Abstract
An ethanolic extract and its ethyl acetate-soluble fraction from leaves of Calendula officinalis L. (Asteraceae) were found to show an inhibitory effect on amylase. From the crude extract fractions, one new phenolic acid glucoside, 6′-O-vanilloyl-β-D-glucopyranose, was isolated, together with twenty-four known compounds including five phenolic acid glucosides, five phenylpropanoids, five coumarins, and nine flavonoids. Their structures were elucidated based on chemical and spectral data. The main components, isoquercitrin, isorhamnetin-3-O-β-D-glucopyranoside, 3,5-di-O-caffeoylquinic acid, and quercetin-3-O-(6′′-acetyl)-β-D-glucopyranoside, exhibited potent inhibitory effects on amylase.
1. Introduction
The Compositae annual herbaceous plant, Calendula officinalis L., commonly called marigold, or pot marigold is widely cultivated as an ornamental, culinary and valuable medicinal herb due to its various pharmacological properties: anti-inflammatory, antioxidant, antibacterial, antifungal, and so forth [1]. A number of chemical investigations have revealed the presence of several classes of compounds, with the main ones being terpenes, flavonoids, carotenoids, lipids, and carbohydrates.
Despite the wide use of C. officinalis flowers, leaves of this species have not currently found a practical application. The productivity of the vegetative foliage is much greater than that of the flowers, making it possible to consider the foliage as a new kind of useful plant material. The presence of various classes of compounds in C. officinalis leaves was determined as a result of chemical investigations. Isorhamnetin, isorhamnetin-3-O-β-d-glucopyranoside, and narcissin [2] belong to the phenolic compounds. Previously it has been shown that phenolic compounds are responsible for the presence of antioxidant and anticholinesterase activity in extracts of C. officinalis [3]. Carotenoids represent 12 compounds, dominated by lutein, β-carotene, and violaxanthin; the total carotenoid content in the leaves of C. officinalis growing in Bulgaria reaches 0.85 mg/g [4]. The most investigated groups of compounds found in the leaves of C. officinalis are triterpene glycosides and sterols. In young leaves the presence of cholestanol, campestanol, stigmastanol, and clerosterol derivatives in free, esterified, and glycosylated forms was revealed [5]. Mono-, di-, tri-, and tetraglucosides of olenolic acid isolated from C. officinalis leaves growing in Poland demonstrated antibacterial and antiparasitic activity [6]. The essential oil of C. officinalis leaves contains a set of compounds, dominated by sesquiterpenes (τ-muurolol, δ-cadinene) and monoterpenes (α-thujene) [7]. A number of neutral components, phospho-, and glycolipids have also been identified in the lipid complex [8].
In our previous investigation we detected some phenylpropanoids (caffeic acid, mono- and di-O-caffeoylquinic acids) and flavonoids (calendoflavoside, isoquercitrin, quercetin-3-O-(6′′-acetyl)-β-d-glucopyranoside, isorhamnetin-3-O-β-d-glucopyranoside, and isorhamnetin-3-O-(6′′-acetyl)-β-d-glucopyranoside) in C. officinalis leaves [9]. That work was realized just for “Orange Big” variety which is one of the most frequently cultivated double-flowered varieties in Russia. However, a number of C. officinalis varieties cultivated in Russia are much more indicating the necessity in extended chemical surveys.
Early, Yoshikawa et al. revealed a high hypoglycemic activity of the extracts and individual compounds from C. officinalis flowers that can be considered marigold as a forthcoming antidiabetic remedy [10]. In the course of our studies on the bioactivity of C. officinalis we found that an ethanolic extract from the leaves of this plant species showed inhibitory effect on the amylase. It is known that inhibitors of amylase, a carbohydrate hydrolyzing enzyme in the small intestine, are relevant to type II diabetes [11].
In this study, we present the results of phytochemical investigation of C. officinalis leaves from nine double-flowered varieties growing in the Russian Federation. As a result, twenty-five compounds were isolated including a new glycoside, 6′-O-vanilloyl-β-d-glucopyranose. In addition, we describe the inhibitory effects of the isolated compounds on amylase.
2. Materials and Methods
2.1. Plant Material
Plants of Calendula officinalis L. in nine double-flowered varieties (“Egypt Sun,” “Flame Dance,” “Geisha,” “Green Heart Orange,” “Indian Prince,” “Radio,” “Red Black Centered,” “Russian Size,” “Touch of Red”) were grown from authenticated seeds obtained from Tsitsin's Main Botanical Garden of the Russian Academy of Science (Moscow, Russian Federation) by cultivation in the fields of the Botanical Garden of the Institute of General and Experimental Biology (IGEB, Ulan-Ude, Russian Federation). The leaves were collected in the middle of August, 2012, and then dried in vacuo at 40°C (12 h) and stored at 4°C in the IGEB Plant Repository.
2.2. General Experimental Procedures
Elemental composition was determined using a MAT 8200 spectrometer (Thermo Finnigan). UV spectra were recorded using a SF-2000 spectrophotometer (OKB Specter). MS spectra were registered on a LCQ mass spectrometer (Thermo Finnigan). NMR spectra were recorded on a VXR 500S spectrometer (Varian). Chromatography was performed over columns of silica gel 60 (NP-SiO2; 230–400 mesh, Merck), Sephadex LH-20 (25–100 μm, Pharmacia), polyamide (Woelm), octadecyl-functionalized silica gel (RP-SiO2; Aldrich), and Amberlite XAD7HP (Sigma). pTLC was performed on Sorbfil-A silica gel TLC plates (layer thickness 2 mm; Imid Ltd.). All chemicals were analytical grade. Alkaline hydrolysis [9], acidic hydrolysis [12], and HPLC analyses of cleavage products [13] were performed as described previously. The Folin method was used to determine total phenolic content (TPC) as described by [14] using chlorogenic acid as a standard compound. Total flavonoid content (TFC) was determined by a spectrophotometric method [15] using isoquercitrin as a standard compound.
