Abstract
BACKGROUND:
A better understanding of costs associated with common and resource-intense conditions such as congenital heart disease has become increasingly important as children’s hospitals face growing pressure to both improve quality and reduce costs. We linked clinical information from a large registry with resource utilization data from an administrative data set to describe costs for common congenital cardiac operations and assess variation across hospitals.
METHODS:
Using linked data from The Society of Thoracic Surgeons and Pediatric Health Information Systems Databases (2006–2010), estimated costs/case for 9 operations of varying complexity were calculated. Between-hospital variation in cost and associated factors were assessed by using Bayesian methods, adjusting for important patient characteristics.
RESULTS:
Of 12 718 operations (27 hospitals) included, median cost/case increased with operation complexity (atrial septal defect repair, [$25 499] to Norwood operation, [$165 168]). Significant between-hospital variation (up to ninefold) in adjusted cost was observed across operations. Differences in length of stay (LOS) and complication rates explained an average of 28% of between-hospital cost variation. For the Norwood operation, high versus low cost hospitals had an average LOS of 50.8 vs 31.8 days and a major complication rate of 50% vs 25.3%. High volume hospitals had lower costs for the most complex operations.
CONCLUSIONS:
This study establishes benchmarks for hospital costs for common congenital heart operations and demonstrates wide variability across hospitals related in part to differences in LOS and complication rates. These data may be useful in designing initiatives aimed at both improving quality of care and reducing cost.
Keywords: congenital heart disease, cost analysis, outcomes
What’s Known on This Subject:
Congenital heart disease is known to be a commonly treated and resource-intense condition across children’s hospitals, yet knowledge regarding the degree of cost variation across hospitals and associated factors is lacking.
What This Study Adds:
Using a linked clinical and administrative data set, we establish benchmarks for hospital costs for common congenital heart operations, and demonstrate wide variation in cost between hospitals related in part to differences in length of stay and complications.
Congenital heart defects are the most common birth defects, and their treatment is known to consume considerable resources.1,2 A recent analysis demonstrated that 6 of the 10 birth defects accounting for the highest hospital charges were congenital heart defects, with hypoplastic left heart syndrome and truncus arteriosus topping the list.1 Another study evaluated the costs of pediatric diseases across 38 children’s hospitals, and found that 9 different congenital heart defects were ranked in the top tier.2 The heart defects identified in both of these studies were generally those requiring surgical intervention early in life.1,2
Despite this, more detailed knowledge regarding resource use in this population is lacking. Previous studies have been limited by the use of administrative data sets and inherent difficulties related to case ascertainment and risk adjustment, such that there are limited data available to hospitals, payers, and policymakers regarding average costs for the most common operations, the range of costs across institutions, and potential mechanisms underlying any variation identified.3–8 This information has become increasingly important in an era of rising health care expenditures, where recent initiatives aim to provide hospitals with incentives to both improve quality of care and reduce costs.9 A better understanding of factors impacting hospital costs for common and resource-intense conditions such as congenital heart disease is critical to the successful design and implementation of such initiatives.
Thus, the purpose of the current study was to link detailed clinical data from the Society of Thoracic Surgeons Congenital Heart Surgery (STS-CHS) Database (a large clinical registry) with resource use information from the Pediatric Health Information Systems (PHIS) Database (an administrative database) to describe costs for common congenital cardiac operations in a large multi-institutional cohort, assess variation in costs across hospitals, and investigate hospital-level factors associated with higher costs.
Methods
Data Source
STS-CHS and PHIS data were linked at the patient level by using the method of indirect identifiers as previously described and verified.10–12 The STS-CHS Database is the largest existing pediatric heart surgery registry and collects perioperative data on all children undergoing heart surgery at >100 North American centers. Data quality is evaluated through intrinsic verification of data (eg, identification and correction of missing/out of range values and inconsistencies in values across fields), and in-person site audits at 7 randomly chosen institutions annually. The PHIS Database is a large administrative database that collects information from the hospital bill from >40 US children’s hospitals. Systematic monitoring occurs to ensure data quality, including bimonthly coding consensus meetings, coding consistency reviews, and quarterly data quality reports. Linking these data sets enabled us to capitalize on the strengths of both data sets, including the detailed clinical and operative data in the STS-CHS Database and resource use information in the PHIS Database.10 This research was not considered human subjects research by the Duke Institutional Review Board.
