Abstract
BACKGROUND AND OBJECTIVE:
Previous studies of survivors of pediatric acute lymphoblastic leukemia (ALL) have drawn heterogeneous conclusions regarding the prevalence of obesity and risk factors for developing obesity in pediatric ALL survivors. We sought to determine the prevalence of obesity in pediatric ALL survivors and examine risk factors for obesity through a systematic review and meta-analysis.
METHODS:
A MEDLINE search was performed from its inception through 2013. Studies met the inclusion criteria if they (1) included at least 10 survivors of pediatric ALL; (2) assessed the prevalence or indicators of obesity; and (3) compared obesity among ALL survivors to a reference population or external control group. Extracted data included patient and treatment characteristics, study design, population used for comparison, and prevalence of obesity.
RESULTS:
Forty-seven studies met the inclusion criteria. Despite significant heterogeneity among the studies (I2 = 96%), the mean BMI z score in 1742 pediatric ALL survivors was 0.83 (95% confidence interval: 0.60–1.06), which corresponds to the 80th BMI percentile, indicating a significantly higher BMI in pediatric ALL survivors than the reference population. Subgroup analyses found a high prevalence of obesity in ALL survivors regardless of survivors’ receipt of cranial irradiation, gender, or age at diagnosis.
CONCLUSIONS:
Obesity is prevalent in pediatric ALL survivors and is independent of patient- and treatment-related characteristics. Clinicians need to screen for obesity and its associated health conditions early in survivorship.
Keywords: obesity, acute lymphoblastic leukemia, pediatric, survivors
Acute lymphoblastic leukemia (ALL) is the most common pediatric cancer, accounting for ∼25% of cancers diagnosed in children aged <20 years.1 More than 80% of the children diagnosed with ALL survive ≥5 years.2,3 This success has translated into a growing population of long-term survivors of pediatric ALL. However, cancer treatment is associated with late effects that substantially contribute to morbidity and mortality of the survivors.4
One increasingly recognized late effect is obesity. Obesity contributes to the already elevated rate of chronic health conditions affecting pediatric ALL survivors.5 Although previous studies have demonstrated a high prevalence of obesity in pediatric ALL survivors, most comprised a small sample size, and the definition of obesity varied across studies. In addition, the association of obesity with various treatment and patient characteristics has been inconsistent. Some studies6–8 demonstrated an increased rate of obesity associated with cranial irradiation therapy (CRT), but others did not.9–11 With the decreasing use of CRT by cooperative groups, it remains unclear whether intrathecal and systemic chemotherapy alone are associated with obesity in pediatric ALL survivors. Female gender and a young age at diagnosis were predictors for obesity in some10–14 but not all studies.7,9,12,15,16 The rate of obesity may also vary by interval from cancer diagnosis. A better understanding of treatment and patient characteristics associated with obesity is needed to guide best clinical care and to inform targeted intervention.
We performed a systematic review and meta-analysis of the prevalence of obesity in pediatric ALL survivors. The primary aim of this study was to systematically evaluate whether survivors of pediatric ALL are more obese than those without cancer. A secondary aim was to explore whether the prevalence of obesity differs by receipt of CRT, gender, age at diagnosis, and interval since treatment completion.
Methods
We followed the Preferred Reporting Items for Systematic Review and Meta-Analyses statement for reporting our results.17 A protocol was developed before the conduct of the systematic review and submitted to PROSPERO, an international prospective register of systematic review protocols.18
Literature Search
We searched MEDLINE from inception through January 29, 2013, to identify studies that investigated obesity in ALL patients or survivors. We used medical subject heading and text words related to obesity (“obesity,” “weight,” “body composition,” “growth,” etc) in combination with survivors (“survivors,” “remission,” “disease-free survival,” etc) and ALL (“acute lymphoblastic leukemia,” “leukemia,” “precursor cell lymphoblastic leukemia-lymphoma,” etc). We consulted a research librarian in specifying the search and searched reference lists of eligible studies and relevant narrative reviews to identify additional studies that met inclusion criteria. Although we did not set any language restrictions for our MEDLINE search, we did not screen studies without abstracts available in English.
Eligibility Criteria and Study Selection
Two authors (FFZ and MK) independently screened the titles and abstracts to identify potentially eligible studies using the software Abstrackr (http://www.cebm.brown.edu/software). Discrepancies were jointly reviewed to reach consensus. These 2 authors then independently screened the full texts of the identified studies to determine which were eligible for review. Studies were eligible if they met the following criteria: (1) were research articles published in peer-reviewed journals; (2) included ≥10 patients who were diagnosed with ALL before age 21 years and who had completed active treatment at the time of the assessment; (3) either assessed prevalence of overweight/obesity, BMI, BMI z score, BMI percentile, or percentage of body fat by using dual radiograph absorptiometry; and (4) compared overweight/obesity among ALL survivors to a reference population (eg, the 2000 Centers for Disease Control and Prevention growth reference19) or an external control group (eg, sibling controls). We excluded review articles, case reports, and studies that assessed only weight or only height as the outcome, as well as studies that did not involve comparisons to a reference population or a control group. We also excluded studies that assessed survivors who received hematopoietic stem-cell transplantation based on the rationale that hematopoietic stem-cell transplantation survivors might experience a different pattern of growth compared with other ALL survivors.
Data Collection and Extraction
Two authors (FFZ and MK) extracted data, and each verified the other’s extracted information. Discrepancies were resolved by consensus. For each eligible study, we extracted the following information: (1) author, year, and country of publication; (2) characteristics of the study population (sample size, treatment period, age at diagnosis, age at study evaluation, years since diagnosis, and percentage receiving CRT); (3) study design (cross-sectional versus longitudinal); (4) type of control used (external control versus normative control and the source of control); (5) outcome measured (BMI, BMI z score, BMI percentile, percentage of body fat, percentage of body fat z score, prevalence of overweight or obesity); and (6) primary findings (overall findings as well as findings by gender and by CRT when available). For those survivors who were children and adolescents at evaluation, BMI z score or BMI percentile was calculated based on age- and gender-specific BMI cutoffs of a reference population because BMI normally changes with age and varies by gender.20 The BMI z score indicates the number of SDs the measurement is away from the age- and gender-specific mean value in the reference population. A BMI z score >0 or BMI percentile >50th indicates a higher-than-average BMI.
Assessment of Study Validity/Quality Assessment
There is no standard scale to assess quality in observational nonrandomized studies.21,22 To describe study-level characteristics associated with obesity in ALL survivors, we modified the Newcastle-Ottawa Scale23 and created a checklist including the following: whether the study adequately described the survivors’ ages at study evaluation, gender distribution, treatment protocols and years at diagnosis, whether the study appropriately selected the reference population or external controls, whether the study clearly defined the obesity outcome, whether the study provided the SD, SE, confidence interval (CI), or P value for the outcome, and whether the study performed subgroup comparisons by CRT and by gender. Longitudinal studies were additionally evaluated to determine if the length of follow-up was stated, and whether sample size at follow-up was provided (Supplemental Table 4).
Statistical Analysis
We performed meta-analysis for 20 studies that reported the mean BMI z score and its SD in a cohort of 1742 survivors of pediatric ALL. These survivors had completed treatment within 10 years at the time of study evaluation.8,12,13,16,24–39 Only 2 studies14,40 examined BMI z score assessed ≥10 years after the completion of treatment (ie, off treatment ≥10 years). Because neither study reported SD/SE of the BMI z score, the meta-analysis did not include survivors who were off treatment beyond 10 years.
