Summary
Preterm birth is the leading cause of perinatal morbidity and mortality worldwide, and its prevention is an important healthcare priority. Preterm parturition is one of the ‘great obstetrical syndromes’ and is caused by multiple etiologies. One of the mechanisms of disease is the untimely decline in progesterone action, which can be manifested by a sonographic short cervix in the midtrimester. The detection of a short cervix in the midtrimester is a powerful risk factor for preterm delivery. Vaginal progesterone can reduce the rate of preterm delivery by 45%, and the rate of neonatal morbidity (admission to neonatal intensive care unit, respiratory distress syndrome, need for mechanical ventilation, etc.). To prevent one case of spontaneous preterm birth <33 weeks of gestation, 12 patients with a short cervix would need to be treated. Vaginal progesterone reduces the rate of spontaneous preterm birth in women with a short cervix both with and without a prior history of preterm birth. In patients with a prior history of preterm birth, vaginal progesterone is as effective as cervical cerclage to prevent preterm delivery. 17α-Hydroxyprogesterone caproate has not been shown to be effective in reducing the rate of spontaneous preterm birth in women with a short cervix.
Keywords: Cervical cerclage, Cervical length, Cervical ultrasound, Short cervix, Ultrasound, Vaginal progesterone
Introduction
Preterm birth (<37 weeks of gestation) is the leading cause of perinatal morbidity and mortality worldwide, and affects 5–18% of all pregnancies.1 In 2009, 13 million neonates were born preterm: 11 million in Africa and Asia and 500 000 in the USA.2 The highest rates of preterm birth are in Africa (11.9%) and North America (10.6%).2 Short- and long-term complications of preterm birth are well known to obstetricians and pediatricians.3–10 The financial cost of preterm birth has been estimated to be $26 billion per year in the USA alone.11 A less-appreciated burden of preterm birth is borne by families caring for preterm infants.