Study Population
Hospitals participating in both the STS-CHS and PHIS Databases from 2006 to 2010 were eligible for inclusion (n = 33 hospitals). Hospitals who did not report resource use data (n = 1 hospital) and those with >15% missing data for any preoperative variables or outcomes data described below (n = 5 hospitals) were excluded. From the remaining 27 hospitals, patients undergoing 9 operations of varying complexity were included. These included the STS benchmark operations: ventricular septal defect (VSD) repair, Tetralogy of Fallot (TOF) repair (excluding pulmonary atresia or absent pulmonary valve, or atrioventricular canal repair), complete atrioventricular canal (CAVC) repair, arterial switch operation (ASO) +/− VSD repair, Fontan operation (including lateral tunnel and extracardiac conduit +/− fenestration; excluding Fontan revision), truncus arteriosus repair (excluding concomitant truncal valve repair/replacement or interrupted aortic arch repair), and the Norwood operation (including either systemic-to-pulmonary artery shunt or right ventricle-to-pulmonary artery conduit).13,14 In addition to these benchmark operations we also included atrial septal defect (ASD) repair and bidirectional Glenn (BDG)/hemi-Fontan. Of these 13 013 eligible patients, those who had missing data for any of the outcomes described below were excluded (n = 295 patients).
Data Collection
Data collected from the STS-CHS Database (data specifications available at: http://www.sts.org/sites/default/files/documents/pdf/CongenitalDataSpecificationsV3_0_20090904.pdf) included demographics, standard preoperative risk factors (cardiovascular, respiratory, neurologic, infectious, renal, and hematologic factors), non-cardiac/genetic abnormalities, presence of prematurity, history of previous cardiothoracic (CT) surgery, diagnosis and procedure data as described previously, postoperative complications and length of stay (LOS), and in-hospital mortality.15 Both the occurrence of any postoperative complication collected in the STS-CHS Database, as well as major complications (as previously defined) were evaluated, including renal failure requiring dialysis, neurologic deficit persisting at discharge, arrhythmia requiring permanent pacemaker, mechanical circulatory support, phrenic nerve injury/paralyzed diaphragm, and unplanned reoperation/reintervention.13,16 Center volume was collected and calculated as the average annual volume of cardiovascular operations (including all index cases with and without cardiopulmonary bypass) during the study period.
Resource use information collected from the PHIS Database included payer type (government, private, and other) and total hospital charges. As described previously, costs were estimated by using hospital and department specific cost-to-charge ratios, adjusted for regional differences by using the Centers for Medicare and Medicaid Services price and wage index, and indexed to 2010 dollars.17
Analysis
Study population characteristics were described by using standard summary statistics. Unadjusted total cost/case was calculated, and the cost distribution in the overall cohort was described for each operation. To assess between-hospital variation in cost, negative binomial Bayesian hierarchical models were used to calculate adjusted hospital-level costs for each operation. Log link functions were used to account for the skewed cost distribution. Our methodology also accounts for increased variability in outcomes from centers with smaller sample size and shrinks estimates from smaller centers toward the population average to provide more stable estimates.18 All models included a center-level random intercept and adjusted for important patient characteristics that may impact resource use across sites, including age, weight, gender, race, prematurity, the presence of any non-cardiac/genetic abnormality or STS-defined preoperative risk factor, previous CT surgery, payer type, and year of surgery.14 VSD repair was also included in the models for ASO, as those undergoing ASO+VSD repair are known to have worse outcomes.19 The presence of concurrent atrioventricular valvuloplasty was included in the models for Fontan and Glenn/hemi-Fontan. A sensitivity analysis was performed to evaluate the potential impact of variation in in-hospital mortality across sites on cost variation. Because it is possible that patients who die (particularly those who die early) may utilize fewer resources, which could potentially impact the degree of cost variation seen across institutions, we repeated the above analyses limiting the study population to hospital survivors and compared these data to our initial results (which included both survivors and non-survivors).
We also fitted additional sets of multilevel models that included postoperative complications and LOS to estimate the amount of between-hospital variation in total cost explained by these variables. We focused on these factors, as previous studies have suggested that they may have an important impact on resource use in this population overall.3,7 To conduct these analyses, the estimated standard deviation of the center level intercept of the models with patient characteristics alone was compared with those from models with complication rates and LOS included. The percent reduction in the estimated standard deviation was used to quantify the amount of between-center variation explained by these variables.