We obtained summary BMI z scores using an inverse variance random effects model.41 For longitudinal studies, we included the outcome with the longest follow-up. Thirteen studies explicitly reported the mean BMI z score for the overall cohort. For the remaining 7 studies, the mean z score was calculated in the following ways: as a weighted average based on subgroup values (n = 5),29,33,34,37,38 estimated based on median and range (n = 1),39 or calculated based on the BMI z score at diagnosis and the change in BMI z score from diagnosis to study follow-up (n = 1).36 Ten studies explicitly reported the SD/SE of the BMI z score. For the remaining 10 studies, the SD was either calculated as a pooled SD based on subgroup values assuming equal variance (n = 5),29,33,34,37,38 calculated from the 95% CI (n = 2),16,27 estimated from median and range (n = 1),39 obtained directly from study authors (n = 1),30 or estimated based on the SD at diagnosis and SD for the change in BMI z score from diagnosis to study evaluation (n = 1).36
To explore the association between patient and treatment characteristics and obesity, we performed subgroup meta-analyses separately by receipt of CRT, gender, and interval since treatment completion. We also performed sensitivity analyses to assess the robustness of our findings. First, we performed the analysis after excluding 7 studies29,33,34,36–39 for which the mean BMI z score or its SD was not explicitly reported or could not be calculated based on subgroup values (for mean BMI z score) or 95% CIs (for SD of BMI z score). We also repeated the analysis after excluding 7 longitudinal studies that did not report sample size of survivors at the follow-up evaluation,8,12,29,33,34,36,38,42 for which we substituted it with the sample size reported at cancer diagnosis assuming loss to follow-up was random. In addition, we performed a “leave-one-out meta-analysis” to evaluate the impact of individual studies on the summary estimates.43
We assessed between-study heterogeneity by using Cochran’s Q statistic44 and the I2 index.45 The Cochran’s Q statistic tests whether there is heterogeneity between the individual study estimates in a meta-analysis and follows a χ2 distribution. The Cochran’s Q was considered statistically significant at PQ < .1. The I2 index represents the proportion of between-study heterogeneity that is beyond chance, ranging between 0% and 100%. Higher values indicate greater inconsistency across studies. All analyses were conducted by using Stata version IC/12.1 (Stata Corp, College Station, TX) and OpenMeta-Analyst (http://www.cebm.brown.edu/software). Statistical significance was defined as a 2-sided P value < .05 for all tests except those for heterogeneity.
Results
Included Studies
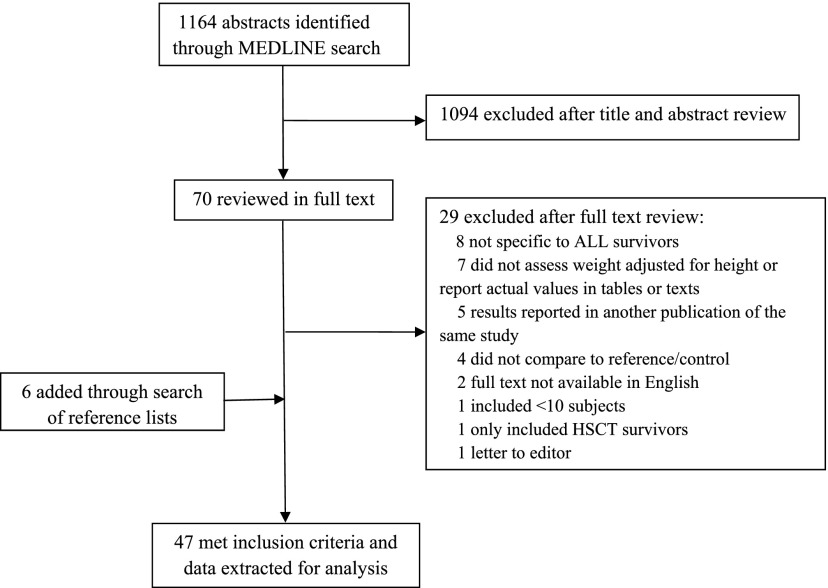
Our initial search identified 1164 studies. After screening titles and abstracts, 70 studies were considered potentially eligible and were retrieved for full text review. Of these, 29 were excluded and 41 were eligible for this systematic review. Six additional studies were identified by a search of the reference lists. As a result, the systematic review included 47 studies reporting on 9223 pediatric ALL survivors for this systematic review (Fig 1). Tables 1, 2, and 3 summarize the characteristics of the eligible studies. The tables are divided by the length of time from completion of treatment to time at which the survivors were assessed.
FIGURE 1.
Search strategy flowchart.
TABLE 1.
Characteristics of Studies That Assessed Obesity in Pediatric ALL Survivors Off Treatment <5 Years
| Author, Year (Location) | Study Design | Type of Controla | Survivors, n /(Control) | Years at Dx | Age at Studyb | Years Since Dxb | % CRT | Major Findingsc | By Genderd | By CRTd |
|---|---|---|---|---|---|---|---|---|---|---|
| BMI z score | ||||||||||
| Odame et al 1994 (UK) | L | Normative (French), external | 40/18 | 1980–1990 | 7.5 | 4.0 | 100 | 1.8/0.6 | M: 1.1, F: 2.4 | N/A |
| Van-Dongen et al 1995 (Netherlands) | L | Normative (French) | 113 | 1978–1990 | 10.3 | 6.5 | 46.0 | 0.9 | M: 0.8, F: 0.9 | CRT+: 0.8, CRT–: 0.9 |
| Birkebaek et al 1998 (Denmark) | L | Normative (French) | 33 | 1973–1984 | 7.3 | 2.5 | 66.7 | 0.2 | N/A | CRT+: 0.2, CRT–: 0.2 |
| Craig et al 1999 (UK) | L | Normative (British) | 298 | 1971–1994 | 7.3 | 2.2 | 71.5 | 0.4 | M: 0.5, F: 0.3 | CRT+: 0.3, CRT–: 0.7 |
| Nysom et al 1999 (Denmark) | L | Normative (French and Danish) | 94 | 1970–1990 | 7.0 | 3.0 | 41.1 | 0.7 | N/A | N/A |
| Arguelles et al 2000 (Spain) | L | Normative (Spain) | 16 | ALL-BFM90 | 7.1 | 3.0 | 50.0 | 1.2 | N/A | N/A |
| Reilly et al 2000 (UK) | L | Normative (British) | 98 | 1990–1997 | 8.2 | 3.0 | 7.1 | 0.8 | None | None |
| Sklar et al 2000 (US) | L | Normative (NHANES II) | 126 | 1969–1982 | 8.9 | 2.5 | 70.0 | 0.4 | N/A | Yes |
| Kourti et al 2005 (Greece) | CS | Normative (NHANES II) | 52 | ALL-BFM90 | 15.2 | 5.6 | 0 | 1.5 | M: 1.6, F: 1.5 | N/A |
| Marinovic et al 2005 (France) | L | Normative (British) and external | 34/74 | 1995–1999 | 9.0 | 5.7 | 0 | 0.7/0.7 | N/A | N/A |
| Baillargeon et al 2007 (US) | L | Normative (CDC) | 307 | 1990–2002 | N/A | 2.5 | 0 | 0.8 | M: 0.8, F: 0.8 | N/A |
| Breene et al 2011 (UK) | L | Normative (British) | 53 | 1997–2007 | 10.1 | 5.5 | 0 | 1.0 | M: 0.8, F: 1.2 | N/A |
| Esbenshade et al 2011 (US) | L | Normative (CDC) | 106 | 2000–2008 | 8.2 | 2.5 | 8.2 | 1.0 | M: 1.1, F: 1.0 | None |
| Bang et al 2012 (Korea) | L | Normative (Korean) | 88 | 2005–2008 | 6.2 | 3.5 | 0 | 0.3 | N/A | N/A |
| BMI percentile | ||||||||||
| Love et al 2011 (Canada) | L | Normative (CDC) | 102 | N/A | 7.3 | 4.0 | 8 | 74.3th | None | N/A |
| Wt for height z score | ||||||||||
