Abstract
BACKGROUND:
The 2011 American Academy of Pediatrics guidelines state that renal and bladder ultrasound (RBUS) should be performed after initial febrile urinary tract infection (UTI) in a young child, with voiding cystourethrogram (VCUG) performed only if RBUS shows abnormalities. We sought to determine test characteristics and predictive values of RBUS for VCUG findings in this setting.
METHODS:
We analyzed 3995 clinical encounters from January 1, 2006 to December 31, 2010 during which VCUG and RBUS were performed for history of UTI. Patients who had previous postnatal genitourinary imaging or history of prenatal hydronephrosis were excluded. Sensitivity, specificity, and predictive values of RBUS for VCUG abnormalities were determined.
RESULTS:
We identified 2259 patients age <60 months who had UTI as the indication for imaging. RBUS was reported as “normal” in 75%. On VCUG, any vesicoureteral reflux (VUR) was identified in 41.7%, VUR grade >II in 20.9%, and VUR grade >III in 2.8%. Sensitivity of RBUS for any abnormal findings on VCUG ranged from 5% (specificity: 97%) to 28% (specificity: 77%). Sensitivity for VUR grade >III ranged from 18% (specificity: 97%) to 55% (specificity: 77%). Among the 1203 children aged 2 to 24 months imaged after a first febrile UTI, positive predictive value of RBUS was 37% to 47% for VUR grade >II (13% to 24% for VUR grade >III); negative predictive value was 72% to 74% for VUR grade >II (95% to 96% for VUR grade >III).
CONCLUSIONS:
RBUS is a poor screening test for genitourinary abnormalities. RBUS and VCUG should be considered complementary as they provide important, but different, information.
Keywords: urinary tract infection, imaging, vesicoureteral reflux, pediatrics
What’s Known on This Subject:
Current guidelines recommend renal ultrasound as a screening test after febrile urinary tract infection, with voiding cystourethrogram (VCUG) only if the ultrasound is abnormal. Few studies have evaluated the accuracy of ultrasound as a screening test for VCUG-identified abnormalities.
What This Study Adds:
This study shows that ultrasound is a poor screening test for genitourinary abnormalities identified on VCUG, such as vesicoureteral reflux. Neither positive nor negative ultrasounds reliably identify or rule out such abnormalities. Ultrasound and VCUG provide different, but complementary, information.
Recommendations regarding appropriate evaluation of infants and young children who have a first febrile urinary tract infection (UTI) continue to evolve. The 1999 American Academy of Pediatrics (AAP) clinical practice guidelines recommended both voiding cystourethrogram (VCUG) and renal and bladder ultrasound (RBUS) in this situation.1 The most recent AAP guidelines, however, state that after an initial febrile UTI in an infant age 2 to 24 months, only RBUS should be performed and that VCUG should only be performed after a second febrile UTI, or if the RBUS “reveals hydronephrosis, scarring, or other findings that would suggest either high-grade vesicoureteral reflux (VUR) or obstructive uropathy.”2
Although the new guidelines do not explicitly frame RBUS as a screening test, the guidelines do suggest that the decision to perform VCUG after a first febrile UTI should be based in part on RBUS findings. The implicit assumption is that RBUS is a useful tool to identify patients likely to have abnormalities on VCUG, and that a normal RBUS effectively rules out clinically significant genitourinary (GU) abnormalities. However, most published studies evaluating RBUS as a screening tool in this context have significant limitations.
The purpose of this study was to assess the test characteristics of RBUS as a screening test for VUR and other GU conditions, and to determine the positive and negative predictive value of RBUS for these conditions, particularly among children age 2 to 24 months who have a history of first febrile UTI.
Methods
Data Source
With Institutional Review Board approval and a waiver of informed consent, we reviewed institutional billing records to identify all clinical encounters between January 1, 2006 and December 31, 2010 during which a patient underwent both a VCUG (Current Procedural Terminology [CPT] code 74455) and RBUS (CPT codes 76700 [abdominal], 76705 [abdominal, limited], 76770 [retroperitoneal], 76775 [retroperitoneal, limited], 76856 [pelvic], or 76857 [pelvic, limited]) on the same day. Results of these studies were abstracted directly from the text of the radiology report in the electronic medical record (images were not reviewed). Clinical information on specific patients was abstracted from the medical record.
Patient Selection
The sample consisted of children age <60 months who underwent VCUG and RBUS on the same day. We excluded patients who had previous postnatal GU imaging (VCUG, RBUS, or other ultrasound or cross-sectional studies during which the urinary tract was imaged), based on review of the medical record. The indication for VCUG/RBUS was categorized as UTI (febrile or nonfebrile, initial or recurrent), history of prenatal GU abnormalities, or “other” indications. We then selected only those patients whose indication for imaging was UTI. Children who had a history of prenatal hydronephrosis or other prenatal GU abnormalities were also excluded, even if they had not previously undergone postnatal GU imaging. Circumcision status was also assessed among males.
RBUS Data Abstraction and Classification
RBUS findings were categorized as renal or ureteral dilation, renal parenchymal findings, bladder findings, and “other.” Synonyms for “hydronephrosis” included pelviectasis, pelvocaliectasis, caliectasis, and pelvic/calyceal dilation. Terms including “extra-renal pelvis,” “fullness,” and “prominence” were considered dilation without hydronephrosis. Synonyms for ureteral dilation included hydroureter, ureterectasis, and megaureter. Dilation was characterized on mild-moderate-severe scale. (At our institution, these terms approximated the Society for Fetal Urology [SFU] hydronephrosis scale3; “mild” = SFU grade 1–2, “moderate” = SFU grade 3, “severe” = SFU grade 4. “Fullness” or “prominence” corresponded to SFU grade 0–1). Renal parenchymal findings included abnormal echogenicity, abnormal cortico-medullary differentiation, cortical thinning/scarring, cysts, ectopia, duplication, hypotrophy or size discrepancy, agenesis, or calcifications. Bladder findings included wall thickening, trabeculation, diverticulum, ureterocele, dilated posterior urethra, and debris. RBUS reports in which none of these findings are identified, or in which the “impression” section states that the ultrasound is “normal,” were considered to represent a “negative” RBUS.