The challenge of the prediction and prevention of preterm birth has been difficult to address. Recent developments suggest that it is possible to identify a subset of patients at risk for preterm delivery, and to prevent this adverse pregnancy outcome with the use of progestogens. In this article, we review an important conceptual framework about spontaneous preterm birth – specifically, the role of progestogens to prevent preterm delivery.
Defining a medical disorder (preterm birth) on the basis of age alone: a pitfall
Preterm birth is defined by the gestational age at which it occurs (conventionally <37 weeks of gestation); yet, age is an unusual way of defining disease in medicine.3 The norm is to identify pathologic conditions associated with discreet symptoms and signs caused by specific mechanisms of disease.3 For example, Mycobacterium tuberculosis is able to induce lung inflammation (pneumonia), which is clinically manifested by fever, a cough, expectoration, etc., and can be cured with the administration of antibiotics. Using age to define a medical condition or disease state recognizes only one of its problems, namely, that the greater the organ immaturity at the time of birth, the higher the risk of death and short- and long-term complications.3 However, the age at birth, by itself, is not informative as to why preterm birth occurred. The causes of preterm birth have important implications for the prognosis of the newborn.12–20
Similarly, at the other end of the life spectrum (i.e. geriatrics), the older an individual, the more likely it is that he/she will have a disease state (secondary to senescence); yet, disease is not defined purely on the basis of age.3 An elderly individual is treated differently if the cause of the symptoms (e.g. cough) is cancer, congestive heart failure, or pneumonia. One of the issues impeding progress in the prevention of preterm birth is the failure to consider the specific causes responsible for this condition, thereby enabling meaningful prevention.21