2.3. Extraction and Isolation
Air-dried, ground leaves of C. officinalis (1.65 kg) were extracted three times with 60% EtOH at 80°C and the extracts were concentrated under reduced pressure to yield 462.7 g of crude extract. The crude extract was resuspended in water (1 : 6, v/v) and successively partitioned with hexane, CHCl3, EtOAc, and n-BuOH. The organic layers were brought to dryness in vacuo to yield 49.5, 79.4, 59.2, and 204.6 g of hexane (F1), CHCl3 (F2), EtOAc (F3), and n-BuOH fraction (F4) residue, respectively. The F2 fraction (54 g) was chromatographed over a Sephadex LH-20 column (6 × 100 cm) and eluted with CHCl3-MeOH (100 : 0→0 : 100) to obtain 10 fractions (frs. F2/1–F2/10). Frs. F2/2-F2/3 were combined and chromatographed on a silica column (3 × 50 cm) eluted with CHCl3-MeOH (100 : 0→70 : 30) to obtain 10 fractions (frs. F2/2-3/1–F2/2-3/10), which were separated using pTLC (solvent-toluene-EtOAc-HCOOH 6 : 3 : 1) to give umbelliferone (12; 6 mg), aesculetin (13; 14 mg) and scopoletin (16; 5 mg) [16]. Frs. F2/5-F2/7 were combined and chromatographed on a silica column (2.5 × 70 cm) and eluted with CHCl3-MeOH (100 : 0→70 : 30) to obtain quercetin (17; 19 mg) and isorhamnetin (21; 9 mg) [17]. Fr. F3 (50 g) was subjected to chromatography on a XAD7HP column (500 g) eluted with H2O (8 L), 40% EtOH (12 L) and 90% EtOH (10 L). The eluates were brought to dryness in vacuo to yield 1.3, 37.9, and 6.4 g of H2O (F3-1), 40% EtOH (F3-2) and 90% EtOH fraction (F3-3) residue, respectively. Fr. F3-2 was chromatographed on a polyamide column (5 × 120 cm) eluted with H2O-MeOH (100 : 0→0 : 100) to obtain 10 fractions (fr. F3-2/1–fr. F3-2/10). Frs. F3-2/2–F3-2/3 were separated on a Sephadex LH-20 column (4 × 140 cm) and eluted with MeOH-H2O (100 : 0→0 : 100) to obtain caffeic acid (7; 24 mg) [18], 3,5-di-O- (9; 184 mg), 1,5-di-O- (10; 62 mg) and 4,5-di-O-caffeoylquinic acid (11; 37 mg) [19]. Frs. F3-2/4–F3-2/7 were separated on a RP-SiO2 column (4 × 140 cm) and eluted with H2O-MeCN (100 : 0→0 : 100) to give 3-O-caffeoylquinic acid (11; 215 mg) [18], aesculin (14; 28 mg), cichoriin (15; 14 mg) [20], isoquercitrin (18; 182 mg), and isorhamnetin-3-O-β-d-glucopyranoside (22; 63 mg) [21]. Fr. F3-3 was subjected to pHPLC chromatography [Summit HPLC-system with a UV-Vis detector (Dionex) using a LiChrosorb RP-18 column (4.6 × 250 mm, 5 μm, Merck), T 35°C, flow rate 1 mL min−1, solvent a linear gradient of 5–40% MeCN in H2O for 90 min, the detector at 360 nm; five runs] to give quercetin-3-O-(6′′-acetyl)-β-d-glucopyranoside (19; 54 mg) [22] and isorhamnetin-3-O-(6′′-acetyl)-β-d-glucopyranoside (23; 25 mg) [9]. Fr. F4 (120 g) was added to a XAD7HP column (800 g) and eluted with H2O (24 L), 40% EtOH (28 L) and 90% EtOH (5 L). The eluates were brought to dryness in vacuo to yield 54.9, 38.4, and 12.5 g of H2O (F4-1), 40% EtOH (F4-2), and 90% EtOH fraction (F4-3) residue, respectively. Fr. F4-2 was chromatographed on a polyamide column (5 × 120 cm) and eluted with H2O-MeOH (100 : 0→0 : 100) to give 10 fractions (F4-2/1–F4-2/10). Frs. F4-2/1–F4-2/2 were separated via pHPLC [Summit HPLC-system with UV-Vis detector (Dionex), LiChrosorb RP-18 column (4.6 × 250 mm, 5 μm, Merck), T 35°C, flow rate 1 mL min−1, solvent a linear gradient of 0–10% MeCN in H2O for 90 min, the detector at 280 nm; four runs] to give 1′-O-p-hydroxybenzoyl-β-d-glucopyranose (1; 24 mg) [23], 6′-O-p-hydroxybenzoyl-β-d-glucopyranose (2; 11 mg), 1′-O-protocatechuoyl-β-d-glucopyranose (3; 18 mg), 6′-O-protocatechuoyl-β-d-glucopyranose (4; 14 mg) [24], 1′-O-vanilloyl-β-d-glucopyranose (5; 18 mg) [25], and compound 6 (27 mg). Frs. F4-2/4–F4-2/6 were separated on a Sephadex LH-20 column (4 × 140 cm) eluted with MeOH-H2O (100 : 0→0 : 100) to obtain rutin (20; 34 mg), narcissin (24; 24 mg) [21] and thyphaneoside (25; 23 mg) [26].
2.4. 6′-O-Vanilloyl-β-d-glucopyranose (6): C14H18O9
UV λ max nm MeOH: 220, 261, 292. HR-ESI-MS, m/z: 353.2764 [M + Na]+ (calcd for C14H18O9Na 353.2840). +FAB-MS, m/z: 331 [M + H]+. 1H-NMR (CD3OD, 500 MHz) and 13C-NMR data (CD3OD, 125 MHz); see Table 2.
Table 2.