| Groot-Loone et al 1996 (Netherlands) | L | Normative (Dutch) | 92 | 1972–1988 | 9.4 | 4.3 | 58.7 | 0.7 | N/A | CRT+: 0.7, CRT–: 0.0* |
| BMI | ||||||||||
| Murphy et al 2006 (UK) | CS | External | 24/24 | MRC ALL97 | 9.6 | 4.3 | 0 | 19.7/17.6* | N/A | N/A |
| BMI % | ||||||||||
| Withycombe et al 2009 (US) | L | Normative (CDC) | 1638 | 1996–2002 | 11.7 | 2.5 | 24.0 | 13.3% | N/A | None |
| % fat | ||||||||||
| Marinovic et al 2005 (France) | L | Normative (British) and external | 37/74 | 1995–1999 | 9.0 | 5.7 | 0 | 20.4/19.4 | N/A | N/A |
| Murphy et al 2006 (UK) | CS | External | 24/24 | MRC ALL97 | 9.6 | 4.3 | 0 | 28.9/24.2 | N/A | N/A |
| % OW/OB | ||||||||||
| Zee et al 1986 (US) | L | Normative (NCHS) | 324 | 1966–1984 | N/A | 4.5 | 100 | OB: 11% | N/A | N/A |
| Odame et al 1994 (UK) | L | Normative (French) and External | 40/18 | 1980–1990 | 7.5 | 4.0 | 100 | OB: 40%/22% | M: 21%, F: 57% | N/A |
| Reilly et al 2000 (UK) | L | Normative (British) | 98 | 1990–1997 | 8.2 | 3.0 | 7.1 | OB: 16% | None | N/A |
| Kourti et al 2005 (Greece) | CS | Normative (NHANES II) | 52 | ALL-BFM90 | 15.2 | 5.6 | 0 | OW: 48%, OB: 6% | N/A | N/A |
| Chow et al 2007 (US) | L | Normative (CDC) | 85 | 1993–2003 | 9.8 | 5.0 | 19.4 | OW: 21.2, OB: 20.0 | N/A | N/A |
| Withycombe et al 2009 (US) | L | Normative (CDC) | 1638 | 1996–2002 | 11.7 | 2.5 | 24.0 | OB: 23% | Higher in F | None |
| Collins et al 2010 (Canada) | L | Normative (N/A) | 162 | 1985–2004 | 8.4 | 2.0 | 60.0 | OW/OB: 40% | N/A | None |
| Pakakasama et al 2010 (Thailand) | CS | Normative (N/A) | 258 | 1990–2009 | 12.2 | 7.2 | 64.7 | OW: 6%, OB: 11% | N/A | Higher in CRT+* |
| Breene et al 2011 (UK) | L | Normative (British) | 53 | 1997–2003 | 10.1 | 5.5 | 0 | OW: 30%, OB: 17% | N/A | N/A |
| Love et al 2011 (Canada) | L | Normative (CDC) | 102 | N/A | 7.3 | 2.5 | 8 | OW/OB: 46% | N/A | N/A |
| Karakurt et al 2012 (Turkey) | L | Normative (CDC) and siblings | 44/32 | 2000–2007 | 11.5 | 5.4 | 59.1 | OW: 21%/16%, OB: 48%/ 13% | N/A | CRT+:50%, CRT–: 44% |
CRT+, survivors treated with CRT; CRT–, survivors treated with no CRT; CS, cross-sectional; Dx, diagnosis; F, female survivors; L, longitudinal; M, male survivors; N/A, values were not presented for subgroups; NCHS, National Center for Health Statistics; OB, obesity; OW, overweight; BFM, Berlin-Frankfurt-MÜnster; MRC, Medical Research Council.
Normative controls are reference populations used to calculate BMI z score or percentile. External controls are usually age- and/or gender-matched healthy unrelated individuals unless sibling controls are indicated.
Age at study and year since Dx are the mean or median as reported or estimated based on the reported age at diagnosis and duration of follow-up (the mean duration of treatment was estimated to be 2.5 y if not indicated by the study).
Weighted average was calculated if values were provided separately for subgroups (eg, M and F, survivors treated with CRT and without CRT).
None: the study indicated no difference by subgroups, but the actual values were not reported for subgroups. Yes: the study indicated a difference by subgroups, but the actual values were not reported for subgroups.
The study reported the subgroup difference was statistically different (P < .05).
TABLE 2.
Characteristics of Studies That Assessed Obesity in Pediatric ALL Survivors Off Treatment 5 to 9 Years
| Author, Year (Location) | Study Design | Type of Controla | Survivors, n /(Control) | Years at Dx | Age at Studyb | Years Since Dxb | % CRT | Major Findingsc | By Genderd | By CRTd |
|---|---|---|---|---|---|---|---|---|---|---|
| BMI z score | ||||||||||
| Birkebaek et al 1998 (Denmark) | L | Normative (French) | 33 | 1973–1984 | 16.4 | 12.1 | 66.7 | 0.6 | N/A | CRT+: 0.6, CRT –: 0.6 |
| Craig et al 1999 (UK) | L | Normative (British) | 213 | 1971–1994 | 17.0 | 13.8 | 100 | 0.4 | M: 0.2, F: 0.6 | N/A |
| Mayer et al 2000 (Germany) | L | Normative (British) | 39 | 1981–1990 | 14.2 | 8.9 | 64.1 | 1.5 | N/A | CRT+: 1.9, CRT–: 0.9 |
| Sklar et al 2000 (US) | L | Normative (NHANES II) | 126 | 1969–1982 | 18.3 | 11.9 | 70.0 | 0.5 | M: 0.5, F: 0.5 | CRT+: 0.7, CRT–: 0.04 |
| van der Sluis et al 2000 (Netherlands) | CS | Normative (British) | 23 | 1984–1988 | 17.2 | 11.8 | 0 | 0.5 | M: 0.6, F: 0.5 | N/A |
| Warner et al 2002 (UK) | CS | Normative (British) and external (sibling) | 35/31 | 1979–1990 | 12.1 | 9.1 | 100 | 1.3/0.7 | M: 0.3, F: 1.6 | N/A |
| Papadia et al 2006 (Brazil) | CS | Normative (British) and external | 27/17 | 2002–2003 | 14.0 | 8.5 | 100 | −0.03/–0.5 | N/A | N/A |
| Chow et al 2007 (US) | L | Normative (CDC) | 24 | 1993–2003 | 11.9 | 10.5 | 19.4 | 0.8 | N/A | None |
| Karaman et al 2010 (Turkey) | CS | Normative (Turkey) and external | 93/50 | 1975–2002 | 15.6 | 12.7 | 79.6 | 1.2/0.7* | None | None |
| Kohler et al 2011 (UK) | CS | Normative (British) and external | 54/51 | MRC UKALL XI ALL 97/99/2003 | 14.0 | 8.3 | 0 | 0.5/0.3 | M: 0.3, F: 0.7* | N/A |
| Aldhafiri et al 2012 (Saudi Arabia) | CS | Normative (WHO) | 56 | 1994–2009 | 13.4 | 9.1 | 0 | −0.04 | M: –0.2, F: 0.2 | N/A |
| BMI percentile | ||||||||||
| Karaman et al 2010 (Turkey) | CS | Normative (Turkey) and external | 93/50 | 1975–2002 | 15.6 | 12.7 | 79.6 | 71.7/62.3 | None | None |
| Love et al 2011 (Canada) | L | Normative (CDC) | 102 | N/A | 14.3 | 9.5 | 8.0 | 66.5 | None | N/A |
| Skoczen et al 2011 (Poland) | L | Normative (British) | 82 | 1985–2005 | 13.2 | 7.8 | 38.0 | 65.5 | N/A | N/A |
| Ratio of BMI/mean BMI | ||||||||||
| Schell et al 1992 (US) | L | Normative (NCHS) | 91 | 1967–1975 | 18.0 | 12.3 | 100 | 1.09 | N/A | N/A |
| BMI | ||||||||||
| Karaman et al 2010 (Turkey) | CS | Normative (Turkey) and external | 93/50 | 1975–2002 | 15.6 | 10.2 | 79.6 | 21.7/20.3* | M: 21.8, F: 21.5 | CRT+: 21.8, CRT–: 20.9 |
| % fat | ||||||||||