Preterm birth is not a single condition
Two-thirds of preterm births occur because women go into spontaneous labor, with intact or ruptured membranes; the other third results from indicated preterm deliveries for potentially life-threatening conditions (e.g. pre-eclampsia) or fetal complications (e.g. intrauterine growth restriction).3 The complexity of the problem extends further – spontaneous preterm labor, prelabor rupture of membranes (PROM), pre-eclampsia, and intrauterine growth restriction are all syndromes caused by multiple etiologies. We have coined the term ‘great obstetrical syndromes’ to reframe the concept of obstetrical disease.21–23 Such syndromes are characterized by: (i) multiple etiologies; (ii) a long preclinical stage; (iii) frequent fetal involvement; (iv) clinical manifestations that are often adaptive in nature; and (v) gene–environment interactions that may predispose to the syndromes.21–23
Preterm parturition syndrome
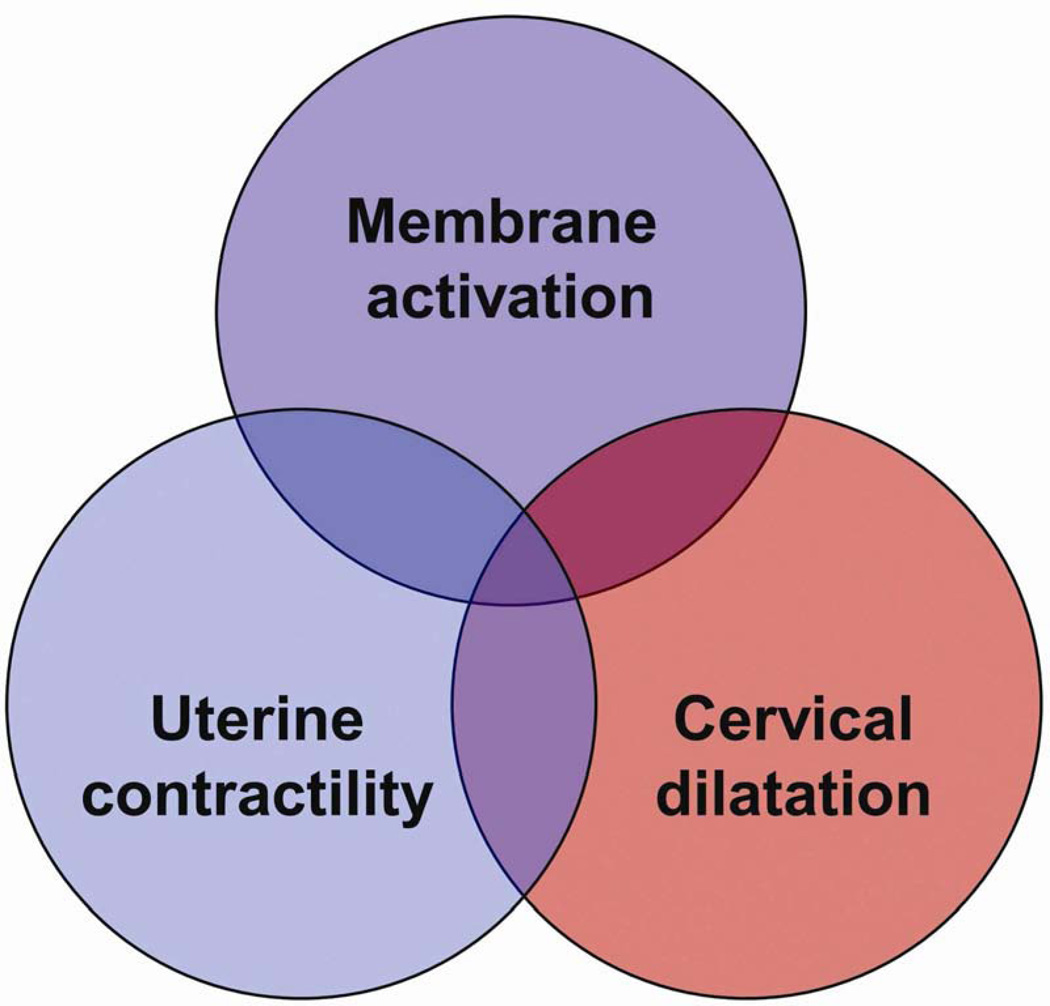
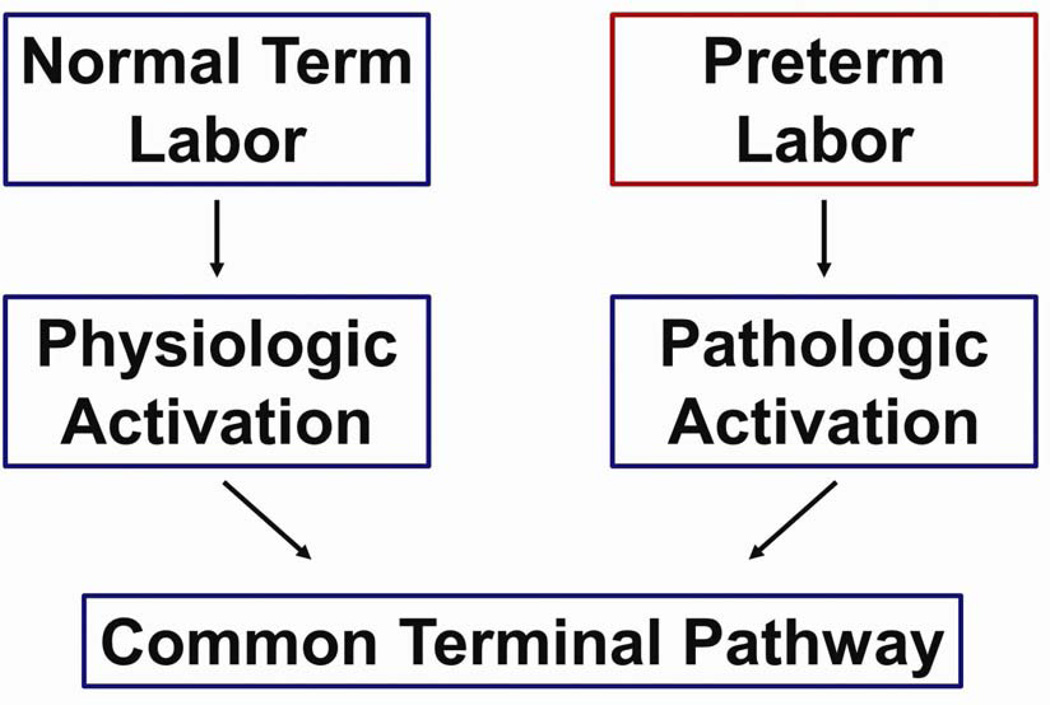
We have proposed that preterm labor is a syndrome characterized by activation of the common pathway of parturition, which we defined as the anatomical, biochemical, endocrinologic, and clinical events that occur in term and preterm parturition.21,24,25 The uterine components of the common pathway include: (i) increased uterine contractility; (ii) cervical ripening; and (iii) decidual membrane activation (Fig. 1).21,24,25 A crucial difference between term and preterm labor is that the former represents ‘physiologic activation of the common pathway’, whereas the latter represents a pathologic process (‘pathologic activation that extemporaneously activates components of the common pathway’) (Fig. 2).24–26
Figure 1.
Uterine components of the common pathway of parturition. Reproduced with permission from Romero et al.24
Figure 2.