1H-NMR and 13C-NMR data of compound 6.
| Atom no. | DEPT | δ H (mult., J in Hz) | δ C |
|---|---|---|---|
| 1 | C | 120.3 | |
| 2 | CH | 7.52 (1H, d, 1.9) | 112.7 |
| 3 | C | 147.5 | |
| 4 | C | 153.4 | |
| 5 | CH | 6.80 (1H, d, 8.0) | 115.4 |
| 6 | CH | 7.63 (1H, dd, 8.0, 1.9) | 125.6 |
| 7 | C | 166.7 | |
| 4-OCH3 | CH3 | 3.97 (3H, s) | 56.3 |
| 1′ | CH | 4.56 (1H, d, 7.2) | 98.3 |
| 2′ | CH | 3.39–3.62 (4H, m) | 73.4 |
| 3′ | CH | 78.7 | |
| 4′ | CH | 70.3 | |
| 5′ | CH | 74.5 | |
| 6′ | CH2 | 4.37 (1H, dd, 11.9, 2.0) 4.05 (1H, dd, 11.9, 7.4) |
64.2 |
2.5. Sample Preparation and Analytical HPLC-UV
The dried and powdered leaves (200 mg) from the different varieties of C. officinalis were extracted with 60% ethanol (5 mL) in an ultrasonic bath for 40 min. The extracted solutions were filtered through a 0.22 μm PTFE syringe filter before injection into the LC system for analysis.
HPLC analysis was performed on a MiLiChrom A-02 microcolumn chromatograph (Econova) coupled with a UV detector, using a ProntoSIL-120-5-C18 AQ column (2 × 75 mm, ∅ 5 μm; Metrohm AG). Mobile phase A was 0.2 M LiClO4 in 0.006 M HClO4 and mobile phase B was acetonitrile. The injection volume was 1 μL, and elution was at 150 μL min−1 with a gradient program (0–7.5 min 11–18% B, 7.5–13.5 min 18% B, 13.5–15 min 18–20% B, 15–18 min 20–25% B, 18–24 min 25% B, and 24–30 min 25–100% B). The detector wavelength was 270 nm. Reference compounds with purity greater than 96% were used for the establishment of calibration curves. This included 3-O-caffeoylquinic acid, 3,5-di-O-caffeoylquinic acid, 1,5-di-O-caffeoylquinic acid, caffeic acid, isoquercitrin, and rutin from Sigma-Aldrich (Missouri, USA); Isorhamnetin-3-O-β-d-glucoside was from Extrasynthese (Lyon, France); 4,5-di-O-caffeoylquinic acid was from ChemFaces (Wuhan, China). Thyphaneoside, quercetin-3-O-(6′′-acetyl)-β-d-glycoside, and isorhamnetin-3-O-(6′′-acetyl)-β-d-glycoside were isolated previously from C. officinalis flowers [9].
2.6. Amylase Inhibition Microplate Assay
Amylase inhibitory activity was measured using a microplate method: 10 μL of a sample solution in DMSO, 30 μL of phosphate buffer (pH 5.0), and 10 μL of amylase from Aspergillus niger (3 U mL−1, Sigma) which were incubated for 20 min at 45°C. Then 10 μL of 2% starch solution, 40 μL of phosphate buffer (pH 5.0), and 100 μL of the reagent were added and incubated for 30 min at 50°C. Absorbance was measured at 510 nm. The reagent was a solution of K2HPO4 (0.8 mM), KH2PO4 (0.4 mM), phenol (220 mM), 4-aminoantipyrine (1.5 μM), glucose oxidase from Aspergillus niger (3 U mL−1; Sigma), and peroxidase from horseradish (0.3 U mL−1) in deionized water. A 2% solution of acarbose was used as a positive control (PC), and water was used as a negative control (NC). The experiment was carried out in triplicate and averaged. The ability to inhibit amylase was calculated using the following equation: inhibitory ability (%) = [(A510 NC − A510 PC) − (A510 Sample − A510 PC)/(A510 NC − A510 PC)] × 100, where A510 NC is the absorbance of the negative control, A510 PC is the absorbance of the positive control, and A510 Sample is the absorbance of the sample solution. The IC50 value is the effective concentration at which amylase activity was inhibited by 50%. Values are expressed as mean obtained from 5 independent experiments.
3. Results and Discussion
3.1. Phenolic Compounds Content in Russian Varieties of C. officinalis Leaves
Preliminary chemical research on the composition of C. officinalis leaves was carried out for nine varieties cultivated widely in the territory of Russia. These varieties are characterized by high productivity and simplicity of cultivation. As a result, it was found that flavonoid content in the leaves examined ranged from 8.49 (“Indian Prince”) to 13.08 mg g−1 (“Radio”), while the total content of phenolic compounds varied from 29.21 (“Russian Size”) to 50.24 mg g−1 (“Radio”) (Figure 1). The maximal content of phenolic compounds was observed for C. officinalis leaves of the “Radio” variety, which were further subjected to detailed chemical study.
Figure 1.
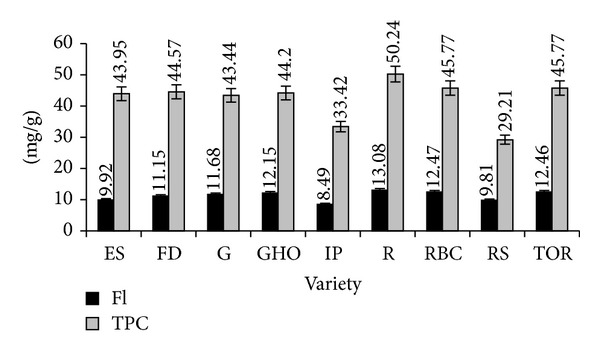
Total flavonoid content (black bars; Fl) and total phenolic content (grey bars; TPC) in nine varieties of C. officinalis leaves. Varieties: “Egypt Sun” (ES), “Flame Dance” (FD), “Geisha” (G), “Green Heart Orange” (GHO), “Indian Prince” (IP), “Radio” (R), “Red Black Centered” (RBC), “Russian Size” (RS), and “Touch of Red” (TOR). Under the bars—means of content, mg g−1.
3.2. Extraction and Isolation of Phenolic Compounds from C. officinalis Leaves of “Radio” Variety
A 60% ethanolic extract of C. officinalis leaves of “Radio” variety was partitioned with CHCl3, EtOAc, and n-BuOH to yield three fractions. The crude extract was found to exhibit inhibitory activity on an amylase with an inhibitory value (IC50) of 38.02 ± 1.29 μg mL−1 (Table 1). In the same bioassay, the ethylacetate-soluble fraction still showed potent inhibitory activity with a lower value, 24.52 ± 0.88 μg mL−1.
