Abstract
Synapses of the hippocampal mossy fiber pathway exhibit several characteristic features, including a unique form of long-term potentiation that does not require activation of the N-methyl-D-aspartate receptor by glutamate, a complex postsynaptic architecture, and sprouting in response to seizures. However, these connections have proven difficult to study in hippocampal slices because of their relative paucity (<0.4%) compared to commissural-collateral synapses. To overcome this problem, we have developed a novel dissociated cell culture system in which we have enriched mossy fiber synapses by increasing the ratio of granule-to-pyramidal cells. As in vivo, mossy fiber connections are composed of large dynorphin A-positive varicosities contacting complex spines (but without a restricted localization). The elementary synaptic connections are glutamatergic, inhibited by dynorphin A, and exhibit N-methyl-D-aspartate-independent long-term potentiation. Thus, the simplicity and experimental accessibility of this enriched in vitro mossy fiber pathway provides a new perspective for studying nonassociative plasticity in the mammalian central nervous system.
Full text
PDF

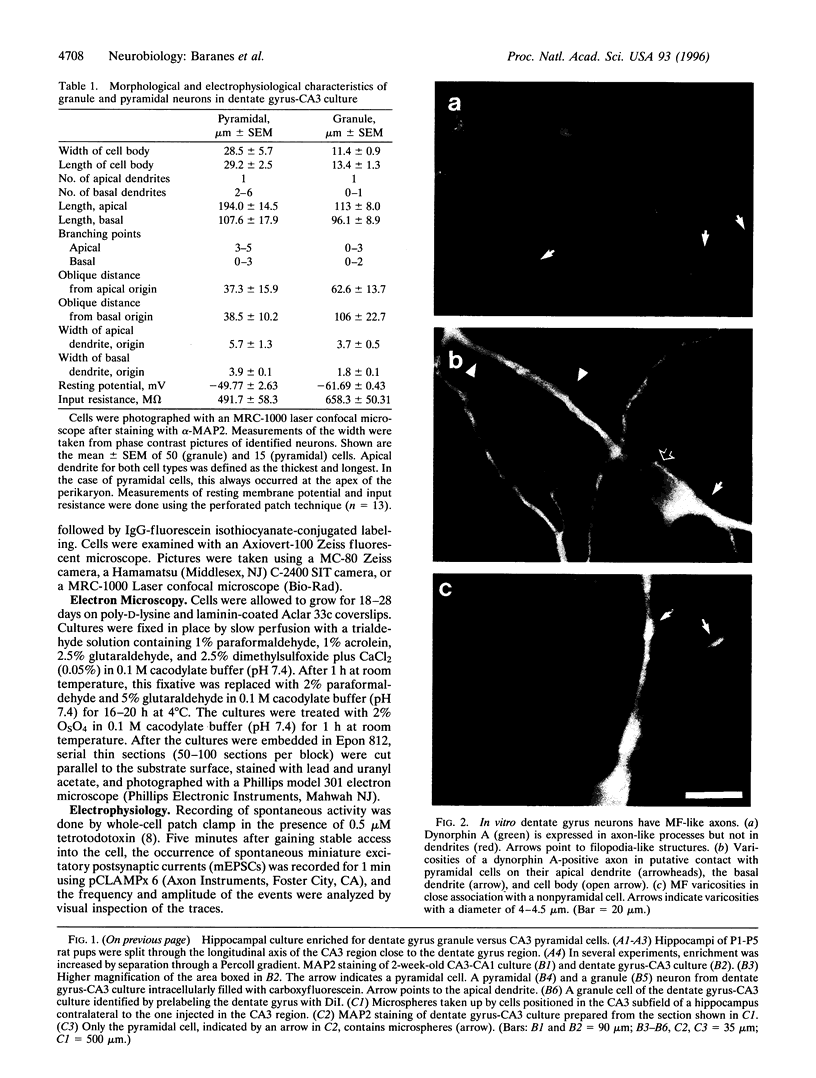
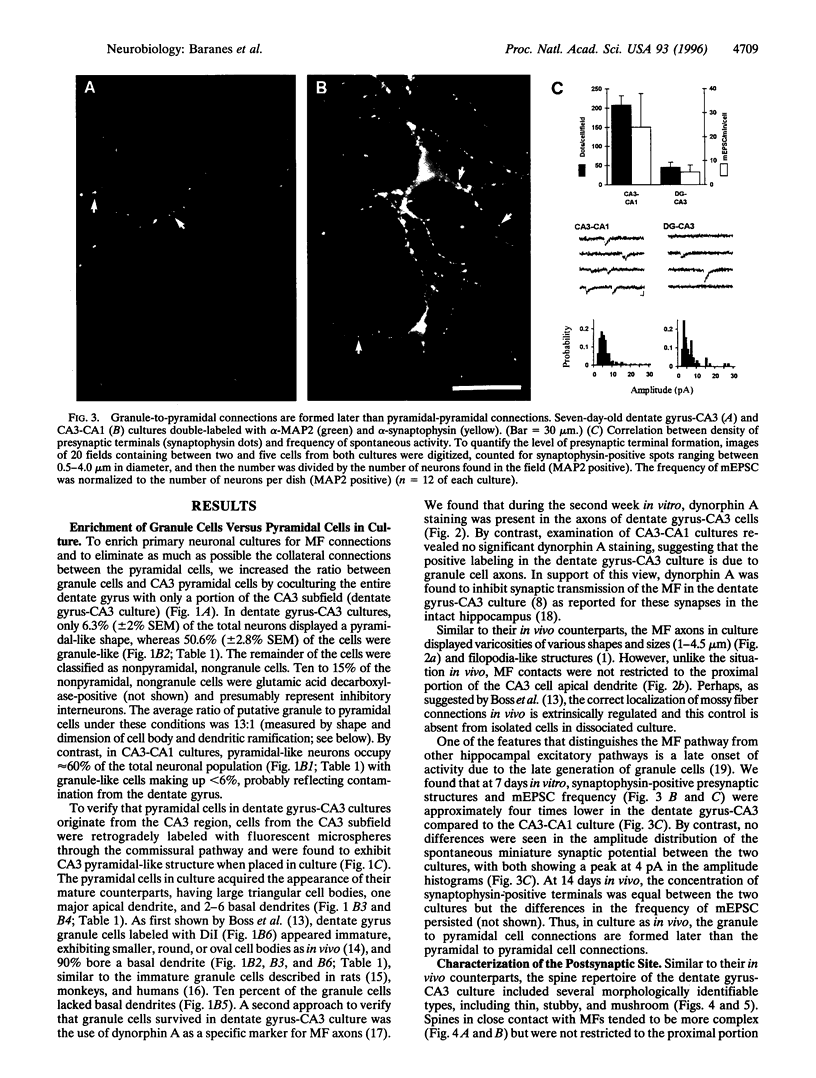


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amaral D. G., Dent J. A. Development of the mossy fibers of the dentate gyrus: I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol. 1981 Jan 1;195(1):51–86. doi: 10.1002/cne.901950106. [DOI] [PubMed] [Google Scholar]
- Bayer S. A. Development of the hippocampal region in the rat. I. Neurogenesis examined with 3H-thymidine autoradiography. J Comp Neurol. 1980 Mar 1;190(1):87–114. doi: 10.1002/cne.901900107. [DOI] [PubMed] [Google Scholar]
- Boss B. D., Gozes I., Cowan W. M. The survival of dentate gyrus neurons in dissociated culture. Brain Res. 1987 Dec 1;433(2):199–218. doi: 10.1016/0165-3806(87)90024-1. [DOI] [PubMed] [Google Scholar]
- Buchhalter J. R., Fieles A., Dichter M. A. Hippocampal commissural connections in the neonatal rat. Brain Res Dev Brain Res. 1990 Nov 1;56(2):211–216. doi: 10.1016/0165-3806(90)90084-c. [DOI] [PubMed] [Google Scholar]