| Warner et al 2002 (UK) | CS | Normative (British) and external (sibling) | 35/32 | 1979–1990 | 12.1 | 9.1 | 100 | 28.7/20.6 | M: 21.5/17.6, F: 33.5/24.5 | N/A |
| % OW/OB | ||||||||||
| Zee et al 1986 (US) | L | Normative (NCHS) | 253 | 1966–1984 | N/A | 7.5 | 100 | OB: 8% | N/A | N/A |
| Schell et al 1992 (US) | L | Normative (NCHS) | 91 | 1967–1975 | 18.0 | 12.3 | 100 | OW/OB: 38% | N/A | N/A |
| Didi et al 1995 (UK) | L | Normative (British) | 114 | 1971–1987 | 16.2 | 10.2 | 100 | OB: 46% | M: 45%, F: 47% | N/A |
| Davies et al 1995 (UK) | L | Normative (French) | 114 | N/A | N/A | N/A | 100 | OW/OB: 47% | M: 45%, F: 48% | N/A |
| Sklar et al 2000 (US) | L | Normative (NHANES II) | 126 | 1969–1982 | 18.3 | 11.9 | 70.0 | OW/OB: 30% | None | CRT+: 39%, CRT–: 11% |
| Mayer et al 2000 (Germany) | L | Normative (British) | 39 | 1981–1990 | 14.2 | 8.9 | 64.1 | OW/OB: 38% | M: 39%, F: 38% | CRT+: 48%, CRT–: 21% |
| Nathan et al 2006 (Canada) | CS | Normative (CDC) | 83 | 1986–1999 | 14.7 | 9.7 | 23.7 | OW: 24%, OB: 14% | M: 41%, F: 34% | None |
| Razzouk et al 2007 (US) | L | Normative (CDC and NHANES II) | 248 | 1979–1984 | 18.4 | 11.9 | 58.1 | OW: 16%, OB: 21% | Yes | None |
| Trimis et al 2007 (Greece) | CS | Normative (Greek) | 80 | 1991–2002 | 13.9 | 8.8 | 22.5 | OW: 44%, OB: 25% | N/A | CRT+: 55%, CRT–: 40% |
| Asner et al 2008 (Switzerland) | L | Normative (Switzerland) | 54 | 1990–2000 | N/A | 8.5 | 0 | OW: 30%, OB: 18% | None | N/A |
| Tylavsky et al 2010 (US) | CS | Normative (CDC) | 164 | 1984–1997 | 19.6 | 9.7 | N/A | OW: 26%, OB: 31% | N/A | Yes |
| Love et al 2011 (Canada) | L | Normative (CDC) | 102 | N/A | 14.3 | 9.5 | 8 | OW/OB: 35% | M: 21%, F: 47%* | N/A |
| Aldhafiri et al 2012 (Saudi Arabia) | CS | Normative (WHO/CDC) | 56 | 1994–2009 | 13.4 | 9.1 | 0 | OW: 18%, OB: 11% | N/A | N/A |
| Skoczen et al 2011 (Poland) | L | Normative (British) | 82 | 1985–2005 | 13.2 | 7.8 | 38.0 | OW/OB: 31% | N/A | CRT+:23%, CRT–: 35% |
CRT+, survivors treated with CRT; CRT–, survivors treated with no CRT; CS, cross-sectional; Dx, diagnosis; F, female survivors; L, longitudinal; M, male survivors; MRC, Medical Research Council; N/A, values were not presented for subgroups; NCHS, National Center for Health Statistics; OB, obesity; OW, overweight; WHO, World Health Organization.
Normative controls are reference populations used to calculate BMI z score or percentile. External controls are usually age- and/or gender-matched healthy unrelated individuals unless sibling controls are indicated.
Age at study and year since Dx are the mean or median as reported or estimated based on the reported age at diagnosis and duration of follow-up (the mean duration of treatment was estimated to be 2.5 y if not indicated by the study).
Weighted average was calculated if values were provided separately for subgroups (eg, M and F, survivors treated with CRT and without CRT).
None: the study indicated no difference by subgroups, but the actual values were not reported for subgroups. Yes: the study indicated a difference by subgroups, but the actual values were not reported for subgroups.
The study reported the subgroup difference was statistically different (P < .05).
TABLE 3.
Characteristics of Studies That Assessed Obesity in Pediatric ALL Survivors Off Treatment ≥10 Years
| Author, Year (Location) | Study Design | Type of Controla | Survivors, n /(Control) | Years at Dx | Age at Studyb | Years Since Dxb | % CRT | Major Findingsc | By Genderd | By CRTd |
|---|---|---|---|---|---|---|---|---|---|---|
| BMI z score | ||||||||||
| Birkebaek et al 1998 (Denmark) | L | Normative (French) | 33 | 1973–1984 | 20.5 | 16.2 | 66.7 | 1.3 | N/A | CRT+: 1.4, CRT –: 1.0 |
| Veringa et al 2012 (Netherlands) | CS | Normative (Dutch) | 68 | 1973–2000 | 25.0 | 18.2 | 45.6 | 0.5 | M: –0.1, F: 1.4 | CRT+: 1.0, CRT–: 0.02 |
| BMI | ||||||||||
| Brennan et al 1999 (UK) | CS | External | 32/35 | MRC UKALL I-X | 23.0 | 17.8 | 100 | 24.3/23.7 | M: 24.0, F: 24.7 | N/A |
| Oeffinger et al 2003 (US) | CS | External (sibling) | 1765/2565 | 1970–1986 | 24.1 | 17.1 | 76.1 | 25.2/25.1 | M: 25.5, F: 24.9 | CRT+: 25.5, CRT–: 24.4 |
| Ness et al 2007 (US) | CS | Normative | 75 | 1970–1986 | 30.2 | 24.6 | 66.7 | 27.4/27.1 | M: 26.8, F: 27.9 | N/A |
| Garmey et al 2008 (US) | CS | External (sibling) | 1451/2167 | 1970–1986 | 32.3 | 25.1 | 76.7 | 27.9/26.8 | M: 27.9, F: 27.9 | CRT+: 28.4, CRT–: 26.4 |
| Geenen et al 2010 (Netherlands) | CS | External (sibling) | 79/69 | 1966–1991 | 24.5 | 20.8 | 60.8 | 24.0/23.9 | N/A | CRT+: 24.7, CRT–: 22.9* |
| % fat | ||||||||||
| Brennan et al 1999 (UK) | CS | External | 32/35 | MRC UKALL I-X | 23.0 | 17.8 | 100 | 32.3/26.2* | M: 25.8, F: 39.7 | N/A |
| Ness et al 2007 (US) | CS | Normative | 75 | 1970–1986 | 30.2 | 24.6 | 66.7 | 33.0/30.0* | M: 26.0, F: 38.0 | N/A |
| % OW/OB | ||||||||||
| Birkebaek et al 1998 (Denmark) | L | Normative (French) | 33 | 1973–1984 | 20.5 | 18.7 | 66.7 | OB: 36% | N/A | None |
| Shaw et al 2000 (UK) | CS | Normative (British) | 33 | 1971–1989 | 23.2 | 17.1 | 100 | OW/OB: 45% | M: 13%, F: 56% | N/A |
| Oeffinger et al 2003 (US) | CS | External (sibling) | 1765/2565 | 1970–1986 | 24.1 | 17.1 | 76.1 | OW: 29%, OB: 17% | M: 51%, F: 49% | CRT+: 47%, CRT–: 37% |
| van Beek et al 2006 (Netherlands) | CS | Normative (Dutch) | 90 | N/A | 21.2 | 12.7 | 21.1 | OW: 26%, OB: 8%, OW/OB: 38% | N/A | N/A |
| Veringa et al 2012 (Netherlands) | CS | Normative (Dutch) | 68 | 1973–2000 | 24.0 | 18.2 | 46.0 | M: 21%, F: 65% | CRT+: 90%, CRT–: 30%* | |
CRT+, survivors treated with CRT; CRT–, survivors treated with no CRT; CS, cross-sectional; Dx, diagnosis; F, female survivors; L, longitudinal; M, male survivors; MRC, Medical Research Council; N/A, values were not presented for subgroups; OB, obesity; OW, overweight.