Table 1.
Amylase inhibiting activity of C. officinalis extract and fractions.
| Sample | IC50 (μg mL−1) |
|---|---|
| Ethanolic extract | 38.02 ± 1.29 |
| CHCl3 fraction | >100 |
| EtAc fraction | 24.52 ± 0.88 |
| BuOH fraction | 72.60 ± 2.61 |
| Acarbosea | 9.54 ± 0.32 |
Values are expressed as mean ± SD obtained from 5 independent experiments.
aReference compounds.
To enhance our knowledge regarding the chemical composition of C. officinalis leaves, all the isolated fractions were separated by chromatographic columns (gel permeation, NP- and RP-SiO2, XAD, and polyamide chromatography), prep. HPLC, and prep. TLC, yielding one new (6) and twenty-four known compounds. The known compounds, including five phenolic acid glucosides [1′-O-p-hydroxybenzoyl-β-d-glucopyranose (1), 6′-O-p-hydroxybenzoyl-β-d-glucopyranose (2), 1′-O-protocatechuoyl-β-d-glucopyranose (3), 6′-O-protocatechuoyl-β-d-glucopyranose (4), and 1′-O-vanilloyl-β-d-glucopyranose (5)], five phenylpropanoids [caffeic acid (7), 3-O- (8), 3,5-di-O- (9), 1,5-di-O- (10), and 4,5-di-O-caffeoylquinic acid (11)], five coumarins [umbelliferone (12), aesculetin (13), aesculin (14), cichoriin (15), scopoletin (16)], and nine flavonoids [quercetin (17), isoquercitrin (18), quercetin-3-O-(6′′-acetyl)-β-d-glucopyranoside (19), rutin (20), isorhamnetin (21), isorhamnetin-3-O-β-d-glucopyranoside (22), isorhamnetin-3-O-(6′′-acetyl)-β-d-glucopyranoside (23), narcissin (24), and thyphaneoside (25)], were identified by comparing their UV, MS, and NMR data with those reported in the literature (Figure 2).
Figure 2.
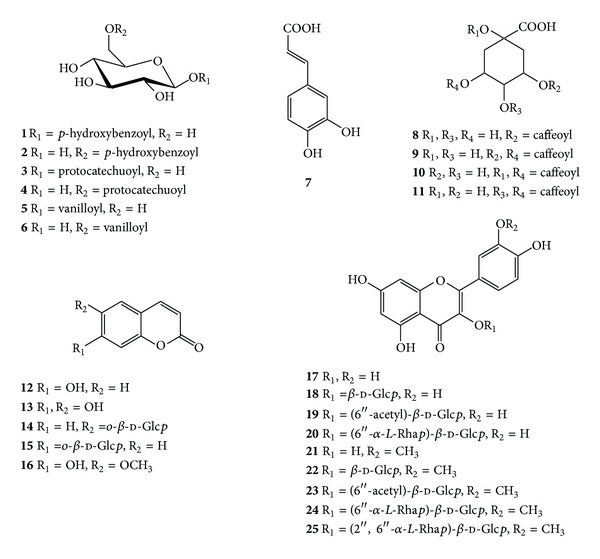
Chemical structures of compounds 1–25 isolated from C. officinalis leaves. Glcp—glucopyranose, Rhap—rhamnopyranose.
Compounds 7, 9, 18, 19, and 21–24 have been previously isolated [2, 9], while compounds 1–5, 8, 10–17, 20, and 25 are detected in C. officinalis leaves for the first time.
3.3. Structure Elucidation of 6′-O-Vanilloyl-β-d-glucopyranose
Compound 6 was isolated as a white amorphous powder. Its molecular formula, C14H18O9, was deduced from its [M + H]+ peak at 331 of positive FAB-MS and the 14 carbon signals in its 13C-NMR spectrum. In its 1H-NMR spectrum the signals of three aromatic protons were observed at δ 7.63 (1H, dd, J = 8.0, 1.9 Hz), 7.52 (1H, d, J = 1.9 Hz), and 6.80 (1H, d, J = 8.0 Hz), which are typical for 1,3,4-trisubstituted benzene rings (Table 2).
Its 13C-NMR spectrum exhibited a carbonyl group at δ 166.7 and a methoxyl group at δ 56.3. In its HMBC spectrum, the carbonyl group (δ 166.7) showed a correlation with the aromatic proton of H-6 at δ 7.63 and the aromatic carbon at δ 147.5 (C-3) correlated with methoxyl protons at δ 3.97 (Figure 3).
Figure 3.
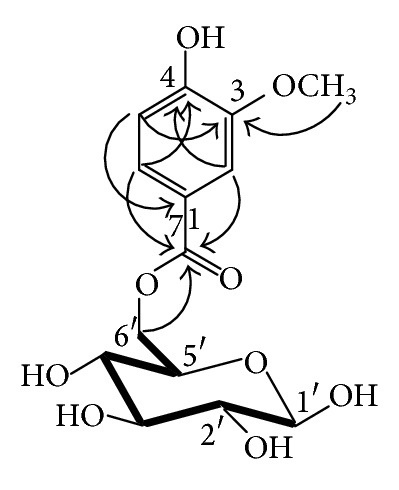
Selected 1H-1H COSY (—)correlations in HMBC spectrum (H→C) of 6.
