Abstract
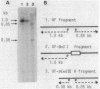
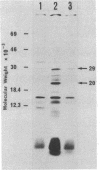
We have determined the sequence of a 1,162-base-pair DNA fragment containing a spo0F gene which is required for an early stage of sporulation in Bacillus subtilis. The sequence has only one long open reading frame consisting of 173 codons, which has been confirmed to be the spo0F cistron by DNA-mediated transformation and in vitro transcription. In UV-irradiated "maxicells" containing pUBSF13, the plasmid that carries cloned spo0F gene, we have observed the synthesis of a 20-kilodalton polypeptide that is absent from cells carrying a vector plasmid pUB110. The molecular weight of this protein is in agreement with the calculated molecular weight of the spo0F gene product (Mr, 19,065). The putative promoter sequences of spo0F gene were 5' T-A-T-A-A-T 3' at -10 and 5' T-T-G-A-T-T 3' at -35. An octamer sequence, 5' A-A-A-G-G-A-G-G 3', situated 8 base pairs prior to the initiation codon was found to be perfectly complementary with the 3' end of 16S ribosomal RNA. This result offers additional evidence for the proposal by Rabinowitz's group that an extensive mRNA-rRNA interaction is a requirement for efficient translation by B. subtilis ribosomes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borer P. N., Dengler B., Tinoco I., Jr, Uhlenbeck O. C. Stability of ribonucleic acid double-stranded helices. J Mol Biol. 1974 Jul 15;86(4):843–853. doi: 10.1016/0022-2836(74)90357-x. [DOI] [PubMed] [Google Scholar]
- Gold L., Pribnow D., Schneider T., Shinedling S., Singer B. S., Stormo G. Translational initiation in prokaryotes. Annu Rev Microbiol. 1981;35:365–403. doi: 10.1146/annurev.mi.35.100181.002053. [DOI] [PubMed] [Google Scholar]
- Haldenwang W. G., Lang N., Losick R. A sporulation-induced sigma-like regulatory protein from B. subtilis. Cell. 1981 Feb;23(2):615–624. doi: 10.1016/0092-8674(81)90157-4. [DOI] [PubMed] [Google Scholar]
- Haldenwang W. G., Losick R. A modified RNA polymerase transcribes a cloned gene under sporulation control in Bacillus subtilis. Nature. 1979 Nov 15;282(5736):256–260. doi: 10.1038/282256a0. [DOI] [PubMed] [Google Scholar]
- Haldenwang W. G., Losick R. Novel RNA polymerase sigma factor from Bacillus subtilis. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7000–7004. doi: 10.1073/pnas.77.12.7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirochika H., Kobayashi Y., Kawamura F., Saito H. Cloning of sporulation gene spoOB of Bacillus subtilis and its genetic and biochemical analysis. J Bacteriol. 1981 May;146(2):494–505. doi: 10.1128/jb.146.2.494-505.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A. Genetics of bacterial sporulation. Adv Genet. 1976;18:69–98. doi: 10.1016/s0065-2660(08)60437-x. [DOI] [PubMed] [Google Scholar]
- Iijima T., Kawamura F., Saito H., Ikeda Y. A specialized transducing phage constructed from Bacillus subtilis phage phi 105. Gene. 1980 Apr;9(1-2):115–126. doi: 10.1016/0378-1119(80)90170-5. [DOI] [PubMed] [Google Scholar]
- Kawamura F., Ito J. Transcription of the genome of bacteriophage phi 29: isolation and mapping of the major early mRNA synthesized in vivo and in vitro. J Virol. 1977 Sep;23(3):562–577. doi: 10.1128/jvi.23.3.562-577.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura F., Saito H., Ikeda Y. A method for construction of specialized transducing phage rho 11 of Bacillus subtilis. Gene. 1979 Feb;5(2):87–91. doi: 10.1016/0378-1119(79)90095-7. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lee G., Talkington C., Pero J. Nucleotide sequence of a promoter recognized by Bacillus subtilis RNA polymerase. Mol Gen Genet. 1980;180(1):57–65. doi: 10.1007/BF00267352. [DOI] [PubMed] [Google Scholar]
- Losick R., Pero J. Cascades of Sigma factors. Cell. 1981 Sep;25(3):582–584. doi: 10.1016/0092-8674(81)90164-1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McLaughlin J. R., Murray C. L., Rabinowitz J. C. Initiation factor-independent translation of mRNAs from Gram-positive bacteria. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4912–4916. doi: 10.1073/pnas.78.8.4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J. R., Murray C. L., Rabinowitz J. C. Unique features in the ribosome binding site sequence of the gram-positive Staphylococcus aureus beta-lactamase gene. J Biol Chem. 1981 Nov 10;256(21):11283–11291. [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., Banner C. D., Haldenwang W. G., Losick R. Promoter for a developmentally regulated gene in Bacillus subtilis. Cell. 1981 Sep;25(3):783–791. doi: 10.1016/0092-8674(81)90186-0. [DOI] [PubMed] [Google Scholar]
- Moran C. P., Jr, Lang N., Losick R. Nucleotide sequence of a Bacillus subtilis promoter recognized by Bacillus subtilis RNA polymerase containing sigma 37. Nucleic Acids Res. 1981 Nov 25;9(22):5979–5990. doi: 10.1093/nar/9.22.5979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray C. L., Rabinowitz J. C. Nucleotide sequences of transcription and translation initiation regions in Bacillus phage phi 29 early genes. J Biol Chem. 1982 Jan 25;257(2):1053–1062. [PubMed] [Google Scholar]
- Piggot P. J., Coote J. G. Genetic aspects of bacterial endospore formation. Bacteriol Rev. 1976 Dec;40(4):908–962. doi: 10.1128/br.40.4.908-962.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhaese H. J., Hoch J. A., Groscurth R. Studies on the control of development: isolation of Bacillus subtilis mutants blocked early in sporulation and defective in synthesis of highly phosphorylated nucleotides. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1125–1129. doi: 10.1073/pnas.74.3.1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T., Saito H. Repair of ultraviolet-induced DNA damage in the subcellular systems of Bacillus subtilis. Mutat Res. 1973 Nov;20(2):159–173. doi: 10.1016/0027-5107(73)90186-3. [DOI] [PubMed] [Google Scholar]
- Shivakumar A. G., Dubnau D. Plasmid replication in DNA Ts mutants of Bacillus subtilis. Plasmid. 1978 Jun;1(3):405–416. doi: 10.1016/0147-619x(78)90055-0. [DOI] [PubMed] [Google Scholar]
- Smith H. O. Recovery of DNA from gels. Methods Enzymol. 1980;65(1):371–380. doi: 10.1016/s0076-6879(80)65048-4. [DOI] [PubMed] [Google Scholar]
- Sterlini J. M., Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969 Jun;113(1):29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Wiggs J. L., Gilman M. Z., Chamberlin M. J. Heterogeneity of RNA polymerase in Bacillus subtilis: evidence for an additional sigma factor in vegetative cells. Proc Natl Acad Sci U S A. 1981 May;78(5):2762–2766. doi: 10.1073/pnas.78.5.2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Lis J., Wu R. Elution of DNA from agarose gels after electrophoresis. Methods Enzymol. 1979;68:176–182. doi: 10.1016/0076-6879(79)68012-6. [DOI] [PubMed] [Google Scholar]