Normative controls are reference populations used to calculate BMI z score or percentile. External controls are usually age- and/or gender-matched healthy unrelated individuals unless sibling controls are indicated.
Age at study and year since Dx are the mean or median as reported or estimated on the basis of the reported age at diagnosis and duration of follow-up (the mean duration of treatment was estimated to be 2.5 y if not indicated by the study).
Weighted average was calculated if values were provided separately for subgroups (eg, M and F, survivors treated with CRT and without CRT).
None: the study indicated no difference by subgroups, but the actual values were not reported for subgroups. Yes: the study indicated a difference by subgroups but the actual values were not reported for subgroups.
The study reported the subgroup difference was statistically different (P < .05).
Meta-Analysis of BMI z Scores
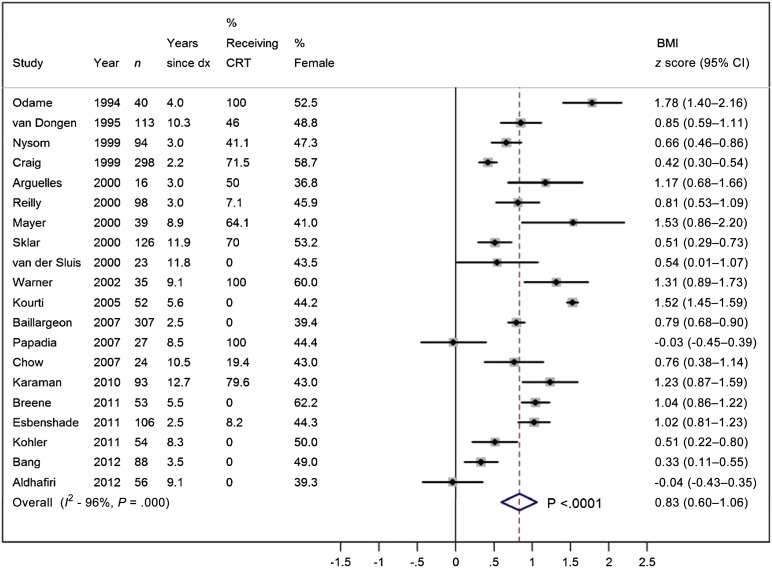
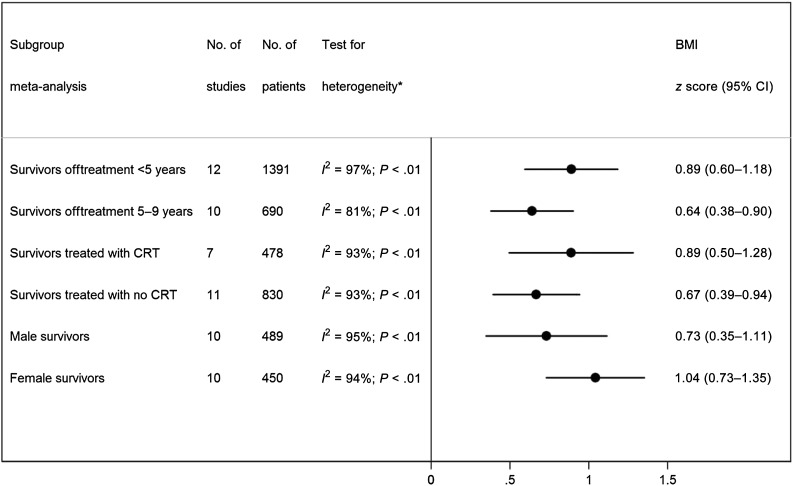
Twenty studies provided data for mean and SD of BMI z score and were included in the meta-analysis. The overall BMI z score in 1742 pediatric ALL survivors off treatment <10 years was 0.83 (95%: 0.60–1.06; Fig 2), which correspond to the 80th BMI percentile, suggesting that pediatric ALL survivors have significantly higher BMIs than children of same age and gender in the reference population. The mean BMI z score of pediatric ALL survivors was also higher than the mean BMI z score of children aged 8 to 18 years examined at the 1999–2004 NHANES, ranging between 0.4 and 0.6.46 However, there was substantial between-study heterogeneity (I2 = 96%; PQ < .01). Subgroup meta-analyses demonstrated that both survivors within 5 years from the completion of treatment (ie, off treatment <5 years) and those at 5 to 9 years from the completion of treatment (ie, off treatment 5–9 years) had a significantly higher BMI z score compared with reference populations; the BMI z score in 1391 survivors off treatment <5 years was 0.89 (95% CI: 0.60–1.18), and the BMI z score in 755 survivors off treatment 5 to 9 years was 0.64 (95% CI: 0.38–0.90). Survivors also had a higher than average BMI regardless of receipt of CRT or gender (Fig 3).
FIGURE 2.
Meta-analysis of BMI z score in survivors of pediatric ALL. dx, diagnosis.
FIGURE 3.
Meta-analysis of BMI z score in survivors of pediatric ALL by interval since treatment completion (off treatment <5 years vs 5–9 years), receipt of CRT (treated with CRT versus treated with no CRT), and gender (male versus female survivors).
Sensitivity Analysis
Sensitivity analysis demonstrated that BMI z score remained unchanged in ALL survivors after excluding studies that did not explicitly report the mean and/or SD of the BMI z score (0.7, 95% CI: 0.5–1.0) or after excluding studies that did not report the sample size of survivors at the follow-up (0.8, 95% CI: 0.5–1.1). Consistent BMI z scores were observed in the leave-one-out meta-analysis, ranging from 0.8 to 0.9 (Supplemental Fig 4).
Assessment of Quality and Reporting
All 47 studies clearly defined the obesity outcome. Most studies clearly stated survivors’ age (89.4%) and gender distribution (97.9%), treatment protocols (93.6%), and adequately described (93.6%) and appropriately selected (90.9%) the reference population or external controls for comparison. Seven of the 47 studies (14.9%) did not explicitly report the SD or did not present sufficient data to allow for the estimation of SD. Twenty-six of the 27 longitudinal studies (96.3%) described the length of follow-up, and 7 of the 26 studies (25.9%) did not provide the sample size at the follow-up. Twenty-two of the 30 studies (73.3%) that included both survivors treated with CRT and without CRT performed subgroup analysis by receipt of CRT, and 28 of the 47 studies (59.6%) performed subgroup analysis by gender (Supplemental Table 4).
Prevalence of Obesity in Pediatric ALL Survivors
Many studies did not report a BMI z score but reported prevalence of overweight or obesity in pediatric ALL survivors (Tables 1–3). This is particularly true for survivors who were off-treatment >10 years, as many had reached adult age at the time of assessment. Five studies directly compared the BMI of the survivors with the external controls. These studies either reported similar ranges of BMI between survivors (24.0–27.4) and controls (23.7–27.1)7,15,47,48 or reported only slightly higher BMI in survivors than in controls (27.9 vs 26.8).6
Although different definitions were used to assess obesity, there was a consistently high prevalence of overweight/obesity in both recent and long-term survivors. The prevalence of overweight/obese survivors exceeded 40% in 11 studies that included pediatric ALL survivors off treatment <5 years (mean/median age = 7.3–15.2 years)27,28,32,34,49–51 (Table 1). The prevalence of overweight/obesity ranged between 29% to 69% in 14 studies that evaluated pediatric ALL survivors off treatment 5 to 9 years (mean/median age = 13.2–19.4 years)8–10,24,33,51–58 except for 1 early study published in 198659 that reported a prevalence of obesity of 8% (Table 2). The prevalence of overweight/obesity was fairly consistent in 5 studies that evaluated pediatric ALL survivors off treatment ≥10 years (mean/median age = 20.5–24.1 years), ranging from 34% to 46%7,14,40,60,61 (Table 3).