Finally, we were also interested in evaluating the association of hospital surgical volume with cost, as volume is a widely studied hospital structure variable and is known to be associated with other important outcomes.20,21 In this portion of the analysis, hospitals were divided into volume categories based on average annual total surgical volume of pediatric cardiac cases (defined previously): <350 (n = 10 hospitals), 350 to 500 (n = 12 hospitals), and ≥500 (n = 5 hospitals) cases/year. These categories are slightly different from those most commonly used in volume analyses in this population, as the PHIS Database contains fewer low-volume centers. Logistic models using the method of generalized estimating equations were used to evaluate the association of hospital surgical volume with total adjusted cost (for the purposes of this portion of the analysis, high cost was defined as >75th percentile for the operation of interest). Models were adjusted for the same patient risk factors as noted earlier. Odds ratios and 95% confidence intervals are presented. Analyses were performed by using SAS version 9.3 (SAS Institute Inc, Cary, NC), R version 2.15.1 (R Foundation for Statistical Computing, Vienna, Austria), and WinBUGS version 1.4.3 (MRC Biostatistics Unit, Cambridge, UK and College School of Medicine at St Mary's, London, UK). A P value <0.05 was considered statistically significant.
Results
Study Population Characteristics
A total of 12 718 patients from 27 hospitals were included. Patient characteristics are displayed in Table 1, along with the proportion of patients undergoing each of the 9 operations included in the analysis. Compared with the overall cohort of hospitals participating in the STS-CHS Database during the study period (n = 108), the 27 included hospitals had a higher average annual volume of pediatric cardiac cases (360 vs 175 cases/year) and included more centers in the Midwest (37% vs 22%).
TABLE 1.
Study Population Characteristics
| Variable | N = 12 718 (27 Centers) |
|---|---|
| Age | 4.4 mo (77 d–2.1 y) |
| Weight | 6.1 kg (4.1–11.0) |
| Sex, female | 5777 (45.4%) |
| Race/ethnicity | |
| Non-Hispanic white | 6636 (52.2%) |
| Other | 6082 (47.8%) |
| Prematurity | 863 (6.8%) |
| Any STS preoperative factor | 2595 (20.4%) |
| Any non-CV/genetic abnormality | 3720 (29.2%) |
| Previous CT surgery | 3457 (27.2%) |
| Payer type | |
| Government | 5620 (44.2%) |
| Private | 4526 (35.6%) |
| Other | 2572 (20.2%) |
| Operation type | |
| ASD repair | 1581 (12.4%) |
| VSD repair | 2669 (21.0%) |
| TOF repair | 1560 (12.3%) |
| Fontan | 1542 (12.1%) |
| BDG/Hemi-Fontan | 1692 (13.3%) |
| CAVC repair | 1150 (9.0%) |
| ASO | 1128 (8.9%) |
| Truncus repair | 226 (1.8%) |
| Norwood | 1170 (9.2%) |
Data are presented as number (%) and median (interquartile range).
Cost/Case in the Overall Cohort
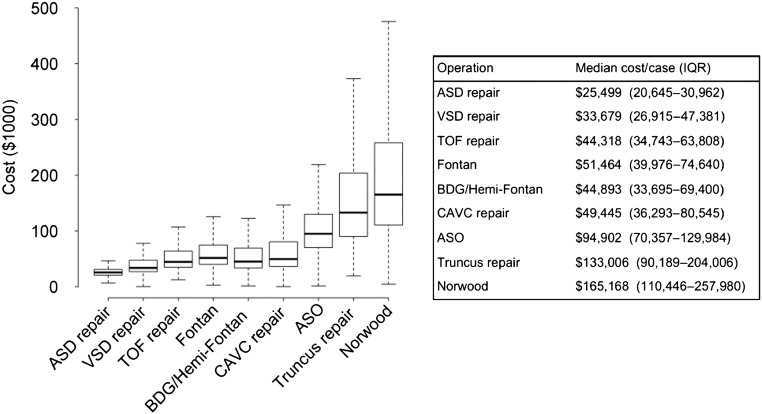
Figure 1 displays data on cost/case in the overall cohort for the operations included in our analysis. Median cost/case increased with operation complexity from $25 499 for ASD repair to $165 168 for the Norwood operation. Variability in cost/case also increased with operation complexity, as displayed in Fig 1.
FIGURE 1.
Distribution of cost/case for each operation in the overall cohort. Box and whisker plot displayed for each operation (middle line, median; ends of box, 25th and 75th percentile; lower whisker, minimum; upper whisker, 75th percentile + 1.5×interquartile range). Operations listed in order of increasing complexity from left to right.
Variation in Costs Across Hospitals
Table 2 displays the distribution of cost across hospitals. These data are adjusted for any potential differences in important patient characteristics across hospitals. Wide variation between hospitals in adjusted cost/case was observed for nearly every operation examined (Table 2). On average there was 5.8-fold variation in costs between hospitals, with a range of 1.2- to 9.5-fold variation across operations. Both high and low complexity operations had significant between-hospital variation in cost.
TABLE 2.
