Abstract
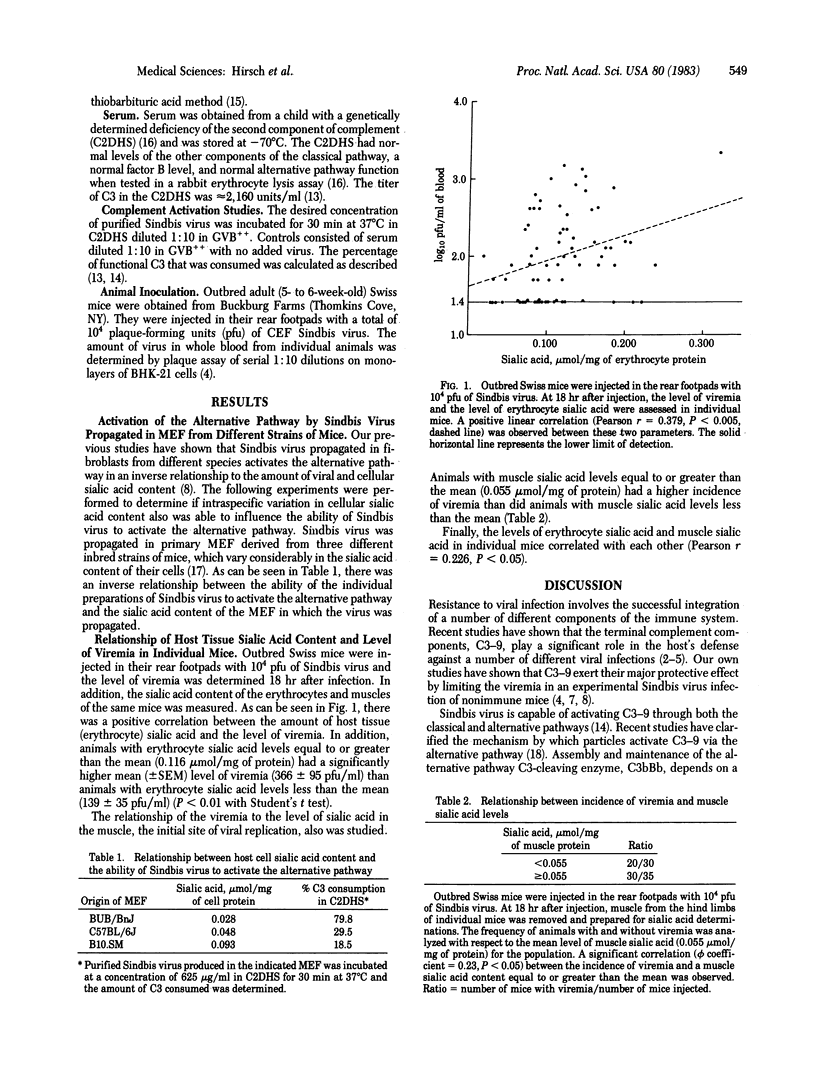
Recent studies have shown that the sialic acid content of Sindbis virus influences both its ability to active the alternative pathway in vitro and its susceptibility to complement dependent clearance from the bloodstream in vivo. Other studies have shown that the sialic acid content of Sindbis virus is determined by the host in which it is propagated. Because individuals vary in their cell surface sialic acid content, it is possible they also vary in their ability to defend themselves against Sindbis virus infection by virtue of their ability to modify the virus sialic acid content and thereby the capacity of the virus to activate the alternative pathway. To test this hypothesis, outbred Swiss mice were injected subcutaneously with Sindbis virus. There was a significant positive correlation between the level of viremia 18 hr after infection and the sialic acid content of the host's erythrocytes. In addition, animals with erythrocyte sialic acid levels equal to or greater than the mean had a higher level of viremia than animals with erythrocyte sialic acid levels less than the mean. Finally, animals that had muscle sialic acid levels equal to or greater than the mean had a higher incidence of viremia than animals with muscle sialic acid levels less than the mean. These studies suggest that the amount of tissue sialic acid in an individual host influences its ability to resist Sindbis virus infection.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fearon D. T. Activation of the alternative complement pathway. CRC Crit Rev Immunol. 1979 Nov;1(1):1–32. [PubMed] [Google Scholar]
- Fearon D. T. Regulation by membrane sialic acid of beta1H-dependent decay-dissociation of amplification C3 convertase of the alternative complement pathway. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1971–1975. doi: 10.1073/pnas.75.4.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks J. T., Ennis F. A., Kim E., Verbonitz M. The importance of an intact complement pathway in recovery from a primary viral infection: influenza in decomplemented and in C5-deficient mice. J Immunol. 1978 Oct;121(4):1437–1445. [PubMed] [Google Scholar]
- Hirsch R. L., Griffin D. E., Winkelstein J. A. Host modification of Sindbis virus sialic acid content influences alternative complement pathway activation and virus clearance. J Immunol. 1981 Nov;127(5):1740–1743. [PubMed] [Google Scholar]