Alkaline hydrolysis of 6 with 1 M KOH gave vanillic acid, which was identified by co-HPLC with an authentic sample and 13C-NMR. These data suggest the presence of a vanilloyl moiety in the structure of 6. The presence of d-glucose was confirmed after acidic hydrolysis followed by HPLC analysis of the hydrolysate. In addition, the remaining 6 carbon signals in its 13C-NMR spectrum are in good agreement with those for β-glucose. The linkage of the vanilloyl moiety and glucose was solved by an analysis of the HMBC spectrum. The carbonyl group (δ166.7) showed correlation with the proton at δ4.05, suggesting that the vanilloyl moiety is connected to C-6′ of the glucose moiety. Moreover, the results of the 13C-NMR analysis showed a downfield shift in the resonance of the glucose C-6′, as well as an upfield shift of the C-5′ resonance comparing with those of free glucose. Assignment of the protons and carbons signals was achieved by a combination of the HMBC and 1H-1H COSY spectral data. Since the anomeric hydroxyl group of the glucose moiety of 6 is unacylated, it may be obtained as anomers (α- and β-forms) mixture. The 13C-NMR data obtained showed a presence of a week signal at δ92.1 that corresponded to α-form of 6. However, the ratio of the peak areas for C-1′ atoms of α- (δ92.1) and β-forms (δ98.3) is about 1 : 92. Thus, we can conclude that the predominant component of mixture is β-forms (total content of α-form is no more than 1.1%). These results enabled compound 6 to be identified as 6′-O-vanilloyl-β-d-glucopyranose.
The moiety of 6 has been detected previously in different compounds like glehlinosides from Glehnia littoralis (Euphorbiaceae) [27], 6′-O-vanilloylsucrose from Saccharum officinarum (Poaceae) [28], 6′-O-vanilloyicariside B5 from Baccaurea ramiflora (Euphorbiaceae) [29], amburoside G from Amburana cearensis (Fabaceae) [30], saccharumosides from Acer saccharum (Aceraceae) [31], and 1′-O-galloyl-6′-O-vanilloyl-β-d-glucopyranose from Alchornea trewioides (Euphorbiaceae) [32]. This is the first case where 6 has been isolated as a separate compound.
3.4. HPLC-UV Analysis of the Main Phenolic Compounds in C. officinalis Leaves
A quantitative analysis of the main phenolic compounds found in C. officinalis leaves was performed using microcolumn HPLC-UV method which allowed separating 15 components (Figure 4). This method was developed previously for analysis of C. officinalis flowers [9].
Figure 4.
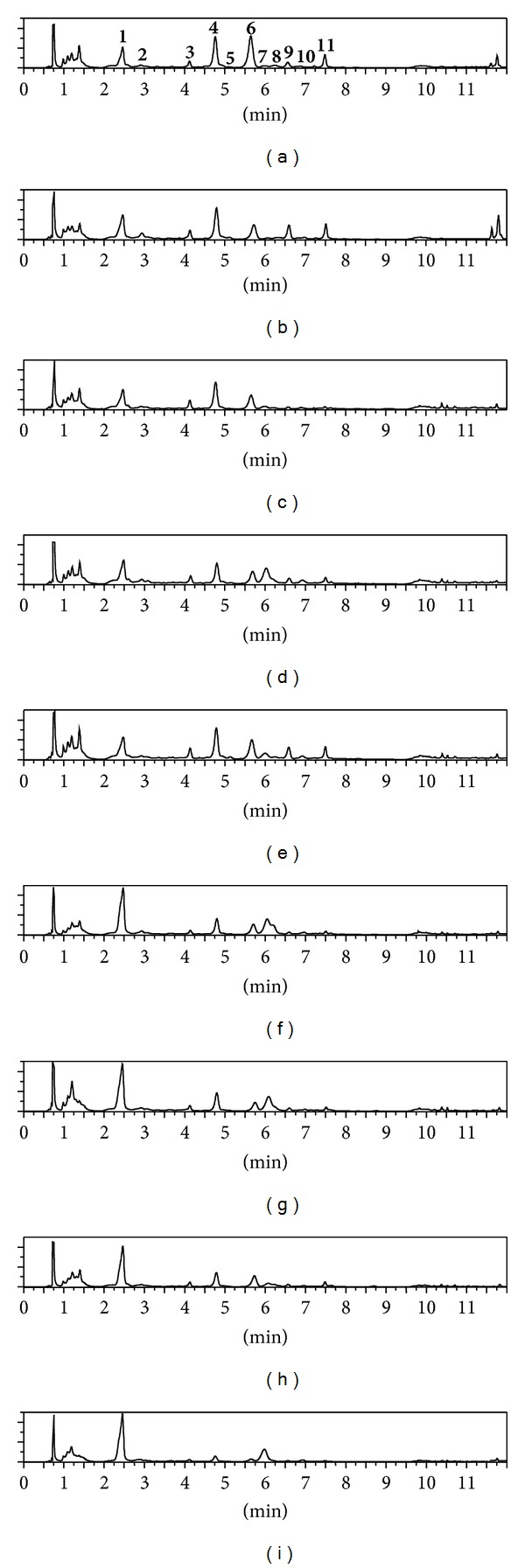
HPLC-UV chromatograms (270 nm) of ethanolic extracts from C. officinalis leaves. Varieties: (a)—“Touch of Red,” (b)—“Green Heart Orange,” (c)—“Russian Size,” (d)—“Indian Prince,” (e)—“Red Black Centered,” (f)—“Radio,” (g)—“Egypt Sun,” (h)—“Geisha,” (i)—“Flame Dance.” Compounds: 1—3-O-caffeoylquinic acid, 2—caffeic acid, 3—thyphaneoside, 4—isoquercitrin, 5—rutin, 6—quercetin-3-O-(6′′-acetyl)-β-d-glycoside, 7—3,5-di-O-caffeoylquinic acid, 8—1,5-di-O-caffeoylquinic acid, 9—isorhamnetin-3-O-β-d-glucoside, 10—4,5-di-O-caffeoylquinic acid, and 11—isorhamnetin-3-O-(6′′-acetyl)-β-d-glycoside.
According to the HPLC data, isoquercitrin—3.44 (“Indian Prince”)—5.34 mg g−1 (“Green Heart Orange”, “Red Black Centered”) was the dominant compound, as well as 3-O-caffeoylquinic acid—6.48 (“Egypt Sun”)—13.25 mg g−1 (“Flame Dance”) (Table 3). The total content of phenylpropanoids ranged from 2.58 mg g−1 in “Touch of Red” to 20.17 mg g−1 in “Flame Dance.” 3-O-caffeoylquinic acid was a major phenylpropanoid component for all species studied. Monocaffeoylquinic acids in all varieties predominated over dicaffeoylquinic acids. The concentration of caffeic acid did not exceed 0.29–1.14 mg g−1. The total content of flavonoids varied from 6.11 mg g−1 in “Flame Dance” to 15.74 mg g−1 in “Touch of Red.”
