res. 1996;78(1):67–72. doi: 10.1016/0166-4328(95)00219-7. [DOI] [PubMed] [Google Scholar]
- Owsley C, Ball K, McGwin G, Jr., Sloane ME, Roenker DL, White MF, et al. Visual processing impairment and risk of motor vehicle crash among older adults. JAMA. 1998;279(14):1083–1088. doi: 10.1001/jama.279.14.1083. [DOI] [PubMed] [Google Scholar]
- Owsley C, McGwin G, Jr., Sloane ME, Stalvey BT, Wells J. Timed instrumental activities of daily living tasks: relationship to visual function in older adults. Optom Vis Sci. 2001;78(5):350–359. doi: 10.1097/00006324-200105000-00019. [DOI] [PubMed] [Google Scholar]
- Owsley C, Sloane M, McGwin G, Jr., Ball K. Timed instrumental activities of daily living tasks: relationship to cognitive function and everyday performance assessments in older adults. Gerontology. 2002;48(4):254–265. doi: 10.1159/000058360. [DOI] [PubMed] [Google Scholar]
- Rasmusson D, Rebok G, Bylsma F, Brandt J. Effects of three types of memory training in normal elderly. Aging Neuropsychol Cognit. 1999;6:56–66. [Google Scholar]
- Rebok G, Balcerak L. Memory self-efficacy and performance differences in young and old adults: The effect of mnemonic training. Dev Psychol. 1989;25(5):714–721. [Google Scholar]
- Rey A. L'examen psychologique dans les cas d'encéphalopathie traumatique. (Les problems.). / The psychological examination in cases of traumatic encepholopathy. Problems. Archives de Psychologie. 1941;28:215–285. [Google Scholar]
- Roenker DL, Cissell GM, Ball KK, Wadley VG, Edwards JD. Speed-of-processing and driving simulator training result in improved driving performance. Hum Factors. 2003;45(2):218–233. doi: 10.1518/hfes.45.2.218.27241. [DOI] [PubMed] [Google Scholar]
- Salthouse T. Mental exercise and mental aging. Evaluating the validity of the “use it or lose it” hypothesis. Perspect Psychol Sci. 2006;1(1):68–87. doi: 10.1111/j.1745-6916.2006.00005.x. doi:10.1111/j.1745-6916.2006.00005. [DOI] [PubMed] [Google Scholar]
- Schaie K. Intellectual Development in Adulthood. The Seattle Longitudinal Study. Cambridge University Press; New York: 1996. [Google Scholar]
- Schaie K. Developmental influences on adult intellectual development: The Seattle Longitudinal Study. Oxford University Press; New York: 2005. [Google Scholar]
- Stalvey B, Owsley C, Sloane M, Ball K. The Life Space Questionnaire: A measure of the extent of mobility of older adults. J Appl Gerontol. 1999;18:460–478. [Google Scholar]
- Strahan GW. An overview of nursing homes and their current residents: data from the 1995 National Nursing Home Survey. Adv Data. 1997;(280):1–12. [PubMed] [Google Scholar]
- Swan GE, Carmelli D, LaRue A. Performance on the digit symbol substitution test and 5-year mortality in the Western Collaborative Group Study. Am J Epidemiol. 1995;141(1):32–40. doi: 10.1093/oxfordjournals.aje.a117342. [DOI] [PubMed] [Google Scholar]
- Thurstone L, Thurstone T. Examiner manual for the SRA Primary Mental Abilities Test (Form 10–14) Science Research Associates; Chicago: 1949. [Google Scholar]
- Unverzagt FW, Kasten L, Johnson KE, Rebok GW, Marsiske M, Koepke KM, et al. Effect of memory impairment on training outcomes in ACTIVE. J Int Neuropsychol Soc. 2007;13(6):953–960. doi: 10.1017/S1355617707071512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- Willis S. Current issues in cognitive training research. In: Lovelace E, editor. Aging and Cognition: Mental Processes, Self Awareness, and Interventions. Elsevier; Amsterdam: 1990. [Google Scholar]
- Willis S, Cornelius S, Blow F, Baltes P. Training in research in aging: Attentional processes. J Educ Psychol. 1983;75:257–270. [Google Scholar]
- Willis S, Nesselroade C. Long term effects of fluid ability training in old-old age. Dev Psychol. 1990;26:905–910. [Google Scholar]
- Willis S, Schaie K. Training the elderly on the ability factors of spatial orientation and inductive reasoning. Psychol Aging. 1986;1:239–247. doi: 10.1037//0882-7974.1.3.239. [DOI] [PubMed] [Google Scholar]
- Willis S, Schaie K. Cognitive training in the normal elderly. In: Forette F, Christen Y, Boller F, editors. Plasticité Cérébrale et Stimulation Cognitiv. Foundation National de Gérontologie; Paris: 1994. [Google Scholar]
- Willis SL. Everyday cognitive competence in elderly persons: conceptual issues and empirical findings. Gerontologist. 1996;36(5):595–601. doi: 10.1093/geront/36.5.595. [DOI] [PubMed] [Google Scholar]
- Willis SL, Allen-Burge R, Dolan MM, Bertrand RM, Yesavage J, Taylor JL. Everyday problem solving among individuals with Alzheimer's disease. Gerontologist. 1998;38(5):569–577. doi: 10.1093/geront/38.5.569. [DOI] [PubMed] [Google Scholar]
- Willis SL, Jay GM, Diehl M, Marsiske M. Longitudinal change and prediction of everyday task competence in the elderly. Res Aging. 1992;14(1):68–91. doi: 10.1177/0164027592141004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SL, Tennstedt SL, Marsiske M, Ball K, Elias J, Koepke KM, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296(23):2805–2814. doi: 10.1001/jama.296.23.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B, Cockburn J, Baddeley A. The Rivermead Behavioural Memory Test. Thames Valley Test Company; 34 The Square, Titchfield, Fareham, Hampshire PO14 4AF: 1985. [Google Scholar]
- Wolinsky FD, Johnson RJ. The use of health services by older adults. J Gerontol. 1991;46(6):S345–357. doi: 10.1093/geronj/46.6.s345. [DOI] [PubMed] [Google Scholar]
- Wolinsky FD, Unverzagt FW, Smith DM, Jones R, Stoddard A, Tennstedt SL. The ACTIVE cognitive training trial and health-related quality of life: protection that lasts for 5 years. J Gerontol A Biol Sci Med Sci. 2006;61(12):1324–1329. doi: 10.1093/gerona/61.12.1324. [DOI] [PubMed] [Google Scholar]