Obesity in Pediatric ALL Survivors by Patient and Treatment Characteristics
CRT
Consistent with the evolution of treatment protocols over time, earlier studies tended to include a higher percentage of ALL survivors treated with CRT than did recent studies. Four studies8,29,33,38 reported separate BMI z scores for survivors treated with and without CRT, 3 studies34,35,39 included exclusively survivors treated with CRT, and 7 studies12,13,24,26,27,32,37 included exclusively survivors treated without CRT. On the basis of these 14 studies, the BMI z score for the 478 survivors treated with CRT was 0.9 (95% CI: 0.5–1.3), and the BMI z score for the 830 survivors treated without CRT was 0.7 (95% CI: 0.4–0.9; Fig 3).
Nineteen studies assessed whether the prevalence of overweight/obesity in ALL survivors differed with receipt of CRT and the findings were inconsistent. Ten studies reported a higher prevalence of overweight/obesity or a higher BMI z score in survivors treated with CRT compared with those treated without CRT,6–8,14,48,50,57,58,62,63 and 9 studies did not find a difference.9–11,28,31,36,40,49
Gender
On the basis of the 10 studies that reported separate BMI z scores for male and female ALL survivors, the BMI z score of the 450 female survivors was 1.0 (95% CI: 0.7–1.4) and the BMI z score of the 489 male survivors was 0.7 (95% CI: 0.3–1.1; Fig 3).
Among the 22 studies that assessed the prevalence of overweight/obesity by gender, 7 reported a higher prevalence of obesity in female than in male survivors.10,11,14,34,47,51,60 However, the other 15 studies did not find evidence of a gender difference.6–8,12,15,28,31,33,36,38,47,52–54,62
Chemotherapy
A few studies examined whether dose or type of glucocorticoids affected obesity. One study reported a sixfold increased risk of obesity with the highest cumulative dose of glucocorticoids,28 whereas 2 other studies reported no dose effects of glucocorticoids.8 Two studies reported a higher prevalence of obesity associated with the use of dexamethasone compared with the use of predinosone.38,62 Three studies did not observe a significant difference in obesity49,61,64 between the 2 glucocorticoids.
Age at Diagnosis
Eleven studies evaluated whether age at diagnosis had an impact on obesity in ALL survivors. Five studies reported a higher prevalence of obesity in association with a young age at diagnosis.6,10–12,36 It should be noted, however, that the method of defining a young age at diagnosis varied across studies. Six studies did not find an effect of age at diagnosis.8,9,16,28,52,54
Weight Status at Diagnosis
A few studies also evaluated weight status at diagnosis, but the evidence is inadequate to warrant a conclusion. Four studies reported that being overweight/obese or having a high BMI z score at diagnosis was associated with a high prevalence of obesity.10,30,51,52 However, 1 study found a low BMI z score at diagnosis predicted obesity after treatment completion.36
Discussion
Although a high obesity rate has been increasingly recognized in pediatric ALL survivors, individual studies have varied appreciably by interval from cancer diagnosis and treatment protocols used for survivors. To our best knowledge, our study is the first systematic review that synthesized the literature in the past 35 years, demonstrating that obesity is prevalent in pediatric ALL survivors. The summary BMI z score in 1742 pediatric ALL survivors corresponds to the 80th BMI percentile, suggesting pediatric ALL survivors have a substantially higher BMI than the standard reference population. Our systematic review also found that obesity is prevalent in pediatric ALL survivors regardless of receipt of CRT, gender, and age at diagnosis.
The strongest evidence for an increased risk of obesity in pediatric ALL survivors came from studies that evaluated survivors who were off treatment <5 years (ie, recent survivors) and were children and preadolescents at the time of study evaluation. This was followed by studies that evaluated survivors who were off treatment 5 to 9 years and were mostly adolescents at the time of the study evaluation. A relatively smaller number of studies examined the prevalence of obesity in long-term survivors, that is, survivors who were off treatment ≥10 years. The largest study was the Childhood Cancer Survivors Study, which compared the BMI of 1451 ALL survivors to 2167 siblings and reported an overall similar BMI in survivors and siblings,6 although subgroup differences were also identified. Additional evidence is needed to determine whether obesity is persistent in long-term ALL survivors.
Previous studies have attributed obesity to CRT provided to patients to prevent central nervous system relapse.7 However, since the 1990s, central nervous system prophylaxis with CRT has gradually been replaced by intrathecal and systemic chemotherapy. Although survivors who received CRT have a slightly higher BMI z score than survivors who received chemotherapy alone, the difference is small and nearly half of the studies did not support a difference in obesity rate by receipt of CRT. In particular, for ALL survivors treated under modern protocols that do not involve CRT, a high prevalence of obesity is also observed. These results suggest that ALL survivors have an elevated risk of being overweight/obese regardless of receipt of CRT.
Corticosteroids, administered as part of the ALL treatment protocol in long-term cycles, are known to play critical roles in regulating energy intake, storage, and mobilization. Two studies examined energy intake in pediatric ALL patients on maintenance therapy,65,66 and both reported a significant increase in energy intake when patients were receiving corticosteroid treatment. However, whether treatment with glucocorticoids has a long-lasting impact on obesity in pediatric ALL survivors is not known. Few studies examined the dose effect of glucocorticoids on obesity, and the current evidence is insufficient to support a link between glucocorticoids dose and obesity in ALL survivors.
Biological mechanisms that modify the risk of obesity by gender and age at diagnosis have been proposed but remain speculative. Female survivors have been found to have a higher prevalence of hyperleptinemia than male survivors,13,31 possibly due to the continuous increase in leptin and body fatness, which occurs during puberty in girls but not in boys. Genetic variations in leptin receptors have also been associated with obesity in female survivors.56 This systematic review suggests that female survivors may have a slightly higher BMI z score than male survivors, but the difference was small, and the overall evidence does not support a clear gender effect. The potential impact of cancer treatment on energy balance, although occurring primarily during active treatment, may last beyond the completion of treatment and become permanent. A young age at diagnosis may be a particular sensitive window for the long-lasting impact of treatment on energy regulation. Although a few studies suggested a young age at diagnosis is associated with a high prevalence of obesity in ALL survivors, the evidence remains inconclusive.
Limitations should be considered when interpreting our findings. Our systematic review comprised heterogeneous studies that included survivors from different countries and used different definitions to characterize obesity. Studies were also conducted over a relatively long period of time over which the treatment protocols have changed. We explored this heterogeneity with subgroup analyses and accounted for unexplained variability through random effects models. Our systematic review did not find substantial differences in BMI z scores when comparing subgroups based on patient- and treatment-related characteristics. However, it is possible that true findings between these subgroups exist but that our subgroup analyses were underpowered to detect them.
Conclusions
Our systematic review suggests that pediatric ALL survivors are more obese than children of the same age and gender in the reference population. The high obesity rate is observed across treatment received, gender, and age at diagnosis, although recent survivors tend to be more obese than long-term survivors. Given that ∼85% of children and adolescents treated for ALL will be cured, our findings have important implications for pediatric oncologists, general pediatricians, and internal medicine/family medicine physicians, all of whom will provide long-term care to this growing population of pediatric ALL survivors. Our findings strongly suggest the need for intensive management of those who are obese, given that ALL survivors are already at increased risk of chronic health conditions. Additional research is needed to elucidate the biologic mechanisms driving the high prevalence of obesity in pediatric ALL survivors, as well as to develop and evaluate interventions targeted at preventing obesity in this at-risk population.
Supplementary Material
Acknowledgments
We thank Drs Mei Chung and Issa Dahabreh for their statistical help and Becca Burns, Nate Bankoff, and Xi Lin for their help in data checking.