Table 3.
Content of phenolic compounds in nine varieties of C. officinalis leaves, mg g−1 from dry plant weight.
| Compound | Variety | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| “Egypt Sun” | “Flame Dance” | “Geisha” | “Green Heart Orange” | “Indian Prince” | “Radio” | “Red Black Centered” | “Russian Size” | “Touch of Red” | |
| Phenylpropanoids | |||||||||
|
| |||||||||
| Caffeic acid | 0.46 | 1.14 | 0.31 | 0.57 | 0.43 | 0.64 | 0.39 | 0.29 | 0.20 |
| 3-O-Caffeoylquinic acid | 6.48 | 13.25 | 6.59 | 2.41 | 2.35 | 8.85 | 2.31 | 1.95 | 1.73 |
| 3,5-Di-O-caffeoylquinic acid | 2.37 | 4.91 | 0.80 | 0.08 | 1.90 | 3.90 | 0.86 | 0.40 | 0.23 |
| 1,5-Di-O-caffeoylquinic acid | 0.46 | 0.33 | 0.34 | 0.10 | 0.52 | 1.53 | 0.24 | 0.19 | 0.27 |
| 4,5-Di-O-caffeoylquinic acid | 0.18 | 0.54 | 0.20 | 0.07 | 0.39 | 0.36 | 0.33 | 0.12 | 0.15 |
| Total phenylpropanoids | 9.95 | 20.17 | 8.24 | 3.23 | 5.59 | 15.28 | 4.13 | 2.95 | 2.58 |
|
| |||||||||
| Flavonoids | |||||||||
|
| |||||||||
| Quercetin derivatives | |||||||||
| Isoquercitrin | 3.93 | 2.73 | 4.32 | 5.34 | 3.44 | 5.02 | 5.34 | 4.59 | 5.14 |
| Rutin | 0.22 | 0.17 | 0.92 | 0.63 | 0.29 | 0.27 | 0.72 | 0.15 | 0.17 |
| Quercetin-3-O-(6′′-acetyl)-β-d-glycoside | 2.50 | 1.68 | 4.36 | 3.14 | 2.67 | 4.25 | 4.30 | 3.03 | 6.85 |
| Total quercetin derivatives | 6.65 | 4.58 | 9.60 | 9.11 | 6.40 | 9.54 | 10.36 | 7.77 | 12.16 |
|
| |||||||||
| Isorhamnetin derivatives | |||||||||
| Thyphaneoside | 0.78 | 0.74 | 1.01 | 1.08 | 0.92 | 0.97 | 1.37 | 2.46 | 0.73 |
| Isorhamnetin-3-O-β-d-glucoside | 0.63 | 0.44 | 0.71 | 2.41 | 0.88 | 0.22 | 2.15 | 0.36 | 1.04 |
| Isorhamnetin-3-O-(6′′-acetyl)-β-d-glycoside | 0.62 | 0.35 | 1.21 | 2.08 | 0.81 | 0.80 | 1.64 | 0.32 | 1.81 |
| Total isorhamnetin derivatives | 2.03 | 1.53 | 2.93 | 5.57 | 2.61 | 1.99 | 5.16 | 3.14 | 3.58 |
| Total flavonoids | 8.68 | 6.11 | 12.53 | 14.68 | 9.01 | 11.53 | 15.52 | 10.91 | 15.74 |
| Total identified | 18.63 | 26.28 | 20.77 | 17.91 | 14.60 | 26.81 | 19.65 | 13.86 | 18.32 |
| Quercetin/isorhamnetin ratio | 3.28 | 2.99 | 3.28 | 1.64 | 2.45 | 4.79 | 2.01 | 2.48 | 3.40 |
| Phenylpropanoid/flavonoid ratio | 1.15 | 3.30 | 0.66 | 0.22 | 0.62 | 1.33 | 0.27 | 0.27 | 0.16 |
The content of quercetin derivatives was higher than the content of derivatives of isorhamnetin, ranging from 1.64- (“Green Heart Orange”) to 4.79-fold (“Radio”). It should be noted that isoquercitrin dominated in almost all species except “Geisha” and “Touch of Red” where quercetin-3-O-(6′′-acetyl)-β-d-glycoside prevailed. Thyphaneoside in “Egypt Sun,” “Flame Dance,” “Indian Prince,” “Radio,” “Russian Size,” isorhamnetin-3-O-β-d-glucoside in “Green Heart Orange” and “Red Black Centered,” and isorhamnetin-3-O-(6′′-acetyl)-β-d-glycoside in “Geisha” and “Touch of Red” are all simple derivatives of isorhamnetin.
According to the data on the phenylpropanoids, flavonoid ratio, C. officinalis varieties studied can be divided into three groups:
varieties with a predominant content of flavonoids (value < 0.5)—“Touch of Red,” “Green Heart Orange,” “Red Black Centered” and “Russian Size”;
varieties with similar contents of phenylpropanoids and flavonoids (value 0.5–1.5)—“Radio,” “Egypt Sun,” “Geisha” and “Indian Prince”;
varieties with a predominance of phenylpropanoids (value > 1.5)—“Flame Dance.”
3.5. Amylase Inhibiting Activity of C. officinalis Phenolic Compounds
All substances isolated were tested for their amylase inhibiting activity by the microplate method. Compounds 7, 13, 17, and 21 showed significantly higher inhibitory activity at concentration ranging 1.02–2.64 μg mL−1 comparing with the reference compound acarbose (9.54 μg mL−1) (Table 4). It should be noted that concentrations of the mentioned compounds in C. officinalis leaves are insignificant; therefore, they cannot be considered as an active substances. Comparative analysis of the inhibitory activities of the dominant compounds showed that the actual antiamylase components in the crude extracts were 18 (isoquercitrin; 15.45 μg mL−1), 22 (isorhamnetin-3-O-β-d-glucopyranoside; 23.41 μg mL−1), 9 (3,5-di-O-caffeoylquinic acid; 25.63 μg mL−1), and 19 (quercetin-3-O-(6′′-acetyl)-β-d-glucopyranoside; 32.70 μg mL−1).