We defined a range of thresholds for a “positive” RBUS screening test, based on presupposed severity of specific findings (Table 1). Using the most stringent threshold, only studies with the most severe findings (eg, “severe hydroureter”) would be considered “positive.” At the most relaxed threshold, an RBUS with any abnormal findings regardless of severity (eg, pelvic “fullness” without hydronephrosis) would be considered “positive.”
TABLE 1.
Criteria for Specific Thresholds for “Abnormal” or (“Positive”) RBUS, Based on Type and Severity of Observed Findings
| Normal ←←←←←←←←←←←←→→→→→→→→→→→→ Abnormal | |||||
|---|---|---|---|---|---|
| No Abnormal Findings Reported | Abnormal Level D (RBUS-D) | Abnormal Level C (RBUS-C) | Abnormal Level Ba (RBUS-B) | Abnormal Level A (RBUS-A) | |
| N = 1694 | N = 41 | N = 135 | N = 298 | N = 91 | |
| 75.0% | 1.8% | 6.0% | 13.2% | 4.0% | |
| Renal collecting system | Normal | Normal | “Fullness” or “prominence” of collecting system without hydronephrosis | Mild hydronephrosis | >Mild hydronephrosis |
| Extra-renal pelvis | Urothelial thickening | ||||
| Ureter | Normal | Normal | Normal | Mild ureteral dilation | >Mild ureteral dilation |
| Renal parenchyma | Normal | Duplication Solitary kidney | Simple cyst (single) | Size discrepancy Renal ectopia | Stone(s) |
| Dysplasia/increased echogenicity | |||||
| Cortical thinning/scar | |||||
| Abnormal cortico-medullary differentiation | |||||
| Multicystic/polycystic kidney | |||||
| Bladder | Normal | Normal | Debris Wall thickening | Trabeculation Diverticulum | Ureterocele Dilated posterior urethra |
The RBUS severity threshold is set according to column furthest to the right that contains finding(s) observed in the RBUS screening test. The screening test is considered “abnormal” (or “positive”) at the threshold of that column, as well as for all those thresholds to the left of that level.
Threshold RBUS-B included findings categorized as “other”: 1 example each of acute pyelonephritis, renal mass, large bladder, urachal remnant, and bilateral enlarged kidneys.
VCUG Data Abstraction and Classification
VCUG findings were divided into 4 categories: VUR, peri-ureteral diverticulum, other bladder findings, and urethral findings. VUR was graded on the 5-point international grading system,4 and laterality and duplication status were recorded. Bladder findings included diverticula, trabeculation, ureterocele, capacity above or below expected volume, or wall thickening, as noted by increased distance from pubic symphysis on bladder filling. Any urethral findings were considered clinically significant. As with RBUS, we defined different VCUG thresholds for a “positive” study, varying primarily by VUR grade (Table 2). At the most stringent threshold, only VUR > grade III indicated a “positive” VCUG, whereas under the most relaxed threshold, any degree of VUR indicated a “positive” VCUG. Other bladder and urethral findings were also included in the threshold definitions. Any VCUG in which the “impression” section stated that the VCUG is “normal” was considered to represent a “negative” VCUG at all thresholds.
TABLE 2.
Criteria for the VCUG Threshold Groups
| VCUG-E | VCUG-D | VCUG-C | VCUG-B | VCUG-A | |
|---|---|---|---|---|---|
| VUR | Any VUR | Any VUR | VUR > Grade I | VUR > Grade II | VUR > Grade III |
| Peri-ureteral (Hutch) diverticulum | Any peri-ureteral diverticulum | Any peri-ureteral diverticulum | Any peri-ureteral diverticulum | Any peri-ureteral diverticulum | Any peri-ureteral diverticulum |
| Bladder | Any abnormalities | “Significant” abnormalities | “Significant” abnormalities | “Significant” abnormalities | “Significant” abnormalities |
| Diverticulum | Diverticulum | Diverticulum | Diverticulum | Diverticulum | |
| Trabeculation | Trabeculation | Trabeculation | Trabeculation | Trabeculation | |
| Ureterocele | Ureterocele | Ureterocele | Ureterocele | Ureterocele | |
| Large volume | |||||
| Small volume | |||||
| Bladder wall thickening | |||||
| Urethra | Any urethral abnormalities | Any urethral abnormalities | Any urethral abnormalities | Any urethral abnormalities | Any urethral abnormalities |
A VCUG is positive for a particular category if any of the findings in that column were reported during the VCUG. If none of the findings in a given column were present, then the VCUG was “negative” for that threshold definition.
Data Analysis: Test Characteristics and Predictive Values
Test characteristics and predictive values were calculated by using the various threshold definitions of RBUS to predict each VCUG outcome. Sensitivity was defined as the proportion of all patients with a “positive” VCUG who had a “positive” RBUS. Specificity was defined as the proportion of all patients with a “negative” VCUG who had a “negative” RBUS. Positive predictive value was the proportion of all patients with a “positive” RBUS who had a “positive” VCUG. Negative predictive value was the proportion of all patients with a “negative” RBUS who had a “negative” VCUG. We calculated test characteristics and predictive values in both the larger sample of all patients age <60 months who had UTI as indication for imaging, as well as in the subset age 2 to 24 months with first febrile UTI (the AAP guidelines population). Receiver Operating Characteristic curves were developed for each of the VCUG outcomes. Analyses were performed by using SAS 9.3 (SAS Institute Inc; Cary, NC) and R 2.15.2 (http://www.R-project.org/).
Results
We identified 3995 clinical encounters during which patients underwent RBUS and VCUG studies on the same date between January 1, 2006 and December 31, 2010. We excluded 930 patients who had previous postnatal GU imaging, leaving 3065 subjects. Of these, 198 were age ≥60 months and were also excluded, leaving 2867 children. Among this group, the indications for imaging were UTI in 2259 (78.8%), prenatally identified abnormalities in 509 (17.8%), and other indications in 99 (3.5%). The 2259 patients who underwent initial GU imaging for UTI are described in Table 3. A total of 79.0% were female, 75.3% were aged 2 to 24 months, and 43% (975/2259) were seen clinically in the Department of Urology at our institution. Among the boys, most were uncircumcised. Among the group aged 2 to 24 months, we confirmed that this was an initial, febrile UTI episode in 1203 patients.
TABLE 3.