- Chavkin C., Shoemaker W. J., McGinty J. F., Bayon A., Bloom F. E. Characterization of the prodynorphin and proenkephalin neuropeptide systems in rat hippocampus. J Neurosci. 1985 Mar;5(3):808–816. doi: 10.1523/JNEUROSCI.05-03-00808.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicurel M. E., Harris K. M. Three-dimensional analysis of the structure and composition of CA3 branched dendritic spines and their synaptic relationships with mossy fiber boutons in the rat hippocampus. J Comp Neurol. 1992 Nov 8;325(2):169–182. doi: 10.1002/cne.903250204. [DOI] [PubMed] [Google Scholar]
- Claiborne B. J., Amaral D. G., Cowan W. M. A light and electron microscopic analysis of the mossy fibers of the rat dentate gyrus. J Comp Neurol. 1986 Apr 22;246(4):435–458. doi: 10.1002/cne.902460403. [DOI] [PubMed] [Google Scholar]
- Claiborne B. J., Xiang Z., Brown T. H. Hippocampal circuitry complicates analysis of long-term potentiation in mossy fiber synapses. Hippocampus. 1993 Apr;3(2):115–121. doi: 10.1002/hipo.450030202. [DOI] [PubMed] [Google Scholar]
- Craig A. M., Blackstone C. D., Huganir R. L., Banker G. The distribution of glutamate receptors in cultured rat hippocampal neurons: postsynaptic clustering of AMPA-selective subunits. Neuron. 1993 Jun;10(6):1055–1068. doi: 10.1016/0896-6273(93)90054-u. [DOI] [PubMed] [Google Scholar]
- Geinisman Y., deToledo-Morrell L., Morrell F. Induction of long-term potentiation is associated with an increase in the number of axospinous synapses with segmented postsynaptic densities. Brain Res. 1991 Dec 6;566(1-2):77–88. doi: 10.1016/0006-8993(91)91683-r. [DOI] [PubMed] [Google Scholar]
- Hatten M. E. Neuronal regulation of astroglial morphology and proliferation in vitro. J Cell Biol. 1985 Feb;100(2):384–396. doi: 10.1083/jcb.100.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner C. H. Plasticity of the dendritic spine. Prog Neurobiol. 1993 Sep;41(3):281–321. doi: 10.1016/0301-0082(93)90002-a. [DOI] [PubMed] [Google Scholar]
- López-García J. C., Arancio O., Kandel E. R., Baranes D. A presynaptic locus for long-term potentiation of elementary synaptic transmission at mossy fiber synapses in culture. Proc Natl Acad Sci U S A. 1996 May 14;93(10):4712–4717. doi: 10.1073/pnas.93.10.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitecka L., Tremblay E., Charton G., Bouillot J. P., Berger M. L., Ben-Ari Y. Maturation of kainic acid seizure-brain damage syndrome in the rat. II. Histopathological sequelae. Neuroscience. 1984 Dec;13(4):1073–1094. doi: 10.1016/0306-4522(84)90289-6. [DOI] [PubMed] [Google Scholar]