Table 4.
Amylase inhibiting activity of individual phenolic compounds.
| Compound | IC50 (μg mL−1) |
|---|---|
| 1 | >100 |
| 2 | >100 |
| 3 | 62.46 |
| 4 | 66.52 |
| 5 | 73.69 |
| 6 | 79.18 |
| 7 | 2.53 |
| 8 | 65.42 |
| 9 | 25.63 |
| 10 | 37.15 |
| 11 | 26.65 |
| 12 | >100 |
| 13 | 1.02 |
| 14 | >100 |
| 15 | >100 |
| 16 | ~100 |
| 17 | 1.79 |
| 18 | 15.45 |
| 19 | 32.70 |
| 20 | 89.85 |
| 21 | 2.64 |
| 22 | 23.41 |
| 23 | ~100 |
| 24 | >100 |
| 25 | >100 |
| Acarbosea | 9.54 |
aReference compounds.
It is known that the phenolic phytosubstances are the perspective natural compounds which exhibited antidiabetic activity [33]. Flavonoids and phenylpropanoids demonstrate a high inhibitory activity on amylase and glucosidase, the key enzymes of carbohydrates digestion process [34]. Some caffeoylquinic acids (chlorogenic acid, isochlorogenic acid) and derivatives of quercetin and isorhamnetin, such as rutin (quercetin-3-O-rutinoside) and narcissin (isorhamnetin-3-O-rutinoside), have been previously reported as inhibitors of glucosidase [35]. The results of our experiments seemed to agree with this finding, probably due to the presence of large amounts of phenolic compounds in C. officinalis leaves.
The present investigation is the first extended study of the phenolic composition from C. officinalis leaves. As a result, twenty-five compounds were isolated, of which sixteen had not previously been reported in this plant source. The presence of undiscovered benzoic acid glucosides early in the Calendula genus, one of which was novel (6′-O-vanilloyl-β-d-glucopyranose), as well as the amylase inhibiting activity of the crude extract and individual compounds, illustrates the need for in-depth research even on very familiar species such as C. officinalis.
Acknowledgment
The authors acknowledge the financial support provided by the Presidium of the Siberian Division of the Russian Academy of Science under the Program “New Medical Technology Centers.”
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Khalid KA, Teixeira da Silva JA. Biology of Calendula officinalis Linn.: focus on pharmacology, biological activities and agronomic practices. Medicinal and Aromatic Plant Science and Biotechnology. 2012;6(1):12–27. [Google Scholar]
- 2.Friedrich H. Untersuchungen über die Isorhamnetinglykoside aus den Blüten von Calendula officinalis L. Archiv der Pharmazie. 1962;295(6):464–471. [Google Scholar]
- 3.Ercetin T, Senol FS, Erdogan Orhan I, Toker G. Comparative assessment of antioxidant and cholinesterase inhibitory properties of the marigold extracts from Calendula arvensis L. and Calendula officinalis L. Industrial Crops and Products. 2012;36(1):203–208. [Google Scholar]
- 4.Bakó E, Deli J, Tóth G. HPLC study on the carotenoid composition of Calendula products. Journal of Biochemical and Biophysical Methods. 2002;53(1-3):241–250. doi: 10.1016/s0165-022x(02)00112-4. [DOI] [PubMed] [Google Scholar]
- 5.Adler G, Kasprzyk Z. Free sterols, steryl esters, glucosides, acylated glucosides and water-soluble complexes in Calendula officinalis . Phytochemistry. 1975;14(3):627–631. [Google Scholar]
- 6.Szakiel A, Ruszkowski D, Grudniak A, et al. Antibacterial and antiparasitic activity of oleanolic acid and its glycosides isolated from marigold (Calendula officinalis) Planta Medica. 2008;74(14):1709–1715. doi: 10.1055/s-0028-1088315. [DOI] [PubMed] [Google Scholar]
- 7.Okoh OO, Sadimenko AP, Asekun OT, Afolayan AJ. The effects of drying on the chemical components of essential oils of Calendula officinalis L. African Journal of Biotechnology. 2008;7(10):1500–1502. [Google Scholar]
- 8.Ul’chenko NT, Glushenkova AI, Mukhamedova KS. Lipids of Calendula officinalis . Chemistry of Natural Compounds. 1998;34(3):272–274. [Google Scholar]
- 9.Olennikov DN, Kashchenko NI. New isorhamnetin glucosides and other phenolic compounds from Calendula officinalis’ . Chemistry of Natural Compounds. 2013;49(5):717–723. [Google Scholar]
- 10.Yoshikawa M, Murakami T, Kishi A, Kageura T, Matsuda H. Medicinal flowers. III. Marigold. (1): Hypoglycemic, gastric emptying inhibitory, and gastroprotective principles and new oleanane-type triterpene oligoglycosides, calendasaponins A, B, C, and D, from Egyptian Calendula officinalis . Chemical and Pharmaceutical Bulletin. 2001;49(7):863–870. doi: 10.1248/cpb.49.863. [DOI] [PubMed] [Google Scholar]
- 11.Rhabasa-Lhoret R, Chiasson JL. Alpha-glucosidase inhibitors. In: Defronzo RA, Ferrannini E, Keen H, Zimmet P, editors. International Textbook of Diabetes Mellitus. Vol. 1. John Wiley, UK: 2004. [Google Scholar]
- 12.Olennikov DN, Rokhin AV, Tankhaeva LM. Lamiaceae carbohydrates. VI. Water-soluble polysaccharides from Lophanthus chinensis . Chemistry of Natural Compounds. 2009;45(3):300–303. [Google Scholar]