Characteristics of Children Undergoing Initial RBUS and VCUG on the Same Day for History of UTI
| Children Age <60 Mo With History of UTI as Indication for Initial GU Imaging (N = 2259) | Children Age 2–24 Mo With First Febrile UTI as Indication for Initial GU Imaging (N = 1203) | |
|---|---|---|
| Gender, (%) | ||
| Female | 1787 (79.1) | 912 (75.8) |
| Male: uncircumcised | 306 (13.6) | 209 (17.4) |
| Male: circumcised | 50 (2.2) | 37 (3.1) |
| Male: circumcision status unknown | 116 (5.1) | 45 (3.7) |
| Age, (%) | ||
| 0–1 mo | 78 (3.45) | 0 (0) |
| 2–6 mo | 591 (26.16) | 463 (38.5) |
| 7–12 mo | 729 (32.27) | 515 (42.8) |
| 13–18 mo | 230 (10.18) | 138 (11.5) |
| 19–24 mo | 151 (6.68) | 87 (7.2) |
| 25–59 mo | 480 (21.25) | 0 (0) |
| Previous UTI history, (%) | ||
| First UTI | 1557 (68.9) | 1203 (100) |
| Recurrent UTI | 176 (7.8) | 0 (0) |
| Recurrence status unknown | 526 (23.3) | 0 (0) |
| UTI fever status, (%) | ||
| Febrile UTI | 2045 (90.5) | 1203 (100) |
| Nonfebrile UTI | 89 (3.9) | 0 (0) |
| Febrile history unknown | 125 (5.5) | 0 (0) |
RBUS findings among both the whole cohort and “initial febrile UTI, age 2 to 24 months” group are shown in Table 4, along with the proportions in each group meeting thresholds for a “positive” RBUS screening test. Depending on the threshold, RBUS was “positive” (abnormal) in 4% (RBUS-A), 17% (RBUS-B), 23% (RBUS-C), or 25% (RBUS-D) of children who had “any UTI.” Positive rates were similar for the “initial febrile UTI” group. Overall, the RBUS findings were notable for the small number of patients who had higher grades of hydronephrosis; only approximately 1.5% of children had hydronephrosis greater than “mild” on either side. Similarly, <5% of children had ureteral dilation noted, in any degree. The most common renal parenchymal findings were size discrepancy and duplication; more discrete renal scarring or other cortical pathology was seen in ∼1% of the group.
TABLE 4.
Findings on RBUS Among Children Undergoing Initial RBUS and VCUG on the Same Day for History of UTI
| Children Age <60 Mo With History of UTI as Indication for Initial GU Imaging (N = 2259) | Children Age 2–24 Mo With First Febrile UTI as Indication for Initial GU Imaging (N = 1203) | |||
|---|---|---|---|---|
| Renal collecting system dilation, (%) | ||||
| None (normal) | 1921 (85.0) | 1013 (84.2) | ||
| “Fullness” or extrarenal pelvis but no hydronephrosis | 113 (5.0) | 57 (4.7) | ||
| “Mild” hydronephrosis | 191 (8.5) | 104 (8.6) | ||
| “Mild-moderate” hydronephrosis | 13 (0.6) | 8 (0.7) | ||
| “Moderate” hydronephrosis | 9 (0.4) | 5 (0.4) | ||
| “Moderate-severe” hydronephrosis | 9 (0.4) | 5 (0.4) | ||
| “Severe” hydronephrosis | 0 (0) | 0 (0) | ||
| Degree not characterized | 3 (0.1) | 1 (0.1) | ||
| Ureteral dilation, (%) | ||||
| None (normal) | 2169 (96.0) | 1144 (95.1) | ||
| “Mild” dilation | 44 (1.95) | 30 (2.5) | ||
| “Mild-moderate” dilation | 9 (0.4) | 6 (0.5) | ||
| “Moderate” dilation | 11 (0.5) | 9 (0.7) | ||
| “Moderate-severe” dilation | 5 (0.2) | 2 (0.2) | ||
| “Severe” dilation | 1 (0.04) | 1 (0.1) | ||
| Degree not characterized | 20 (0.9) | 11 (0.9) | ||
| Renal Parenchyma, (%) | ||||
| None (normal) | 2015 (89.2) | 1075 (89.4) | ||
| Size discrepancy/atrophy | 90 (4.0) | 48 (4.0) | ||
| Duplication of collecting system | 80 (3.5) | 43 (3.6) | ||
| Urothelial thickening | 42 (1.9) | 19 (1.6) | ||
| Cortical thinning/scarring | 18 (0.8) | 9 (0.8) | ||
| Renal cyst, single | 14 (0.6) | 9 (0.8) | ||
| Increased echogenicity/dysplasia | 10 (0.4) | 8 (0.7) | ||
| Renal ectopia | 9 (0.4) | 4 (0.3) | ||
| Stones/calcification | 4 (0.2) | 3 (0.3) | ||
| Abnormal corticomedullary differentiation | 3 (0.1) | 1 (0.1) | ||
| Multiple renal cysts | 2 (0.1) | 2 (0.2) | ||
| Solitary kidney | 1 (0.04) | 1 (0.1) | ||
| Bladder, (%) | ||||
| None (normal) | 2197 (97.3) | 1183 (98.3) | ||
| Debris | 36 (1.6) | 11 (0.9) | ||
| Bladder wall thickening | 21 (0.9) | 6 (0.5) | ||
| Trabeculation | 5 (0.2) | 3 (0.3) | ||
| Ureterocele | 4 (0.2) | 1 (0.1) | ||
| Diverticulum | 0 (0) | 0 (0) | ||
| Dilated posterior urethra | 0 (0) | 0 (0) | ||
| Other GU findings | 5 (0.2) | 2 (0.2) | ||
| Counts of patients at various thresholds for “positive” RBUS, (%) | “Positive” test (abnormal) | “Negative” test (normal) | “Positive” test (abnormal) | “Negative” test (normal) |
| RBUS-A threshold (most stringent criteria) | 91 (4.0) | 2168 (96.0) | 55 (4.6) | 1148 (95.4) |
| RBUS-B threshold | 389 (17.2) | 1870 (82.8) | 215 (17.9) | 988 (82.1) |
| RBUS-C threshold | 524 (23.2) | 1735 (76.8) | 287 (23.9) | 916 (76.1) |
| RBUS-D threshold (most relaxed criteria) | 565 (25.0) | 1694 (75.0) | 310 (25.8) | 893 (74.2) |
VCUG findings among both patient groups are shown in Table 5, along with the proportions in each group meeting each threshold for a “positive” VCUG. Abnormalities of any kind were identified in 43.9% of studies (49.2% of the initial febrile UTI group), and VUR was identified in 41.7% (47.5%). Significant numbers of children had dilating VUR, with VUR grade >II seen in 20.9% (26.9%). However, high-grade VUR was uncommon: VUR grade >III was present in just 2.7% (2.6%). As with RBUS, the proportion of children who had a positive VCUG depended on the threshold used: VCUG was “positive” (abnormal) in 7% (VCUG-A), 23% (VCUG-B), 39% (VCUG-C), or 43% (VCUG-D) of the “any UTI, age <60 months” group. Positive rates were slightly higher for the “initial febrile UTI, age 2 to 24 months” group.