Variation in Adjusted Cost/Case Across Hospitals
| Operation | Hospital-Specific Adjusted Cost/Case, $ | Max/Min | 75th/25th | Pa | ||||
|---|---|---|---|---|---|---|---|---|
| Min | 25th Percentile | Median | 75th Percentile | Max | ||||
| ASD repair | 14 548 | 29 402 | 34 866 | 41 628 | 116 744 | 8.0 | 1.4 | <.001 |
| VSD repair | 31 720 | 42 268 | 49 494 | 60 736 | 107 784 | 3.4 | 1.4 | <.0001 |
| TOF repair | 22 722 | 50 622 | 64 933 | 94 529 | 215 642 | 9.5 | 1.9 | <.0001 |
| Fontan | 26 504 | 46 405 | 81 312 | 105 844 | 182 890 | 6.9 | 2.3 | <.0001 |
| BDG/Hemi-Fontan | 23 949 | 55 275 | 71 029 | 124 718 | 203 033 | 8.5 | 2.3 | <.0001 |
| CAVC repair | 38 286 | 63 807 | 88 718 | 115 400 | 216 082 | 5.6 | 1.8 | <.0001 |
| ASO | 56 711 | 104 817 | 129 221 | 151 432 | 308 722 | 5.4 | 1.4 | <.0001 |
| Truncus repair | 193 462 | 207 181 | 211 299 | 217 221 | 234 686 | 1.2 | 1.1 | .17 |
| Norwood | 99 983 | 190 545 | 238 959 | 286 659 | 376 555 | 3.8 | 1.5 | <.0001 |
Adjusted costs calculated for each hospital; data displayed demonstrate the distribution of costs across hospitals.
Bayesian probability; max versus min.
In a sensitivity analysis in which those who died before hospital discharge were excluded and the analysis was repeated including only hospital survivors, the between-hospital variation in adjusted cost/case was slightly less, but overall largely unchanged compared with the initial analysis (average 5.3-fold variation, range 1.6- to 7.9-fold variation across operations).
Factors Associated With Between-Hospital Variation in Cost
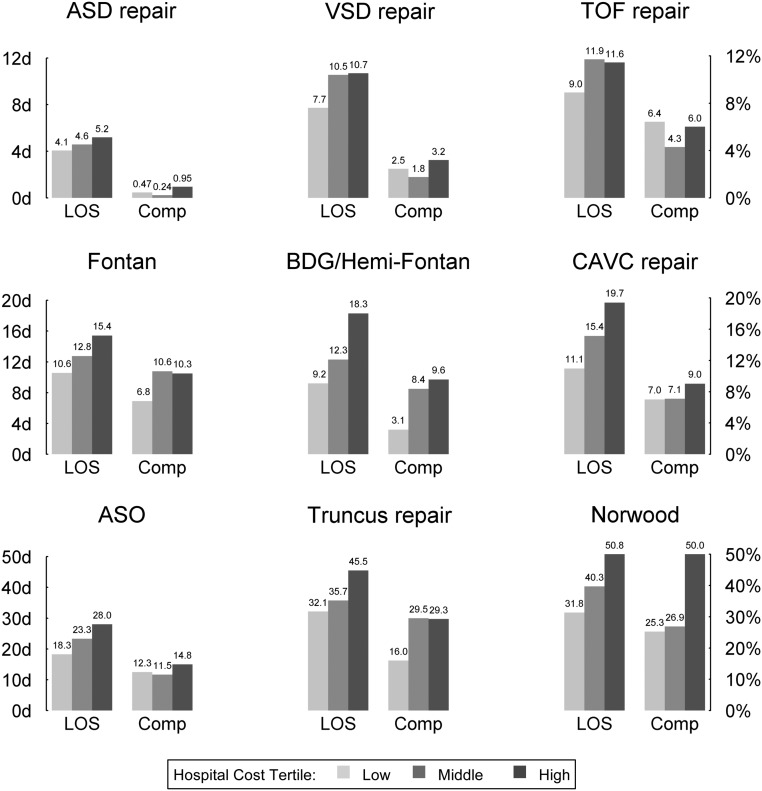
The association of several factors with the observed between-hospital variation in cost was evaluated. Data for postoperative LOS and complication rates are shown in Table 3 and Fig 2. Differences in postoperative LOS alone accounted for an average of 23% of the variation in costs between hospitals (range, 2.6%–45.5% across operations) (Table 3). The degree of cost variation explained by complication rates was generally higher for the more complex operations. The combination of both complication rate and LOS explained an average of 28% of the between-hospital cost variation (range, 4.8%–49.6% across operations) (Table 3).
TABLE 3.