- Hirsch R. L., Griffin D. E., Winkelstein J. A. Role of complement in viral infections: participation of terminal complement components (C5 to C9) in recovery of mice from Sindbis virus infection. Infect Immun. 1980 Dec;30(3):899–901. doi: 10.1128/iai.30.3.899-901.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch R. L., Griffin D. E., Winkelstein J. A. The effect of complement depletion on the course of Sindbis virus infection in mice. J Immunol. 1978 Oct;121(4):1276–1278. [PubMed] [Google Scholar]
- Hirsch R. L., Griffin D. E., Winkelstein J. A. The role of complement in viral infections. II. the clearance of Sindbis virus from the bloodstream and central nervous system of mice depleted of complement. J Infect Dis. 1980 Feb;141(2):212–217. doi: 10.1093/infdis/141.2.212. [DOI] [PubMed] [Google Scholar]
- Hirsch R. L. The complement system: its importance in the host response to viral infection. Microbiol Rev. 1982 Mar;46(1):71–85. doi: 10.1128/mr.46.1.71-85.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch R. L., Winkelstein J. A., Griffin D. E. The role of complement in viral infections. III. Activation of the classical and alternative complement pathways by Sindbis virus. J Immunol. 1980 May;124(5):2507–2510. [PubMed] [Google Scholar]
- Hyatt A. C., Altenburger K. M., Johnston R. B., Jr, Winkelstein J. A. Increased susceptibility to severe pyogenic infections in patients with an inherited deficiency of the second component of complement. J Pediatr. 1981 Mar;98(3):417–419. doi: 10.1016/s0022-3476(81)80708-1. [DOI] [PubMed] [Google Scholar]
- Keegstra K., Sefton B., Burke D. Sindbis virus glycoproteins: effect of the host cell on the oligosaccharides. J Virol. 1975 Sep;16(3):613–620. doi: 10.1128/jvi.16.3.613-620.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSharry J. J., Pickering R. J., Caliguiri L. A. Activation of the alternative complement pathway by enveloped viruses containing limited amounts of sialic acid. Virology. 1981 Oct 30;114(2):507–515. doi: 10.1016/0042-6822(81)90230-0. [DOI] [PubMed] [Google Scholar]
- Nydegger U. E., Fearon D. T., Austen K. F. Autosomal locus regulates inverse relationship between sialic acid content and capacity of mouse erythrocytes to activate human alternative complement pathway. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6078–6082. doi: 10.1073/pnas.75.12.6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin H. S., Mayer M. M. The third component of the guinea pig complement system. II. Kinetic study of the reaction of EAC'4,2a with guinea pig C'3. Enzymatic nature of C'3 comsumption, multiphasic character of fixation, and hemolytic titration of C'3. Biochemistry. 1968 Aug;7(8):2997–3002. doi: 10.1021/bi00848a041. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]