TABLE 5.
Findings on VCUG Among Children With UTI as Indication for Initial GU Imaging
| Children Age <60 Mo With History of UTI as Indication for Initial GU Imaging (N = 2259) | Children Age 2–24 Mo With First Febrile UTI as Indication for Initial GU Imaging (N = 1203) | |||
|---|---|---|---|---|
| VUR laterality, (%) | ||||
| No VUR | 1317 (58.3) | 632 (52.5) | ||
| Unilateral right VUR | 192 (8.5) | 108 (9.0) | ||
| Unilateral left VUR | 298 (13.2) | 154 (12.8) | ||
| Bilateral VUR | 452 (20.0) | 309 (25.7) | ||
| Highest VUR grade, (%) | ||||
| No VUR | 1317 (58.3) | 632 (52.5) | ||
| I | 112 (5.0) | 53 (4.4) | ||
| I–II | 5 (0.2) | 3 (0.25) | ||
| II | 352 (15.6) | 193 (16.0) | ||
| II–III | 150 (6.6) | 111 (9.2) | ||
| III | 260 (11.5) | 180 (15.0) | ||
| III–IV | 30 (1.3) | 14 (1.2) | ||
| IV | 23 (1.0) | 11 (0.9) | ||
| IV–V | 3 (0.1) | 3 (0.25) | ||
| V | 3 (0.1) | 0 (0) | ||
| Not graded (concurrent obstruction) | 4 (0.2) | 3 (0.25) | ||
| Peri-ureteral (Hutch) diverticulum, (%) | ||||
| No (normal) | 2193 (97.1) | 1165 (96.8) | ||
| Yes | 65 (2.9) | 37 (3.1) | ||
| Not reported | 1 (0.04) | 1 (0.08) | ||
| Bladder abnormality, (%) | ||||
| Normal | 2211 (97.9) | 1185 (98.5) | ||
| Minor findinga | 28 (1.2) | 7 (0.6) | ||
| Major findingb | 18 (0.8) | 9 (0.7) | ||
| Not reported | 2 (0.1) | 2 (0.2) | ||
| Urethral abnormality, (%) | ||||
| Normal | 2139 (94.7) | 1139 (94.7) | ||
| Abnormalityc | 11 (0.5) | 6 (0.5) | ||
| Urethra not reported | 109 (4.8) | 58 (4.8) | ||
| Counts of patients at various thresholds for “positive” VCUG, (%) | “Positive” test (abnormal) | “Negative” test (normal) | “Positive” test (abnormal) | “Negative” test (normal) |
| VCUG-A threshold (urethral findings or major bladder finding or VUR >III) | 137 (6.1) | 2122 (93.9) | 71 (5.9) | 1132 (94.1) |
| VCUG-B threshold (urethral findings or major bladder finding or VUR >II) | 528 (23.4) | 1731 (76.6) | 351 (29.2) | 852 (70.8) |
| VCUG-C threshold (urethral findings or major bladder finding or VUR >I) | 873 (38.65) | 1386 (61.35) | 542 (45.05) | 661 (54.95) |
| VCUG-D threshold (urethral findings or major bladder finding or any VUR) | 975 (43.2) | 1284 (56.8) | 590 (49.0) | 613 (51.0) |
| VCUG-E threshold (any abnormal finding on VCUG) | 992 (43.9) | 1267 (56.1) | 592 (49.2) | 611 (50.8) |
Minor bladder finding: volume higher or lower than predicted; bladder wall thickening.
Major bladder finding: trabeculation, ureterocele, or diverticulum.
Urethral findings: posterior urethral valves (5), dilated urethra (2), anterior urethral diverticulum (1), spinning top (1), utricle (1), urethral prolapsed (1).
Test characteristics and predictive values for RBUS as a screening test for VCUG findings are shown in Table 6. Receiver Operating Characteristic curves are shown in Fig 1 A–D. As expected, there was little difference in test characteristics (sensitivity and specificity) between the “any UTI” and “initial febrile UTI” groups. Predictive values differed somewhat more, particularly for negative predictive value. RBUS is not a sensitive test regardless of the threshold used, with a maximum sensitivity of 55% (RBUS-D for the VCUG-A outcome). Specificity did reach high levels (maximum 97%) but only at extremely low levels of sensitivity (<10%). Positive predictive values were also low, suggesting that only a fraction of those who have a positive RBUS have VCUG findings at any “positivity” level. Finally, negative predictive values were high, but only for the highest grades of VUR.
TABLE 6.