Relationship of Complication Rates and LOS With Between-Hospital Variation in Cost
| Operation | Proportion of Between-Hospital Variation in Cost Explained by the Indicated Variable(s) | ||||
|---|---|---|---|---|---|
| LOS | Complication Rate | Major Complication Rate | LOS + Complication Rate | LOS + Major Complication Rate | |
| ASD repair | 2.6 | 4.2 | — | 4.8 | — |
| VSD repair | 24.8 | 20.7 | — | 40.9 | 18.9 |
| TOF repair | 15.5 | — | 3.4 | 20.1 | 9.4 |
| Fontan | 17.0 | 3.9 | 1.2 | 22.5 | 17.4 |
| BDG/Hemi-Fontan | 45.5 | 11.1 | 10.0 | 49.6 | 41.7 |
| CAVC repair | 24.7 | — | 4.2 | 21.1 | 24.6 |
| ASO | 43.0 | 4.9 | 1.9 | 44.9 | 38.7 |
| Truncus repair | 6.9 | — | 7.2 | 14.5 | 17.4 |
| Norwood | 28.8 | 7.2 | 15.5 | 31.1 | 35.1 |
All data are presented as percentages.
FIGURE 2.
Association of hospital complication rates and LOS with adjusted cost. Hospital complication rates and LOS displayed across hospital cost tertiles. Operations listed in order of increasing complexity, with higher complexity operations at the bottom of the plot.
To further investigate the relationship between LOS, complications, and cost, hospitals were grouped into tertiles based on adjusted costs, and data regarding major complication rates and postoperative LOS were displayed across tertiles (Fig 2). Hospitals with higher costs generally had longer postoperative LOS and higher major complication rates. These relationships were more prominent and consistent for the higher complexity operations. For example, for the Norwood operation, hospitals in the high versus low cost group had a mean postoperative LOS of 50.8 days versus 31.8 days, and a major complication rate of 50% versus 25.3%. In the Norwood cohort, there was a relatively strong positive correlation between hospital major complication rate and mean LOS (Spearman rank correlation = 0.61). Few hospitals (n = 3) had lower major complication rates (<30%) but longer mean LOS (>40 days).
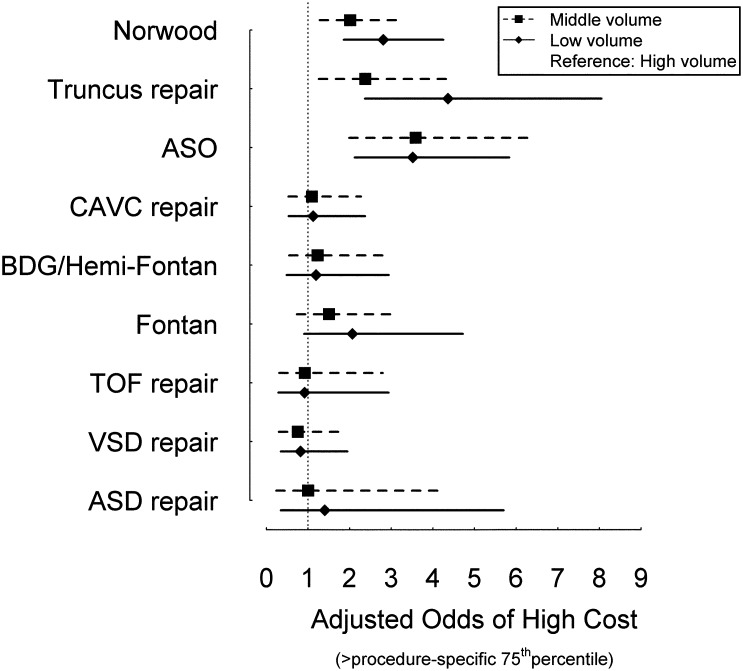
Finally, the association between hospital cardiac surgery volume and cost was examined. Compared with high-volume centers, low- and medium-volume centers had higher adjusted odds of increased cost (procedure-specific cost >75th percentile) for the most complex operations (Norwood operation, truncus arteriosus repair, and ASO; Fig 3). For less complex operations, there was no significant association between hospital costs and cardiac surgery volume.
FIGURE 3.
Association of hospital surgical volume with adjusted cost. Adjusted odds of high cost (procedure-specific cost >75th percentile) and 95% confidence intervals in middle (box) and low (diamond) volume versus high-volume hospitals (reference). Operations are listed in order of increasing complexity with higher complexity operations at the top of the plot.
Discussion
This study establishes benchmarks regarding hospital costs across a variety of common congenital heart operations of varying complexity. In addition, we demonstrate that costs vary widely across institutions despite adjustment for important patient characteristics. This variation can be explained in part by differences in LOS and postoperative complication rates.