Normal spontaneous labor at term results from physiologic activation of the common pathway of parturition. By contrast, preterm labor begins because of a pathologic insult, resulting in the initiation of labor. Reproduced with permission from Romero et al.25
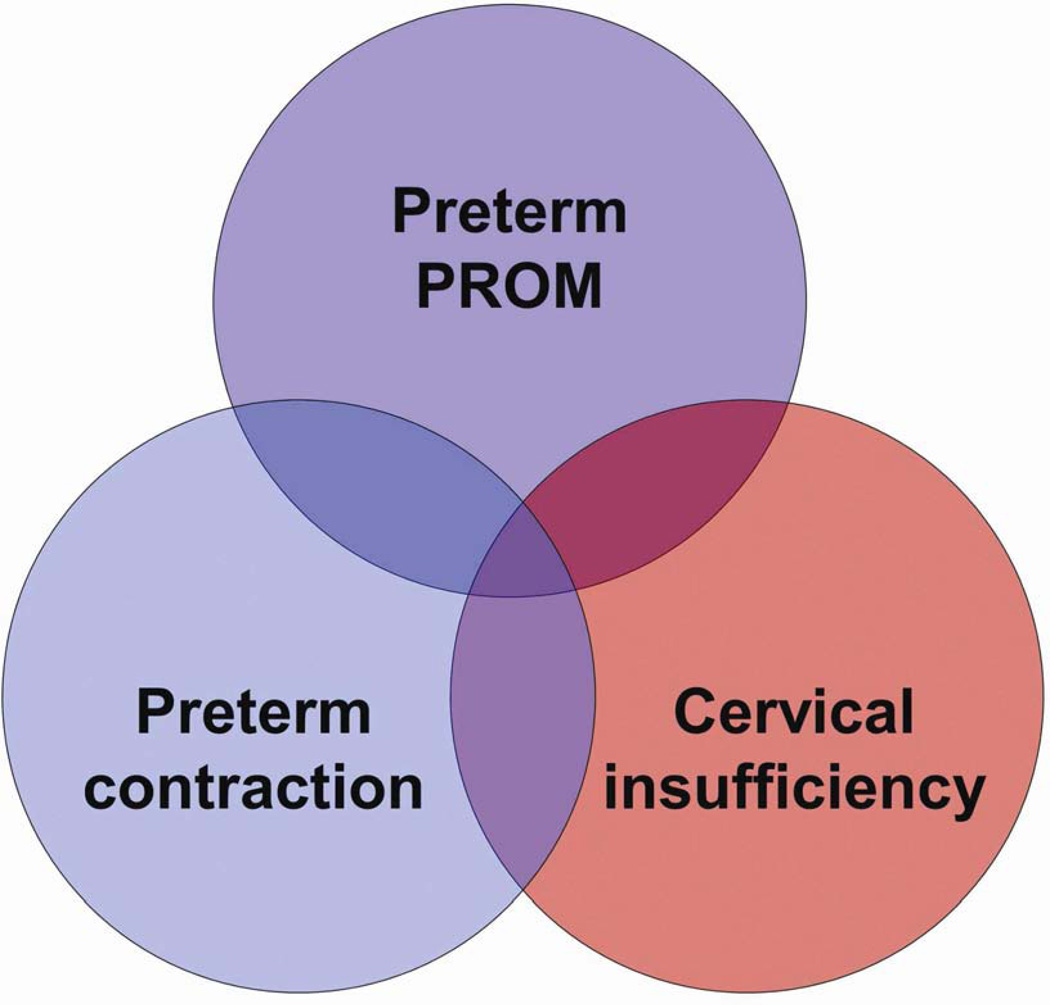
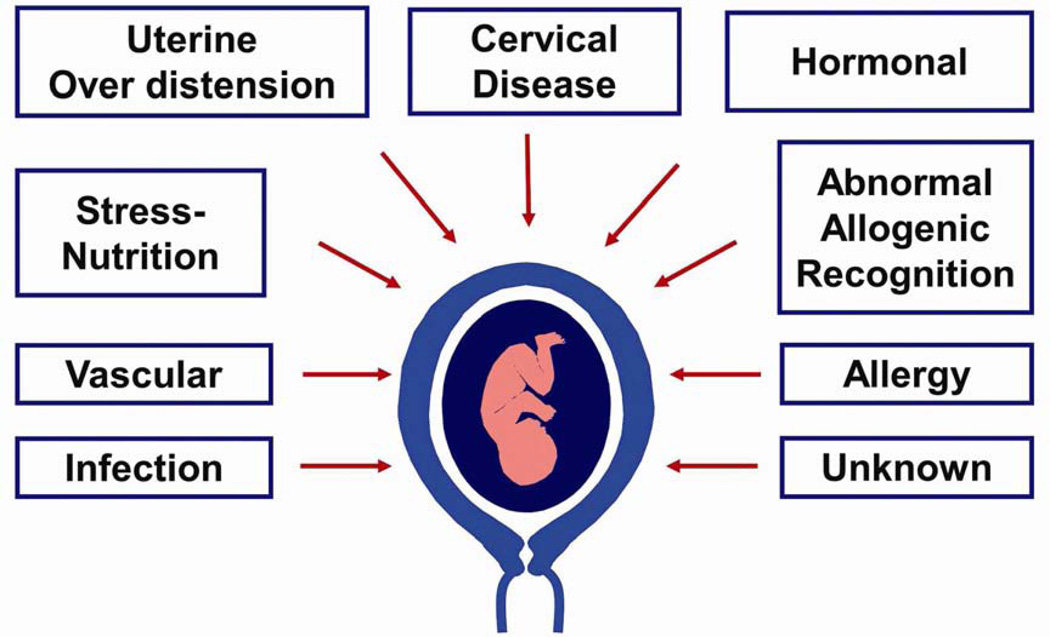
Activation of the different uterine components of the common pathway of parturition may be synchronous or asynchronous.27 Synchronous activation results in clinical spontaneous preterm labor, whereas asynchronous results in a different clinical presentation (referred to, by some, as a phenotype). For instance, predominant activation of the membranes would lead to preterm prelabor rupture of membranes (PPROM), of the cervix to cervical insufficiency, and of the myometrium to increased preterm uterine contractions (Fig. 3).24 The activation of each component confers a different risk for impending preterm delivery. For example, rupture of membranes is followed by the onset of labor, in most cases, within a short period of time. By contrast, most patients who present with increased uterine contractility at an early gestational age deliver at term. Acute cervical insufficiency (formerly called ‘cervical incompetence’) may lead to a late spontaneous abortion or early preterm delivery within days or weeks after diagnosis.25,28–31 An isolated short cervix in the midtrimester is an example of asynchronous activation of the common pathway of parturition, because in general, patients do not have increased uterine contractility or evidence of ruptured membranes. The mechanisms of disease responsible for the preterm parturition syndrome are shown in Fig. 4,32 and the evidence in support of this has been reviewed elsewhere.21