Test Characteristics and Predictive Values of Each RBUS “Positive” Threshold for Each of the VCUG Thresholds
| Sensitivity (95% CI) | Specificity (95% CI) | Positive Predictive Value (95% CI) | Negative Predictive Value (95% CI) | |
|---|---|---|---|---|
| VCUG-E threshold (any abnormal finding on VCUG) | ||||
| RBUS-D (most relaxed criteria) | 28.0(25.2–30.9)* / 26.7(23.2–30.4)** | 77.3(74.9–79.6)* / 75.1 (71.5–78.5)** | 49.2(45.0–53.4)* / 51.0(45.3–56.7)** | 57.9(55.5–60.2)* / 51.4(48.1–54.7)** |
| RBUS-C | 25.5(22.8–28.3)* / 24.2(20.8–27.8)** | 78.6(76.2–80.8)* / 76.4 (72.9–79.7)** | 48.3(43.9–52.7)* / 49.8(43.9–55.8)** | 57.4(55.0–59.7)* / 51.0(47.7–54.3)** |
| RBUS-B | 19.1(16.7–21.6)* / 18.8(15.7–22.1)** | 84.2(82.1–86.2)* / 83.0 (79.8–85.9)** | 48.6(43.5–53.7)* / 51.6(44.7–58.5)** | 57.1(54.8–59.3)* / 51.3(48.1–54.5)** |
| RBUS-A (most stringent criteria) | 4.9(3.7–6.5)* / 5.2(3.6–7.4)** | 96.7(95.5–97.6)* / 96.1 (94.2–97.5)** | 53.8(43.1–64.4)* / 56.4(42.3–69.7)** | 56.5(54.4–58.6)* / 51.1(48.2–54.1)** |
| VCUG-D threshold (urethral findings or major bladder finding or any VUR) | ||||
| RBUS-D (most relaxed criteria) | 28.1(25.3–31.0)* / 26.6(23.1–30.4)** | 77.3(74.9–79.6)* / 75.0(71.4–78.4)** | 48.5(44.3–52.7)* / 50.6(44.9–56.3)** | 58.6(56.2–61.0)* / 51.5(48.2–54.8)** |
| RBUS-C | 25.5(22.8–28.4)* / 24.1(20.7–27.7)** | 78.6(76.2–80.8)* / 76.3(72.8–79.7)** | 47.5(43.2–51.9)* / 49.5(43.6–55.4)** | 58.2(55.8–60.5)* / 51.1(47.8–54.4)** |
| RBUS-B | 19.0(16.6–21.6)* / 18.6(15.6–22)** | 84.1(82.0–86.1)* / 82.9(79.7–85.8)** | 47.6(42.5–52.7)* / 51.2(44.3–58)** | 57.8(55.5–60.0)* / 51.4(48.2–54.6)** |
| RBUS-A (most stringent criteria) | 4.9(3.7–6.5)* / 5.3(3.6–7.4)** | 96.7(95.5–97.6)* / 96.1(94.2–97.5)** | 52.7(42.0–63.3)* / 56.4(42.3–69.7)** | 57.2(55.1–59.3)* / 51.3(48.4–54.2)** |
| VCUG-C threshold (urethral findings or major bladder finding or VUR >I) | ||||
| RBUS-D (most relaxed criteria) | 29.0(26.0–32.1)* / 27.3 (23.6–31.3)** | 77.5(75.2–79.7)* / 75.5(72.0–78.7)** | 44.8(40.6–49.0)* / 47.7(42.1–53.5)** | 63.4(61.1–65.7)* / 55.9(52.6–59.2)** |
| RBUS-C | 26.5(23.6–29.5)* / 24.7(21.1–28.6)** | 78.9(76.6–81.0) / 76.9(73.4–80.0) | 44.1(39.8–48.5) / 46.7(40.8–52.6) | 63.0(60.7–65.3) / 55.5(52.2–58.7) |
| RBUS-B | 19.6(17.0–22.4)* / 19.0(15.8–22.6)** | 84.3(82.2–86.1) / 83.1(80.0–85.8) | 44.0(39.0–49.0) / 47.9(41.1–54.8) | 62.5(60.2–64.7) / 55.6(52.4–58.7) |
| RBUS-A (most stringent criteria) | 5.2(3.8–6.8)* / 5.4(3.6–7.6)** | 96.7(95.6–97.6) / 96.1(94.3–97.4) | 49.5(38.8–60.1) / 52.7(38.8–66.3) | 61.8(59.7–63.9) / 55.3(52.4–58.2) |
| VCUG-B threshold (urethral findings or major bladder finding or VUR >II) | ||||
| RBUS-D (most relaxed criteria) | 35.8(31.7–40.0)* / 32.8 (27.9–37.9)** | 78.3(76.3–80.2)* / 77.1(74.1–79.9)** | 33.5(29.6–37.5)* / 37.1(31.7–42.7)** | 80.0(78.0–81.9)* / 73.6(70.5–76.4)** |
| RBUS-C | 33.1(29.1–37.3)* / 29.9(25.2–35.0)** | 79.8(77.9–81.7)* / 78.6(75.7–81.3)** | 33.4(29.4–37.6)* / 36.6(31.0–42.4)** | 79.7(77.7–81.5)* / 73.1(70.1–76.0)** |
| RBUS-B | 25.2(21.5–29.1)* / 23.1(18.8–27.8)** | 85.2(83.5–86.9)* / 84.3(81.7–86.7)** | 34.2(29.5–39.1)* / 37.7(31.2–44.5)** | 78.9(77.0–80.7)* / 72.7(69.8–75.4)** |
| RBUS-A (most stringent criteria) | 7.8(5.6–10.4)* / 7.4(4.9–10.7)** | 97.1(96.2–97.8)* / 96.6(95.1–97.7)** | 45.1(34.6–55.8)* / 47.3(33.7–61.2)** | 77.5(75.7–79.3)* / 71.7(69.0–74.3)** |
| VCUG-A threshold (urethral findings or major bladder finding or VUR >III) | ||||
| RBUS-D (most relaxed criteria) | 54.7(46.0–63.3)* / 54.9(42.7–66.8)** | 76.9(75.1–78.7)* / 76.1(73.5–78.5)** | 13.3(10.6–16.4)* / 12.6(9.1–16.8)** | 96.3(95.3–97.2)* / 96.4(95.0–97.5)** |
| RBUS-C | 53.3(44.6–61.9)* / 53.5(41.3–65.5)** | 78.7(76.9–80.5)* / 78.0(75.5–80.4)** | 13.9(11.1–17.2)* / 13.2(9.5–17.7)** | 96.3(95.3–97.1)* / 96.4(95.0–97.5)** |
| RBUS-B | 44.5(36.0–53.3)* / 46.5(34.5–58.7)** | 84.5(82.9–86.1)* / 83.9(81.7–86.0)** | 15.7(12.2–19.7)* / 15.3(10.8–20.9)** | 95.9(94.9–96.8)* / 96.2(94.8–97.3)** |
| RBUS-A (most stringent criteria) | 18.2(12.2–25.7)* / 18.3(10.1–29.3)** | 96.9(96.1–97.6)* / 96.3(95.0–97.3)** | 27.5(18.6–37.8)* / 23.6(13.2–37.0)** | 94.8(93.8–95.7)* / 94.9(93.5–96.1)** |
See Table 2 for explanation of RBUS threshold criteria. *Values among children age <120 mo with history of UTI as indication for imaging (N = 2259). **Values among children age 2–24 mo with initial febrile UTI as the indication for GU imaging (N = 1203). CI, 95% confidence interval.