The few previous studies that have examined resource use in this population have relied on administrative data sets, which can be limited with regard to case ascertainment, risk adjustment, and assessment of postoperative complications.8,22–25 This study highlights an alternative approach involving linking an administrative data set with clinical registry data. The detailed information regarding diagnoses and procedures in the STS-CHS Database allowed a precise evaluation of several operations across varying levels of complexity, and collected preoperative characteristics allowed for more detailed risk adjustment. We were also able to assess complications specific to congenital heart surgery.3–7 Standardized information collected across hospitals within the PHIS Database allowed calculation of total hospital costs, which better estimate the resources consumed to carry out the service provided, compared with charge data reported in previous studies, which only reflect what the hospital billed for a service, may vary across hospitals, and overestimate cost.6,7 These types of data linkages can maximize the strengths and mitigate the weaknesses of each data set, and allow for analyses not possible within each individual data set alone.26,27
The results of our analysis establish benchmarks for in-hospital costs across a broad sample of congenital operations of varying levels of complexity. This information has previously not been available to stakeholders involved in the design and implementation of initiatives aimed at reducing costs. Furthermore, we found a significant degree of variation between hospitals in total costs, even after accounting for important patient characteristics, as demonstrated in similar analyses in other patient populations.28 This variation suggests that there is ample room for improvement, and mirrors findings related to variation in clinical outcomes documented in other studies.14,29 Our results also support previous analyses that have evaluated the volume-outcome relationship, and suggest that this relationship also plays a role when evaluating the outcome of resource utilization.20,21,30
In addition, we investigated factors associated with the observed variation in cost across hospitals. Previous studies have suggested that LOS and postoperative complications are important drivers of high cost on a patient level; however, the impact of these variables on resource use on a hospital level has not been previously examined.3,7 Together these variables accounted for more than a quarter of the total observed variation in our study. The impact of complications was less clear for the lower complexity operations, which may be related in part to the lower prevalence of complications in general in these patients. Further study is needed to assess which specific complications are associated with the highest costs. Overall, these data suggest that recent federal initiatives aimed at reducing costs may consider targeting LOS and complications.9 This type of strategy would have the potential to both improve clinical outcomes and reduce costs. As an initial step, mechanisms to provide hospitals with integrated data regarding resource use and outcomes benchmarked to national norms would need to be developed, which would likely necessitate further collaboration between clinical and administrative data sets such as STS-CHS and PHIS. Second, a better understanding of differences in LOS and complication rates across hospitals, and how to best achieve improvements, is needed. A previous single center report demonstrated that LOS (and associated hospital costs) could be reduced by standardization of care involving practices aimed at early extubation and mobilization in children undergoing certain types of heart surgery.5 In adult surgical subspecialties, multicenter quality improvement collaboratives have proven to be successful models for sharing these types of best practices across hospitals, and reducing variation in clinical outcomes and costs.31–33 A regional collaborative in Michigan is estimated to reduce complications after general and vascular surgery in ∼2500 patients each year, translating into annual savings of ∼$20 million.31 New initiatives in the field of pediatric cardiology and cardiac surgery such as those sponsored by the Pediatric Heart Network and the Pediatric Cardiac Critical Care Consortium (PC4) aim to employ similar approaches.
While this analysis represents the most comprehensive evaluation of variation in costs across hospitals in this population to date, our results may not be generalizable to all of the ∼125 US pediatric heart surgery programs.34 Further expansion of linkages between registry and administrative data can facilitate inclusion of additional centers in the future. In our assessment of factors associated with variation in cost across hospitals, we focused primarily on an evaluation of postoperative complication rate and LOS.3,7 Further studies are needed to provide additional insight into other factors, such as potential differences in use of laboratory testing and imaging.4 In addition, resource use can be assessed through a variety of different methods, each of which are associated with certain strengths and limitations. We used standard methodology involving cost-to-charge ratios. Further development of methods that standardize line item costs may allow delineation of whether the observed cost variability is related more directly to differences in the volume of resources consumed versus inter-institutional differences in item costs.2 In addition, future study of payments/reimbursements from large payer data sets or combination of Medicaid data sets across states may allow further analysis from the payer or consumer’s perspective. Finally, a complete understanding of resource use associated with congenital heart surgery will require additional analyses beyond the in-hospital period, further study of specific patient factors associated with resource utilization, as well as a better understanding of “value” or the relationship between cost and measures of quality such as mortality and important morbidities.
Conclusions
This study establishes benchmarks for hospital costs associated with common congenital heart operations and demonstrates wide variation across hospitals. Differences across hospitals in postoperative complication rates and LOS were found to explain more than a quarter of this variation. These data and other recent findings suggest that initiatives that provide feedback to hospitals regarding their costs and outcomes benchmarked to peer institutions, and provide a mechanism for sharing of best practices to reduce LOS and complication rates may allow for both improved quality of care and reduced costs.