- 13.Olennikov DN, Agafonova SV, Rokhin AV, Penzina TA, Borovskii GB. Branched glucan from the fruiting bodies of Piptoporus betulinus (Bull.:Fr) Karst. Applied Biochemistry and Microbiology. 2012;48(1):65–70. [PubMed] [Google Scholar]
- 14.Shetty K, Curtis OF, Levin RE, Witkowsky R, Ang W. Prevention of vitrification associated with in vitro shoot culture of oregano (Origanum vulgare) by Pseudomonas spp. Journal of Plant Physiology. 1995;147(3-4):447–451. [Google Scholar]
- 15.Chirikova NK, Olennikov DN, Tankhaeva LM. Quantitative determination of flavonoid content in the aerial part of Baical scullcap (Scutellaria baicalensis Georgi) Russian Journal of Bioorganic Chemistry. 2010;36(7):915–922. [Google Scholar]
- 16.Olennikov DN, Tankhaeva LM. Biologically active substances from Cacalia hastata leaves. 5. Coumarins and triterpenes. Chemistry of Natural Compounds. 2005;41(5):600–601. [Google Scholar]
- 17.Olennikov DN, Tankhaeva LM, Agafonova SV. Antioxidant components of Laetiporus sulphureus (Bull.: Fr.) Murr. fruit bodies. Applied Biochemistry and Microbiology. 2011;47(4):419–425. [PubMed] [Google Scholar]
- 18.Olennikov DN, Tankhaeva LM, Stolbikova AV, Petrov EV. Phenylpropanoids and polysaccharides from Plantago depressa and P. media growing in Buryatia. Chemistry of Natural Compounds. 2011;47(2):165–169. [Google Scholar]
- 19.Satake T, Kamiya K, An Y, Oishi T, Yamamoto J. The anti-thrombotic active constituents from Centella asiatica . Biological and Pharmaceutical Bulletin. 2007;30(5):935–940. doi: 10.1248/bpb.30.935. [DOI] [PubMed] [Google Scholar]
- 20.Malikov VM, Saudkhodzhaev AI. Coumarins: plants, structures, properties. Chemistry of Natural Compounds. 1998;34(5):517–548. [Google Scholar]
- 21.Olennikov DN, Partilkhaev VV. Isolation and densitometric HPTLC analysis of rutin, narcissin, nicotiflorin, and isoquercitrin in Caragana spinosa shoots. Journal of Planar Chromatography. 2012;25(1):30–35. [Google Scholar]
- 22.Wang YB, Pu JX, Ren HY, et al. New acetylated flavonol glycosides from Knoxia corymbosa . Chinese Chemical Letters. 2003;14(12):1268–1270. [Google Scholar]
- 23.Du Q, Xu Y, Li L, Zhao Y, Jerz G, Winterhalter P. Antioxidant constituents in the fruits of Luffa cylindrica (L.) Roem. Journal of Agricultural and Food Chemistry. 2006;54(12):4186–4190. doi: 10.1021/jf0604790. [DOI] [PubMed] [Google Scholar]
- 24.Olennikov DN, Zilfikarov IN, Khodakova SE. Phenolic compounds from Serenoa repens fruit. Chemistry of Natural Compounds. 2013;49(3):526–529. [Google Scholar]
- 25.Choi JW, Kim KH, Lee IK, Choi SU, Lee KR. Phytochemical constituents of Amomum xanthioides . Natural Product Sciences. 2009;15(1):44–49. [Google Scholar]
- 26.Vidal-Ollivier E, Elias R, Faure F, et al. Flavonol glycosides from Calendula officinalis flowers. Planta Medica. 1989;55(1):73–74. doi: 10.1055/s-2006-961831. [DOI] [PubMed] [Google Scholar]
- 27.Yuan Z, Tezuka Y, Fan W, Kadota S, Li X. Constituents of the underground parts of Glehnia littoralis . Chemical and Pharmaceutical Bulletin. 2002;50(1):73–77. doi: 10.1248/cpb.50.73. [DOI] [PubMed] [Google Scholar]
- 28.Takara K, Matsui D, Wada K, Ichiba T, Nakasone Y. New antioxidative phenolic glycosides isolated from Kokuto non-centrifuged cane sugar. Bioscience, Biotechnology and Biochemistry. 2002;66(1):29–35. doi: 10.1271/bbb.66.29. [DOI] [PubMed] [Google Scholar]
- 29.Yang X-W, He H-P, Ma Y-L, et al. Three new vanilloid derivatives from the stems of baccaurea ramiflora. Planta Medica. 2010;76(1):88–90. doi: 10.1055/s-0029-1185901. [DOI] [PubMed] [Google Scholar]
- 30.Canuto KM, Lima MAS, Silveira ER. Amburosides c-h and 6-o-protocatechuoyl coumarin from Amburana cearensis . Journal of the Brazilian Chemical Society. 2010;21(9):1746–1753. [Google Scholar]
- 31.Yuan T, Wan C, González-Sarrías A, Kandhi V, Cech NB, Seeram NP. Phenolic glycosides from sugar maple (Acer saccharum) bark. Journal of Natural Products. 2011;74(11):2472–2476. doi: 10.1021/np200678n. [DOI] [PubMed] [Google Scholar]
- 32.Quin RD, Cheng W, Zhang QY, Liang H. Phenolic acid derivatives from Alchornea trewioides . Acta Pharmaceutica Sinica. 2012;47(7):926–929. [PubMed] [Google Scholar]
- 33.Xiao J, Ni X, Kai G, Chen X. A review on structure-activity relationship of dietary polyphenols inhibiting α-amylase. Critical Reviews in Food Science and Nutrition. 2013;53(5):497–506. doi: 10.1080/10408398.2010.548108. [DOI] [PubMed] [Google Scholar]
- 34.Bahadoran Z, Mirmiran P, Azizi F. Dietary polyphenols as potential nutraceuticals in management of diabetes: a review. Journal of Diabetes and Metabolic Disorders. 2013;12(43) doi: 10.1186/2251-6581-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Sales PM, de Souza PM, Simeoni LA, Magalhães PDO, Silveira D. α-amylase inhibitors: a review of raw material and isolated compounds from plant source. Journal of Pharmacy and Pharmaceutical Sciences. 2012;15(1):141–183. doi: 10.18433/j35s3k. [DOI] [PubMed] [Google Scholar]