Figure 3.
Clinical manifestations of preterm activation of the common pathway of parturition. PROM, prelabor rupture of membranes. Reproduced with permission from Romero et al.24
Figure 4.
Pathological processes implicated in the preterm parturition syndrome. Reproduced with permission from Romero et al.32
Progesterone: a key hormone for pregnancy maintenance
Progesterone was discovered as a hormone produced by the corpus luteum, essential for pregnancy maintenance.33–39 Removal of the ovary with the corpus luteum in the first trimester of pregnancy leads to spontaneous abortion,40 unless progesterone is replaced. The name of the hormone reflects this understanding (‘pro’: in favor; ‘-gest’: gestation; ‘-one’: ketone chemical structure). In most mammalian species, progesterone concentrations in peripheral blood decrease before the onset of labor at term – a progesterone withdrawal.41–49 Nonetheless, this does not occur in humans, and therefore, the role of progesterone in pregnancy maintenance beyond the first trimester has been controversial.41,43,46–48,50–55 However, an important concept is that subsets of women who have a preterm delivery also have a progesterone deficiency, which is largely subclinical in nature but can be sonographically detected by a midtrimester short cervix.3 The basis for this hypothesis is that the administration of progesterone receptor antagonists (e.g. RU-486) any time in gestation leads to cervical ripening (which includes shortening), and sometimes, the onset of labor.56–61 For these reasons, it has been proposed that a progesterone deficiency can be corrected by the administration of this hormone in close anatomic proximity to the cervix.