Glossary
- ASD
atrial septal defect
- ASO
arterial switch operation
- BDG
bidirectional Glenn
- CAVC
complete atrioventricular canal
- CT
cardiothoracic
- LOS
length of stay
- PHIS
Pediatric Health Information Systems
- STS-CHS
Society of Thoracic Surgeons Congenital Heart Surgery
- TOF
Tetralogy of Fallot
- VSD
ventricular septal defect
Footnotes
Dr Pasquali conceptualized and designed the study, directed the analysis, reviewed and interpreted the data, and drafted the manuscript; Drs M. Jacobs, Shah, Peterson, Hall, Gaynor, Hill, Mayer, J. Jacobs, and Li participated in the design of the study, reviewed and interpreted the study data, and reviewed and revised the manuscript; Mr He participated in the design of the study, conducted the statistical analysis, reviewed and interpreted the study data, and reviewed and revised the manuscript; and all authors approved the final manuscript to be submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported in part by the National Heart, Lung, and Blood Institute (1K08HL103631, Principal Investigator, Dr Pasquali; 1RC1HL099941, co-Principal Investigators, Drs J. Jacobs and Li). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: Dr J Jacobs, Chair, STS-CHS Database Task Force; Dr Peterson, Principal Investigator, STS National Databases Analytic Center; Dr Shah, Children’s Hospital Association, Executive Council Member of the Pediatric Research in Inpatient Settings Network. The other authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Robbins JM, Bird TM, Tilford JM, et al. Centers for Disease Control and Prevention (CDC) . Hospital stays, hospital charges, and in-hospital deaths among infants with selected birth defects—United States, 2003. MMWR Morb Mortal Wkly Rep. 2007;56(2):25–29 [PubMed] [Google Scholar]
- 2.Keren R, Luan X, Localio R, et al. Pediatric Research in Inpatient Settings (PRIS) Network . Prioritization of comparative effectiveness research topics in hospital pediatrics. Arch Pediatr Adolesc Med. 2012;166(12):1155–1164 [DOI] [PubMed] [Google Scholar]
- 3.Pasquali SK, Sun JL, d’Almada P, et al. Center variation in hospital costs for patients undergoing congenital heart surgery. Circ Cardiovasc Qual Outcomes. 2011;4(3):306–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smith AH, Gay JC, Patel NR. Trends in resource utilization associated with the inpatient treatment of neonatal congenital heart disease [published online ahead of print June 5, 2013]. Congenit Heart Dis. 10.1111.chd.12103 [DOI] [PubMed]
- 5.Lawrence EJ, Nguyen K, Morris SA, et al. Economic and safety implications of introducing fast tracking in congenital heart surgery. Circ Cardiovasc Qual Outcomes. 2013;6(2):201–207 [DOI] [PubMed] [Google Scholar]
- 6.Dean PN, Hillman DG, McHugh KE, Gutgesell HP. Inpatient costs and charges for surgical treatment of hypoplastic left heart syndrome. Pediatrics. 2011;128(5). Available at: www.pediatrics.org/cgi/content/full/128/5/e1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benavidez OJ, Connor JA, Gauvreau K, Jenkins KJ. The contribution of complications to high resource utilization during congenital heart surgery admissions. Congenit Heart Dis. 2007;2(5):319–326 [DOI] [PubMed] [Google Scholar]
- 8.Pasquali SK, Peterson ED, Jacobs JP, et al. Differential case ascertainment in clinical registry vs. administrative data and impact on outcomes assessment in pediatric heart surgery. Ann Thorac Surg. 2013;95:197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Accountable care organizations (ACOs) and pediatricians: evaluation and engagement. AAP News. Vol. 32, No. 1: January 1, 2011
- 10.Pasquali SK, Jacobs JP, Shook GJ, et al. Linking clinical registry data with administrative data using indirect identifiers: implementation and validation in the congenital heart surgery population. Am Heart J. 2010;160(6):1099–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pasquali SK, Li JS, He X, et al. Comparative analysis of antifibrinolytic medications in pediatric heart surgery. J Thorac Cardiovasc Surg. 2012;143(3):550–557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pasquali SK, Li JS, He X, et al. Perioperative methylprednisolone and outcome in neonates undergoing heart surgery. Pediatrics. 2012;129(2). Available at: www.pediatrics.org/cgi/content/full/129/2/e385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacobs JP, Jacobs ML, Austin EH, III, et al. Quality measures for congenital and pediatric cardiac surgery. World J Pediatr Congenit Heart Surg. 2012;3(1):32–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jacobs JP, O’Brien SM, Pasquali SK, et al. Richard E. Clark Paper: Variation in outcomes for benchmark operations: An analysis of the STS Congenital Heart Surgery Database. Ann Thorac Surg. 2011;92:2184–2192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Database Full Specifications STS. Available at: www.sts.org/sites/default/files/documents/pdf/CongenitalDataSpecificationsV3_0_20090904.pdf. Accessed May 28, 2013