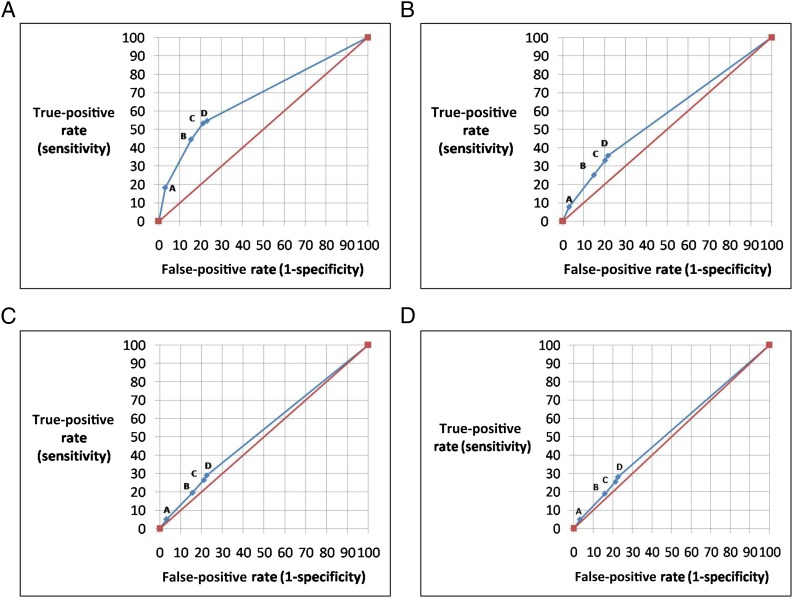
FIGURE 1.
ROC curves for RBUS as a screening test for GU abnormalities on VCUG. Each graph represents specific VCUG outcome threshold. Points on each curve represent each RBUS thresholds A through D (see Table 1). A, ROC curve for VCUG-A (urethral findings or major bladder findings or VUR > grade III). Area under curve (AUC) = 0.674. B, ROC curve for VCUG-B (urethral findings or major bladder findings or VUR > grade II). AUC = 0.573. C, ROC curve for VCUG-C (urethral findings or major bladder findings or VUR > grade I). AUC = 0.532. D, ROC curve for VCUG-D (urethral findings or major bladder findings or any VUR). AUC = 0.527.
Discussion
The 2011 AAP guidelines regarding the evaluation of infants who have a first febrile UTI represent significant evolution in management, with the most significant change being the recommendation that VCUG be deferred until after a second febrile UTI.2 The discussion regarding VCUG timing has been vigorous5 and is beyond the scope of this paper. Much less attention has been paid to the recommendation that VCUG be performed if the RBUS is abnormal. Although RBUS is not explicitly characterized in the guidelines as a screening test, and although the guidelines acknowledge that RBUS is insensitive for VUR, the practical result of this recommendation is to sort children into groups who should or should not proceed to VCUG after first febrile UTI, based on RBUS results. Use of RBUS in this manner bears many features of a screening test, yet there has been very little discussion regarding the value of RBUS as a screening test in such circumstances.
Although the AAP guidelines committee recommended RBUS, they graded the supporting evidence with a “C” grade. They felt that, although the RBUS would only identify “abnormalities that would lead to action” in 1% to 2% of cases, the potential benefit outweighed the potential harm. However, few primary references are cited.
Before this investigation, several large studies have reported test characteristics of RBUS as a screening test for GU abnormalities, and although findings have varied widely, none have found RBUS to be an accurate screening test in this setting. Sensitivity has ranged from 18% to 79% and specificity from 41% to 99%, depending on how a “positive” RBUS was defined and what VUR outcome was assessed (eg, any VUR, “dilating VUR,” “high-grade VUR”).6–11 Many other groups have reported GU imaging findings among children who have a history of UTI. However, most of these papers have limitations that make it impossible to determine the test characteristics of RBUS; most common is that many studies do not provide sufficient data to directly compare RBUS findings with VCUG findings in individual patients.12–19 Other studies focus on the value of RBUS to predict renal scintigraphic findings (scarring).13,20–22
Verification bias is a common weakness in the published literature. For example, Foresman et al assessed the correlation between RBUS and VCUG among patients hospitalized for acute pyelonephritis.23 RBUS was performed in all patients during the hospitalization; however, not all patients subsequently underwent VCUG, and performance of the VCUG varied depending on RBUS findings, with 67% of patients who had normal RBUS having VCUG, but 87% of patients who had abnormal RBUS having VCUG. Such differential assessment introduces an inherent bias into the data. Many studies have similar limitations.6,7,9,19,24 Other studies have limited generalizability owing to narrow10,25 or broad26–28 age ranges, or small sample size.8,26–28 One study looked specifically at the predictive value of ureteral dilation as an isolated finding on RBUS.29
Specific features of GU imaging practice at our institution during the study period address some previous studies’ weaknesses. First, routine practice in our region (before 2011) was to obtain both RBUS and VCUG in children who had febrile UTI. Such universal assessment is a key characteristic of evaluation of any screening test: both the screening test and the gold standard test must be performed in all subjects. Differential ascertainment can result in verification bias, because patients who have certain (usually negative) findings will be systematically excluded from the sample of patients undergoing both tests. In the current study we have limited this bias by including only patients scheduled a priori for RBUS and VCUG on the same day; performance of either test was not dependent on the findings of the other. Second, the sample includes a large number of patients followed at community practices, and seen at our institution only for imaging (only 43% [975/2259] were ever seen in our Urology Department). Our sample therefore is more representative of the population of children who had UTI, and does not only contain patients treated at a tertiary center. Such patients can be expected to have a higher disease severity than the overall study population; inclusion of community patients therefore reduces the selection bias that often compromises clinical studies at tertiary centers.