Glossary
- ALL
acute lymphoblastic leukemia
- CI
confidence interval
- CRT
cranial irradiation therapy
Footnotes
Dr Zhang conceptualized and designed the study, performed the systematic review and meta-analysis, and drafted the initial manuscript; Dr Kelly conceptualized and designed the study, performed the systematic review and meta-analysis, and reviewed and revised the manuscript; Drs Saltzman, Must, and Roberts conceptualized the study and reviewed and revised the manuscript; Dr Parsons conceptualized the study, coordinated the systematic review, and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: All phases of this study were supported by Boston Nutrition Obesity Research Center grant P30DK46200, National Center for Research Resources grant UL1 RR025752, the National Center for Advancing Translational Sciences, and National Institutes of Health grant UL1 TR000073. The funding source had no role in the design, conduct, or analysis of this study or the decision to submit the manuscript for publication. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354(2):166–178 [DOI] [PubMed] [Google Scholar]
- 2.Pulte D, Gondos A, Brenner H. Trends in 5- and 10-year survival after diagnosis with childhood hematologic malignancies in the United States, 1990–2004. J Natl Cancer Inst. 2008;100(18):1301–1309 [DOI] [PubMed] [Google Scholar]
- 3.Pui CH, Robison LL, Look AT. Acute lymphoblastic leukaemia. Lancet. 2008;371(9617):1030–1043 [DOI] [PubMed] [Google Scholar]
- 4.Mody R, Li S, Dover DC, et al. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. Blood. 2008;111(12):5515–5523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oeffinger KC, Mertens AC, Sklar CA, et al. Childhood Cancer Survivor Study . Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582 [DOI] [PubMed] [Google Scholar]
- 6.Garmey EG, Liu Q, Sklar CA, et al. Longitudinal changes in obesity and body mass index among adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2008;26(28):4639–4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oeffinger KC, Mertens AC, Sklar CA, et al. Childhood Cancer Survivor Study . Obesity in adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2003;21(7):1359–1365 [DOI] [PubMed] [Google Scholar]
- 8.Sklar CA, Mertens AC, Walter A, et al. Changes in body mass index and prevalence of overweight in survivors of childhood acute lymphoblastic leukemia: role of cranial irradiation. Med Pediatr Oncol. 2000;35(2):91–95 [DOI] [PubMed] [Google Scholar]
- 9.Nathan PC, Jovcevska V, Ness KK, et al. The prevalence of overweight and obesity in pediatric survivors of cancer. J Pediatr. 2006;149(4):518–525 [DOI] [PubMed] [Google Scholar]
- 10.Razzouk BI, Rose SR, Hongeng S, et al. Obesity in survivors of childhood acute lymphoblastic leukemia and lymphoma. J Clin Oncol. 2007;25(10):1183–1189 [DOI] [PubMed] [Google Scholar]
- 11.Withycombe JS, Post-White JE, Meza JL, et al. Weight patterns in children with higher risk ALL: a report from the Children’s Oncology Group (COG) for CCG 1961. Pediatr Blood Cancer. 2009;53(7):1249–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baillargeon J, Langevin AM, Lewis M, et al. Demographic correlates of body size changes in children undergoing treatment for acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;49(6):793–796 [DOI] [PubMed] [Google Scholar]
- 13.Kohler JA, Moon RJ, Wright S, Willows E, Davies JH. Increased adiposity and altered adipocyte function in female survivors of childhood acute lymphoblastic leukaemia treated without cranial radiation. Horm Res Paediatr. 2011;75(6):433–440 [DOI] [PubMed] [Google Scholar]
- 14.Veringa SJ, van Dulmen-den Broeder E, Kaspers GJ, Veening MA. Blood pressure and body composition in long-term survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2012;58(2):278–282 [DOI] [PubMed] [Google Scholar]
- 15.Ness KK, Baker KS, Dengel DR, et al. Body composition, muscle strength deficits and mobility limitations in adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;49(7):975–981 [DOI] [PubMed] [Google Scholar]
- 16.Nysom K, Holm K, Michaelsen KF, Hertz H, Müller J, Mølgaard C. Degree of fatness after treatment for acute lymphoblastic leukemia in childhood. J Clin Endocrinol Metab. 1999;84(12):4591–4596 [DOI] [PubMed] [Google Scholar]
- 17.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012 [DOI] [PubMed] [Google Scholar]
- 18.Black AE, Goldberg GR, Jebb SA, Livingstone MB, Cole TJ, Prentice AM. Critical evaluation of energy intake data using fundamental principles of energy physiology: 2. Evaluating the results of published surveys. Eur J Clin Nutr. 1991;45(12):583–599 [PubMed] [Google Scholar]
- 19.Centers for Disease Control and Prevention (CDC). 2000 CDC Growth Charts. Atlanta, GA: CDC; 2000
- 20.Barlow SE, Expert Committee . Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120(suppl 4):S164–S192 [DOI] [PubMed] [Google Scholar]
- 21.Deeks JJ, Dinnes J, D’Amico R, et al. International Stroke Trial Collaborative Group. European Carotid Surgery Trial Collaborative Group . Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7(27):iii–x, 1–173 [DOI] [PubMed] [Google Scholar]
- 22.Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol. 2007;36(3):666–676 [DOI] [PubMed] [Google Scholar]
- 23.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-analyses. Ottawa, Canada: Ottawa Hospital Research Institute; 2013
- 24.Aldhafiri F, Al-Nasser A, Al-Sugair A, Al-Mutairi H, Young D, Reilly JJ. Obesity and metabolic syndrome in adolescent survivors of standard risk childhood acute lymphoblastic leukemia in Saudi Arabia. Pediatr Blood Cancer. 2012;59(1):133–137 [DOI] [PubMed] [Google Scholar]
- 25.Argüelles B, Barrios V, Pozo J, Muñoz MT, Argente J. Modifications of growth velocity and the insulin-like growth factor system in children with acute lymphoblastic leukemia: a longitudinal study. J Clin Endocrinol Metab. 2000;85(11):4087–4092 [DOI] [PubMed] [Google Scholar]
- 26.Bang KW, Seo SY, Lee JW, et al. Evaluation of changes in random blood glucose and body mass index during and after completion of chemotherapy in children with acute lymphoblastic leukemia. Korean J Pediatr. 2012;55(4):121–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Breene RA, Williams RM, Hartle J, Gattens M, Acerini CL, Murray MJ. Auxological changes in UK survivors of childhood acute lymphoblastic leukaemia treated without cranial irradiation. Br J Cancer. 2011;104(5):746–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chow EJ, Pihoker C, Hunt K, Wilkinson K, Friedman DL. Obesity and hypertension among children after treatment for acute lymphoblastic leukemia. Cancer. 2007;110(10):2313–2320 [DOI] [PubMed] [Google Scholar]
- 29.Craig F, Leiper AD, Stanhope R, Brain C, Meller ST, Nussey SS. Sexually dimorphic and radiation dose dependent effect of cranial irradiation on body mass index. Arch Dis Child. 1999;81(6):500–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Esbenshade AJ, Simmons JH, Koyama T, Koehler E, Whitlock JA, Friedman DL. Body mass index and blood pressure changes over the course of treatment of pediatric acute lymphoblastic leukemia. Pediatr Blood Cancer. 2011;56(3):372–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karaman S, Ercan O, Yildiz I, et al. Late effects of childhood ALL treatment on body mass index and serum leptin levels. J Pediatr Endocrinol Metab. 2010;23(7):669–674 [DOI] [PubMed] [Google Scholar]
- 32.Kourti M, Tragiannidis A, Makedou A, Papageorgiou T, Rousso I, Athanassiadou F. Metabolic syndrome in children and adolescents with acute lymphoblastic leukemia after the completion of chemotherapy. J Pediatr Hematol Oncol. 2005;27(9):499–501 [DOI] [PubMed] [Google Scholar]