Sonographic short cervix: a risk factor for preterm delivery
Many studies have now provided compelling evidence that women with a short cervix, detected by transvaginal ultrasound in the midtrimester of pregnancy (18–24 weeks), are at risk for spontaneous preterm delivery.28,62–69 The cervix should be longer than 30 mm during normal pregnancy. Women with a cervix of ≤15 mm have a 50% chance of preterm delivery at <33 weeks of gestation.66 The shorter the cervix, the greater the risk of preterm delivery. It is possible to estimate the individualized risk of preterm delivery based upon cervical length in the midtrimester, a prior history of preterm birth, and other maternal characteristics.70 However, sonographic cervical length is not a screening test for spontaneous preterm delivery, since only a fraction of patients (<60%) who will have a spontaneous preterm birth have a short cervix in the midtrimester. Yet, sonographic cervical length is a method for risk assessment for spontaneous preterm delivery. It is the single most powerful predictor for preterm birth in the index pregnancy,66,71 and is far more informative than a history of prior preterm birth.66,72,73
Vaginal progesterone to prevent preterm birth: randomized clinical trials
The first randomized clinical trial (RCT) to examine the effects of vaginal progesterone on the prevention of preterm birth in women with a short cervix was reported by da Fonseca et al.74 on behalf of the Fetal Medicine Foundation Second Trimester Screening Group of the United Kingdom. In this trial, women with a short cervix (defined as ≤15 mm by transvaginal ultrasound) between 20 and 25 weeks of gestation were allocated to receive either vaginal progesterone (200 mg of micronized progesterone) or placebo (safflower oil). The duration of treatment was from 24 to 34 weeks of gestation. The primary outcome of the trial was the frequency of spontaneous preterm delivery at <34 weeks of gestation. Patients allocated to receive vaginal progesterone had a lower rate of preterm delivery than those in the placebo group [19.2% (24/125) vs 34.4% (43/125)]. The rate of adverse events was similar. The trial was not designed to test whether progesterone administration could reduce neonatal morbidity, and such a reduction was not observed. Twins were included in this trial; however, the number of twin gestations was small.
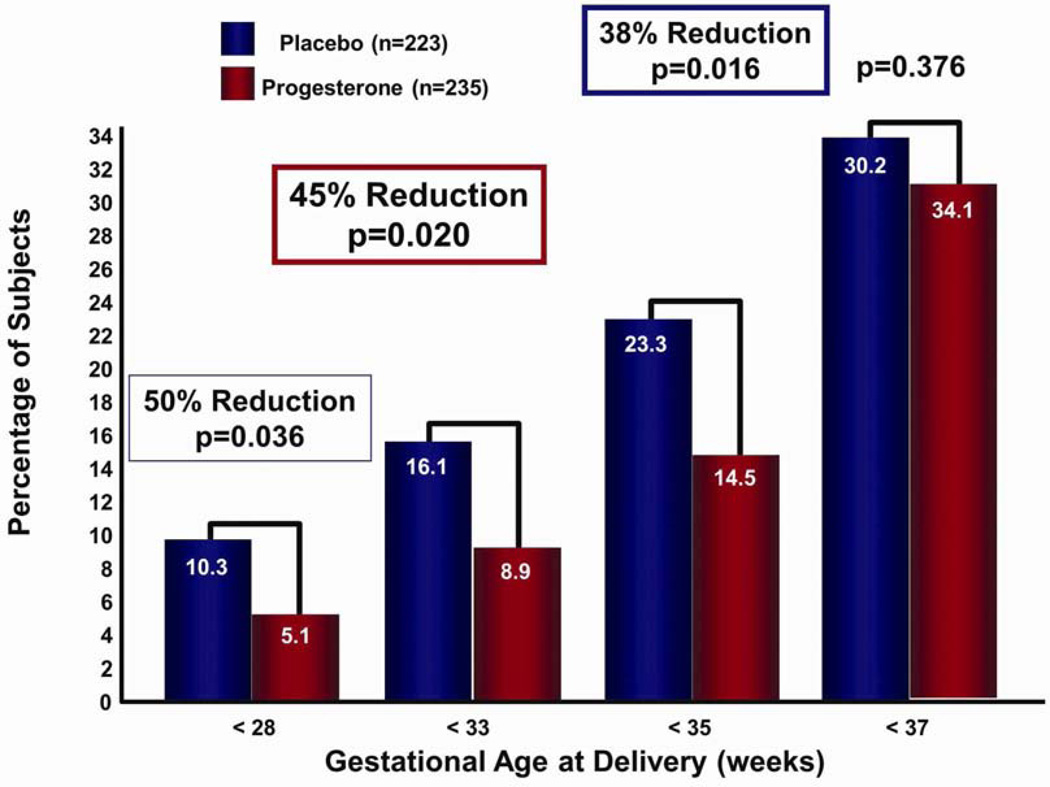
The second trial designed to determine the effects of vaginal progesterone on the rate of preterm birth in women with a sonographic short cervix was the PREGNANT trial.75 This was a multicenter, randomized, double-blind, placebo-controlled trial that enrolled asymptomatic women with a singleton gestation and a sonographic short cervix (10–20 mm) at 19–236/7 weeks of gestation. Patients were randomly allocated to receive a vaginal progesterone gel (90 mg) vs placebo