Additionally, the imaging studies were almost entirely performed after the acute pyelonephritis episode. (Among a 10% random sample, mean time from UTI to imaging was 41 days, and only 7% were imaged within 5 days of UTI). Thus, the RBUS test characteristics we observed may differ from RBUS performed during the acute infection. Although the AAP guidelines state that RBUS may be useful during the first 2 days of treatment to identify complications (eg, abscess), the guidelines are also clear that acute-phase imaging may actually be misleading.2
The low overall incidence of significant GU anomalies in our sample warrants comment. Previous studies have reported a similar phenomenon. Hoberman et al examined imaging results among 309 children who had febrile UTI, and noted that patients who had high-grade VUR were under-represented in their sample, and stated that “the validity of renal ultrasonography in identifying such children [with high-grade VUR] warrants further study.”30 A relatively low incidence of high-grade VUR has been seen in other series as well.8 Although our sample was much larger than these studies, we too noted that <3% of our sample had high-grade VUR (grade >III). Similarly, <1.5% of children had higher-grade hydronephrosis on RBUS. The low prevalence of severe abnormalities may be attributable to the effect of prenatal screening. We excluded children who had a history of prenatally diagnosed abnormalities because (1) the AAP imaging guidelines can be read as being applicable to children who have no history of prenatal abnormalities, and (2) such children usually undergo GU imaging as newborns, and we excluded children who had a history of previous GU imaging. Many, although not all, cases of high-grade disease are likely detected prenatally, reducing the incidence of such anomalies among children presenting with de novo UTI postnatally. Fifty years ago, we presumably would have observed much higher rates of such anomalies, because many of the children who are now diagnosed in utero would have presented postnatally with clinical UTI.
The results of our investigation should be interpreted in light of its limitations. This study was retrospective, subject to the limitations of this design. For example, study subjects all had history of UTI, but many of these diagnoses were made elsewhere and could not be independently verified. As some patients may have been misdiagnosed, the results may not reflect those that would be seen among children who had strictly defined and confirmed UTI. However, our radiologists take a detailed history before VCUG, to verify the history. Furthermore, irrespective of the diagnostic details, these are the patients being referred for GU imaging, and so reflect the “real-world” screening population seen in practice. With respect to imaging findings, we relied on the final interpretations of the imaging studies as dictated by the clinical radiologist; independent confirmatory review of images was not performed. The findings therefore are subject to the relative variability of interpretation (eg, grading of VUR) that occurs in all clinical care. Furthermore, the radiologists reading each study were not systematically blinded to the findings of the other test, so it is possible that interpretation of 1 study could have been influenced by knowledge of the findings on the other test. However, the radiologist interpreting the VCUG was usually a different individual than that radiologist interpreting the RBUS, and in most patients the RBUS was performed (and usually read) before the VCUG, which would minimize the impact of such unblinded interpretation. Furthermore, most findings on both RBUS and VCUG are relatively objective. As noted, verification bias is a concern in any evaluation of a screening test. To minimize this, we included only patients who underwent both VCUG and RBUS on the same day. However, it is likely that some patients underwent RBUS or VCUG separately, or underwent 1 test but not the other; such patients would be excluded from our sample. If such patients were numerous, and if the decision not to obtain the second test was based on the results of the first test, then bias could have been introduced into our sample. As we also noted, however, several features of our practice environment during the study period make this less likely, including the widespread adherence within our institution and community to the 1999 AAP guidelines, the routine practice of scheduling both RBUS and VCUG on the same day for patients who have a history of UTI, and the routine completion of both tests, regardless of findings.
Conclusions
Among young children who have a history of UTI, RBUS is a poor screening test for GU abnormalities, with low sensitivity/specificity. A negative RBUS does not rule out significant GU pathology (particularly VUR grades III and higher), whereas a positive RBUS is a poor predictor. In such children, RBUS and VCUG should be considered complementary as they provide important, but different, information.
Glossary
- AAP
American Academy of Pediatrics
- GU
genitourinary
- SFU
Society for Fetal Urology
- RBUS
renal and bladder ultrasound
- UTI
urinary tract infection
- VCUG
voiding cystourethrogram
- VUR
vesicoureteral reflux
Footnotes
Dr Nelson conceptualized and refined the study design, performed a substantial portion of data collection and interpretation, and drafted the initial manuscript; Dr Johnson contributed substantially to data collection and interpretation, critically reviewed the manuscript, and incorporated revisions from all the authors; Dr Logvinenko performed data analysis and critically reviewed the manuscript; Dr Chow contributed to conceptualization and refinement of the study design and data interpretation and critically reviewed the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Dr Nelson is supported by grant K23-DK088943 from the National Institute of Diabetes and Digestive and Kidney Diseases. Dr Johnson is supported by AHRQ/ARRA Recovery Act 2009 T32 HS19485 National Research Service Award in Expanding Training in Comparative Effectiveness for Child Health Researchers. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found on page 535, and online at www.pediatrics.org/cgi/doi/10.1542/peds.2013-4158.