- 16.Jacobs ML, O’Brien SM, Jacobs JP, et al. An empirically based tool for analyzing morbidity associated with operations for congenital heart disease. J Thorac Cardiovasc Surg. 2013;145(4):1046–1057, e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasquali SK, He X, Jacobs ML, et al. Hospital variation in postoperative infection and outcome after congenital heart surgery. Ann Thorac Surg. 2013;96(2):657–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dimick JB, Staiger DO, Birkmeyer JD. Ranking hospitals on surgical mortality: the importance of reliability adjustment. Health Serv Res. 2010;45(6 pt 1):1614–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Brien SM, Clarke DR, Jacobs JP, et al. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg. 2009;138(5):1139–1153 [DOI] [PubMed] [Google Scholar]
- 20.Hornik CP, He X, Jacobs JP, et al. Relative impact of surgeon and center volume on early mortality after the Norwood operation. Ann Thorac Surg. 2012;93(6):1992–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pasquali SK, Li JS, Burstein DS, et al. Association of center volume with mortality and complications in pediatric heart surgery. Pediatrics. 2012;129(2). Available at: www.pediatrics.org/cgi/content/full/129/2/e370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strickland MJ, Riehle-Colarusso TJ, Jacobs JP, et al. The importance of nomenclature for congenital cardiac disease: implications for research and evaluation. Cardiol Young. 2008;18(suppl 2):92–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cronk CE, Malloy ME, Pelech AN, et al. Completeness of state administrative databases for surveillance of congenital heart disease. Birth Defects Res A Clin Mol Teratol. 2003;67(9):597–603 [DOI] [PubMed] [Google Scholar]
- 24.Frohnert BK, Lussky RC, Alms MA, Mendelsohn NJ, Symonik DM, Falken MC. Validity of hospital discharge data for identifying infants with cardiac defects. J Perinatol. 2005;25(11):737–742 [DOI] [PubMed] [Google Scholar]
- 25.Lawson EH, Louie R, Zingmond DS, et al. A comparison of clinical registry versus administrative claims data for reporting of 30-day surgical complications. Ann Surg. 2012;256(6):973–981 [DOI] [PubMed] [Google Scholar]
- 26.Newgard CD. Validation of probabilistic linkage to match de-identified ambulance records to a state trauma registry. Acad Emerg Med. 2006;13(1):69–75 [DOI] [PubMed] [Google Scholar]
- 27.Hernandez AF, Hammill BG, Peterson ED, et al. Relationships between emerging measures of heart failure processes of care and clinical outcomes. Am Heart J. 2010;159(3):406–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Dartmouth Atlas of Health Care. Available at: www.dartmouthatlas.org/publications/reports.aspx. Accessed August 26, 2013
- 29.Jacobs JP, O’Brien SM, Pasquali SK, et al. Variation in outcomes for risk-stratified pediatric cardiac surgical operations: an analysis of the STS Congenital Heart Surgery Database. Ann Thorac Surg. 2012;94(2):564–571, discussion 571–572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirsch JC, Gurney JG, Donohue JE, Gebremariam A, Bove EL, Ohye RG. Hospital mortality for Norwood and arterial switch operations as a function of institutional volume. Pediatr Cardiol. 2008;29(4):713–717 [DOI] [PubMed] [Google Scholar]
- 31.Share DA, Campbell DA, Birkmeyer N, et al. How a regional collaborative of hospitals and physicians in Michigan cut costs and improved the quality of care. Health Aff (Millwood). 2011;30(4):636–645 [DOI] [PubMed] [Google Scholar]
- 32.Prager RL, Armenti FR, Bassett JS, et al. Michigan Society of Thoracic and Cardiovascular Surgeons . Cardiac surgeons and the quality movement: the Michigan experience. Semin Thorac Cardiovasc Surg. 2009;21(1):20–27 [DOI] [PubMed] [Google Scholar]
- 33.Likosky DS. Lessons learned from the Northern New England Cardiovascular Disease Study Group. Prog Pediatr Cardiol. 2012;33:53–56 [Google Scholar]
- 34.Jacobs ML, Daniel M, Mavroudis C, et al. Report of the 2010 Society of Thoracic Surgeons Congenital Heart Surgery Practice and Manpower Survey. Ann Thorac Surg. 2011;92(2):762–768, discussion 768–769 [DOI] [PubMed] [Google Scholar]