- 33.Mayer EI, Reuter M, Dopfer RE, Ranke MB. Energy expenditure, energy intake and prevalence of obesity after therapy for acute lymphoblastic leukemia during childhood. Horm Res. 2000;53(4):193–199 [DOI] [PubMed] [Google Scholar]
- 34.Odame I, Reilly JJ, Gibson BE, Donaldson MD. Patterns of obesity in boys and girls after treatment for acute lymphoblastic leukaemia. Arch Dis Child. 1994;71(2):147–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Papadia C, Naves LA, Costa SS, Vaz JA, Domingues L, Casulari LA. Incidence of obesity does not appear to be increased after treatment of acute lymphoblastic leukemia in Brazilian children: role of leptin, insulin, and IGF-1. Horm Res. 2007;68(4):164–170 [DOI] [PubMed] [Google Scholar]
- 36.Reilly JJ, Ventham JC, Newell J, Aitchison T, Wallace WH, Gibson BE. Risk factors for excess weight gain in children treated for acute lymphoblastic leukaemia. Int J Obes Relat Metab Disord. 2000;24(11):1537–1541 [DOI] [PubMed] [Google Scholar]
- 37.van der Sluis IM, van den Heuvel-Eibrink MM, Hählen K, Krenning EP, de Muinck Keizer-Schrama SM. Bone mineral density, body composition, and height in long-term survivors of acute lymphoblastic leukemia in childhood. Med Pediatr Oncol. 2000;35(4):415–420 [DOI] [PubMed] [Google Scholar]
- 38.Van Dongen-Melman JE, Hokken-Koelega AC, Hählen K, De Groot A, Tromp CG, Egeler RM. Obesity after successful treatment of acute lymphoblastic leukemia in childhood. Pediatr Res. 1995;38(1):86–90 [DOI] [PubMed] [Google Scholar]
- 39.Warner JT, Evans WD, Webb DK, Gregory JW. Body composition of long-term survivors of acute lymphoblastic leukaemia. Med Pediatr Oncol. 2002;38(3):165–172 [DOI] [PubMed] [Google Scholar]
- 40.Birkebaek NH, Clausen N. Height and weight pattern up to 20 years after treatment for acute lymphoblastic leukaemia. Arch Dis Child. 1998;79(2):161–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188 [DOI] [PubMed] [Google Scholar]
- 42.Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72(11):1605–1621 [DOI] [PubMed] [Google Scholar]
- 43.Olkin I. Diagnostic statistical procedures in medical meta-analyses. Stat Med. 1999;18(17–18):2331–2341 [DOI] [PubMed] [Google Scholar]
- 44.Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129 [Google Scholar]
- 45.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558 [DOI] [PubMed] [Google Scholar]
- 46.Heo M, Wylie-Rosett J, Pietrobelli A, Kabat GC, Rohan TE, Faith MS. US pediatric population-level associations of DXA-measured percentage of body fat with four BMI metrics with cutoffs. Int J Obes (Lond). 2014,38(1):60–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brennan BM, Rahim A, Blum WF, Adams JA, Eden OB, Shalet SM. Hyperleptinemia in young adults following cranial irradiation in childhood: growth hormone deficiency or leptin insensitivity? Clin Endocrinol (Oxf). 1999;50(2):163–169 [DOI] [PubMed] [Google Scholar]
- 48.Geenen MM, Bakker PJ, Kremer LC, Kastelein JJ, van Leeuwen FE. Increased prevalence of risk factors for cardiovascular disease in long-term survivors of acute lymphoblastic leukemia and Wilms tumor treated with radiotherapy. Pediatr Blood Cancer. 2010;55(4):690–697 [DOI] [PubMed] [Google Scholar]
- 49.Collins L, Zarzabal LA, Nayiager T, Pollock BH, Barr RD. Growth in children with acute lymphoblastic leukemia during treatment. J Pediatr Hematol Oncol. 2010;32(8):e304–e307 [DOI] [PubMed] [Google Scholar]
- 50.Karakurt H, Sarper N, Kılıç SÇ, Gelen SA, Zengin E. Screening survivors of childhood acute lymphoblastic leukemia for obesity, metabolic syndrome, and insulin resistance. Pediatr Hematol Oncol. 2012;29(6):551–561 [DOI] [PubMed] [Google Scholar]
- 51.Love E, Schneiderman JE, Stephens D, et al. A cross-sectional study of overweight in pediatric survivors of acute lymphoblastic leukemia (ALL). Pediatr Blood Cancer. 2011;57(7):1204–1209 [DOI] [PubMed] [Google Scholar]
- 52.Asner S, Ammann RA, Ozsahin H, Beck-Popovic M, von der Weid NX. Obesity in long-term survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2008;51(1):118–122 [DOI] [PubMed] [Google Scholar]
- 53.Davies HA, Didcock E, Didi M, Ogilvy-Stuart A, Wales JK, Shalet SM. Growth, puberty and obesity after treatment for leukaemia. Acta Paediatr Suppl. 1995;411:45–50, discussion 51 [DOI] [PubMed] [Google Scholar]
- 54.Didi M, Didcock E, Davies HA, Ogilvy-Stuart AL, Wales JK, Shalet SM. High incidence of obesity in young adults after treatment of acute lymphoblastic leukemia in childhood. J Pediatr. 1995;127(1):63–67 [DOI] [PubMed] [Google Scholar]
- 55.Schell MJ, Ochs JJ, Schriock EA, Carter M. A method of predicting adult height and obesity in long-term survivors of childhood acute lymphoblastic leukemia. J Clin Oncol. 1992;10(1):128–133 [DOI] [PubMed] [Google Scholar]
- 56.Skoczen S, Tomasik PJ, Bik-Multanowski M, et al. Plasma levels of leptin and soluble leptin receptor and polymorphisms of leptin gene -18G > A and leptin receptor genes K109R and Q223R, in survivors of childhood acute lymphoblastic leukemia. J Exp Clin Cancer Res. 2011;30:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trimis G, Moschovi M, Papassotiriou I, Chrousos G, Tzortzatou-Stathopoulou F. Early indicators of dysmetabolic syndrome in young survivors of acute lymphoblastic leukemia in childhood as a target for preventing disease. J Pediatr Hematol Oncol. 2007;29(5):309–314 [DOI] [PubMed] [Google Scholar]
- 58.Tylavsky FA, Smith K, Surprise H, et al. Nutritional intake of long-term survivors of childhood acute lymphoblastic leukemia: evidence for bone health interventional opportunities. Pediatr Blood Cancer. 2010;55(7):1362–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zee P, Chen CH. Prevalence of obesity in children after therapy for acute lymphoblastic leukemia. Am J Pediatr Hematol Oncol. 1986;8(4):294–299 [DOI] [PubMed] [Google Scholar]
- 60.Shaw MP, Bath LE, Duff J, Kelnar CJ, Wallace WH. Obesity in leukemia survivors: the familial contribution. Pediatr Hematol Oncol. 2000;17(3):231–237 [DOI] [PubMed] [Google Scholar]
- 61.van Beek RD, de Muinck Keizer-Schrama SM, Hakvoort-Cammel FG, et al. No difference between prednisolone and dexamethasone treatment in bone mineral density and growth in long term survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2006;46(1):88–93 [DOI] [PubMed] [Google Scholar]
- 62.Groot-Loonen JJ, Otten BJ, van’t Hof MA, Lippens RJ, Stoelinga GB. Influence of treatment modalities on body weight in acute lymphoblastic leukemia. Med Pediatr Oncol. 1996;27(2):92–97 [DOI] [PubMed] [Google Scholar]
- 63.Pakakasama S, Veerakul G, Sosothikul D, et al. Late effects in survivors of childhood acute lymphoblastic leukemia: a study from Thai Pediatric Oncology Group. Int J Hematol. 2010;91(5):850–854 [DOI] [PubMed] [Google Scholar]
- 64.Murphy AJ, Wells JC, Williams JE, Fewtrell MS, Davies PS, Webb DK. Body composition in children in remission from acute lymphoblastic leukemia. Am J Clin Nutr. 2006;83(1):70–74 [DOI] [PubMed] [Google Scholar]
- 65.Jansen H, Postma A, Stolk RP, Kamps WA. Acute lymphoblastic leukemia and obesity: increased energy intake or decreased physical activity? Support Care Cancer. 2009;17(1):103–106 [DOI] [PubMed] [Google Scholar]
- 66.Reilly JJ, Brougham M, Montgomery C, Richardson F, Kelly A, Gibson BE. Effect of glucocorticoid therapy on energy intake in children treated for acute lymphoblastic leukemia. J Clin Endocrinol Metab. 2001;86(8):3742–3745 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.