References
- 1.American Academy of Pediatrics. Committee on Quality Improvement. Subcommittee on Urinary Tract Infection . Practice parameter: the diagnosis, treatment, and evaluation of the initial urinary tract infection in febrile infants and young children. Pediatrics. 1999;103(4 pt 1):843–852 [DOI] [PubMed] [Google Scholar]
- 2.Roberts KB, Subcommittee on Urinary Tract Infection, Steering Committee on Quality Improvement and Management . Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128(3):595–610 [DOI] [PubMed] [Google Scholar]
- 3.Fernbach SK, Maizels M, Conway JJ. Ultrasound grading of hydronephrosis: introduction to the system used by the Society for Fetal Urology. Pediatr Radiol. 1993;23(6):478–480 [DOI] [PubMed] [Google Scholar]
- 4.Medical versus surgical treatment of primary vesicoureteral reflux: report of the International Reflux Study Committee. Pediatrics. 1981;67(3):392–400 [PubMed] [Google Scholar]
- 5.Wan J, Skoog SJ, Hulbert WC, et al. Executive Committee, Section on Urology, American Academy of Pediatrics . Section on Urology response to new Guidelines for the diagnosis and management of UTI. Pediatrics. 2012;129(4). Available at: www.pediatrics.org/cgi/content/full/129/4/e1051–e1053 [DOI] [PubMed] [Google Scholar]
- 6.Rickwood AM, Carty HM, McKendrick T, et al. Current imaging of childhood urinary infections: prospective survey. BMJ. 1992;304(6828):663–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zamir G, Sakran W, Horowitz Y, Koren A, Miron D. Urinary tract infection: is there a need for routine renal ultrasonography? Arch Dis Child. 2004;89(5):466–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahant S, Friedman J, MacArthur C. Renal ultrasound findings and vesicoureteral reflux in children hospitalised with urinary tract infection. Arch Dis Child. 2002;86(6):419–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JH, Kim MK, Park SE. Is a routine voiding cystourethrogram necessary in children after the first febrile urinary tract infection? Acta Paediatr. 2012;101(3):e105–e109 [DOI] [PubMed] [Google Scholar]
- 10.Tsai JD, Huang CT, Lin PY, et al. Screening high-grade vesicoureteral reflux in young infants with a febrile urinary tract infection. Pediatr Nephrol. 2012;27(6):955–963 [DOI] [PubMed] [Google Scholar]
- 11.Preda I, Jodal U, Sixt R, Stokland E, Hansson S. Normal dimercaptosuccinic acid scintigraphy makes voiding cystourethrography unnecessary after urinary tract infection. J Pediatr. 2007;151(6):581–584 [DOI] [PubMed]
- 12.Alon US, Ganapathy S. Should renal ultrasonography be done routinely in children with first urinary tract infection? Clin Pediatr (Phila). 1999;38(1):21–25 [DOI] [PubMed] [Google Scholar]
- 13.Björgvinsson E, Majd M, Eggli KD. Diagnosis of acute pyelonephritis in children: comparison of sonography and 99mTc-DMSA scintigraphy. AJR Am J Roentgenol. 1991;157(3):539–543 [DOI] [PubMed] [Google Scholar]
- 14.Giorgi LJ, Jr, Bratslavsky G, Kogan BA. Febrile urinary tract infections in infants: renal ultrasound remains necessary. J Urol. 2005;173(2):568–570 [DOI] [PubMed] [Google Scholar]
- 15.Jakobsson B, Nolstedt L, Svensson L, Söderlundh S, Berg U. 99mTechnetium-dimercaptosuccinic acid scan in the diagnosis of acute pyelonephritis in children: relation to clinical and radiological findings. Pediatr Nephrol. 1992;6(4):328–334 [DOI] [PubMed] [Google Scholar]
- 16.Jahnukainen T, Honkinen O, Ruuskanen O, Mertsola J. Ultrasonography after the first febrile urinary tract infection in children. Eur J Pediatr. 2006;165(8):556–559 [DOI] [PubMed] [Google Scholar]
- 17.Kass EJ, Fink-Bennett D, Cacciarelli AA, Balon H, Pavlock S. The sensitivity of renal scintigraphy and sonography in detecting nonobstructive acute pyelonephritis. J Urol. 1992;148(2 Pt 2):606–608 [DOI] [PubMed] [Google Scholar]
- 18.Lavocat MP, Granjon D, Allard D, Gay C, Freycon MT, Dubois F. Imaging of pyelonephritis. Pediatr Radiol. 1997;27(2):159–165 [DOI] [PubMed] [Google Scholar]
- 19.Montini G, Zucchetta P, Tomasi L, et al. Value of imaging studies after a first febrile urinary tract infection in young children: data from Italian renal infection study 1. Pediatrics. 2009;123(2). Available at: www.pediatrics.org/cgi/content/full/123/2/e239–e246 [DOI] [PubMed] [Google Scholar]
- 20.Sreenarasimhaiah V, Alon US. Uroradiologic evaluation of children with urinary tract infection: are both ultrasonograpy and renal cortical scintigraphy necessary? J Pediatr. 1995;127(3):373–377 [DOI] [PubMed] [Google Scholar]
- 21.Rosenberg AR, Rossleigh MA, Brydon MP, Bass SJ, Leighton DM, Farnsworth RH. Evaluation of acute urinary tract infection in children by dimercaptosuccinic acid scintigraphy: a prospective study. J Urol. 1992;148(5 pt 2):1746–1749 [DOI] [PubMed] [Google Scholar]
- 22.Biggi A, Dardanelli L, Pomero G, et al. Acute renal cortical scintigraphy in children with a first urinary tract infection. Pediatr Nephrol. 2001;16(9):733–738 [DOI] [PubMed] [Google Scholar]
- 23.Foresman WH, Hulbert WC, Jr, Rabinowitz R. Does urinary tract ultrasonography at hospitalization for acute pyelonephritis predict vesicoureteral reflux? J Urol. 2001;165(6 pt 2):2232–2234 [DOI] [PubMed] [Google Scholar]
- 24.Ben-Ami T, Rozin M, Hertz M. Imaging of children with urinary tract infection: a tailored approach. Clin Radiol. 1989;40(1):64–67 [DOI] [PubMed] [Google Scholar]
- 25.Goldman M, Lahat E, Strauss S, et al. Imaging after urinary tract infection in male neonates. Pediatrics. 2000;105(6):1232–1235 [DOI] [PubMed] [Google Scholar]
- 26.Smellie JM, Rigden SP, Prescod NP. Urinary tract infection: a comparison of four methods of investigation. Arch Dis Child. 1995;72(3):247–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson CE, Shurin PA, Marchant CD, et al. Identification of children requiring radiologic evaluation for urinary infection. Pediatr Infect Dis. 1985;4(6):656–663 [DOI] [PubMed] [Google Scholar]
- 28.Tappin DM, Murphy AV, Mocan H, et al. A prospective study of children with first acute symptomatic E. coli urinary tract infection. Early 99mtechnetium dimercaptosuccinic acid scan appearances. Acta Paediatr Scand. 1989;78(6):923–929 [DOI] [PubMed] [Google Scholar]
- 29.Kenney IJ, Negus AS, Miller FN. Is sonographically demonstrated mild distal ureteric dilatation predictive of vesicoureteric reflux as seen on micturating cystourethrography? Pediatr Radiol. 2002;32(3):175–178 [DOI] [PubMed] [Google Scholar]
- 30.Hoberman A, Charron M, Hickey RW, Baskin M, Kearney DH, Wald ER. Imaging studies after a first febrile urinary tract infection in young children. N Engl J Med. 2003;348(3):195–202 [DOI] [PubMed] [Google Scholar]