- Okazaki M. M., Nadler J. V. Protective effects of mossy fiber lesions against kainic acid-induced seizures and neuronal degeneration. Neuroscience. 1988 Sep;26(3):763–781. doi: 10.1016/0306-4522(88)90097-8. [DOI] [PubMed] [Google Scholar]
- Rayport S., Sulzer D., Shi W. X., Sawasdikosol S., Monaco J., Batson D., Rajendran G. Identified postnatal mesolimbic dopamine neurons in culture: morphology and electrophysiology. J Neurosci. 1992 Nov;12(11):4264–4280. doi: 10.1523/JNEUROSCI.12-11-04264.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rihn L. L., Claiborne B. J. Dendritic growth and regression in rat dentate granule cells during late postnatal development. Brain Res Dev Brain Res. 1990 Jun 1;54(1):115–124. doi: 10.1016/0165-3806(90)90071-6. [DOI] [PubMed] [Google Scholar]
- Schuster T., Krug M., Wenzel J. Spinules in axospinous synapses of the rat dentate gyrus: changes in density following long-term potentiation. Brain Res. 1990 Jul 16;523(1):171–174. doi: 10.1016/0006-8993(90)91654-y. [DOI] [PubMed] [Google Scholar]
- Seress L., Mrzljak L. Basal dendrites of granule cells are normal features of the fetal and adult dentate gyrus of both monkey and human hippocampal formations. Brain Res. 1987 Mar 3;405(1):169–174. doi: 10.1016/0006-8993(87)91003-1. [DOI] [PubMed] [Google Scholar]
- Seress L., Pokorny J. Structure of the granular layer of the rat dentate gyrus. A light microscopic and Golgi study. J Anat. 1981 Sep;133(Pt 2):181–195. [PMC free article] [PubMed] [Google Scholar]
- Tarrant S. B., Routtenberg A. The synaptic spinule in the dendritic spine: electron microscopic study of the hippocampal dentate gyrus. Tissue Cell. 1977;9(3):461–473. doi: 10.1016/0040-8166(77)90006-4. [DOI] [PubMed] [Google Scholar]
- Tauck D. L., Nadler J. V. Evidence of functional mossy fiber sprouting in hippocampal formation of kainic acid-treated rats. J Neurosci. 1985 Apr;5(4):1016–1022. doi: 10.1523/JNEUROSCI.05-04-01016.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf M. G., Zalutsky R. A., Nicoll R. A. The opioid peptide dynorphin mediates heterosynaptic depression of hippocampal mossy fibre synapses and modulates long-term potentiation. Nature. 1993 Apr 1;362(6419):423–427. doi: 10.1038/362423a0. [DOI] [PubMed] [Google Scholar]