daily, starting between 20 and 236/7 weeks of gestation until 366/7 weeks of gestation, rupture of membranes, or delivery, whichever occurred first. The primary endpoint was preterm birth before 33 weeks of gestation. Of the women randomized, 458 were available for analysis. Patients allocated to receive vaginal progesterone had a significantly lower rate of preterm birth before 33 weeks of gestation than those allocated to placebo [8.9% vs 16.1%; relative risk (RR): 0.55; 95% confidence interval (CI): 0.33–0.92; P = 0.02 (when adjusted for pooled study site and a history of previous preterm birth, RR: 0.54; 95% CI: 0.33–0.89; P = 0.01)]. It was estimated that 14 women with a cervical length between 10 and 20 mm would need to be treated with vaginal progesterone to prevent one case of preterm birth before 33 weeks of gestation. In addition, there was a significant decrease in the rate of preterm delivery <35 and <28 weeks of gestation (Fig. 5). Importantly, neonates born to mothers allocated to receive vaginal progesterone gel had a significantly lower frequency of respiratory distress syndrome (RDS) than those allocated to placebo (3% vs 7.6%; RR: 0.39; 95% CI: 0.17–0.92; P = 0.03). The number of patients needed to treat to prevent one case of RDS was 22. The reduction in RDS remained significant after adjusting for pooled study site and a history of preterm birth (RR: 0.40; 95% CI: 0.17–0.94; P = 0.03). Frequencies of other neonatal adverse outcomes were not statistically significant. Adverse events were similar, and there was no evidence of a potential safety signal.
Figure 5.
In women with a short cervix, those receiving vaginal progesterone (vs placebo) had a significant decrease in the rate of preterm delivery <28, <33, and <35 weeks of gestation. Reproduced with permission from Hassan et al.75
An individual patient meta-analysis of vaginal progesterone in women with a short cervix to prevent preterm birth
An individual patient meta-analysis is a specific type of systematic review in which original research data from each participant in a study are obtained directly from the investigators.76 This method is considered the ‘gold standard’ to summarize evidence across clinical trials, since it offers several advantages (both statistically and clinically) over conventional meta-analyses that use aggregated data.77
An individual patient meta-analysis was recently published. The primary endpoint was to determine whether the use of vaginal progesterone in asymptomatic women with a short cervix in the midtrimester (≤25 mm) reduces the rate of preterm birth, and improves neonatal morbidity and mortality.78 The pre-specified primary outcome was preterm birth <33 weeks of gestation. Secondary outcomes included: preterm birth at <37, <36, <35, <34, <30, and <28 weeks of gestation; RDS; birth weight <1500 g; admission to the neonatal intensive care unit (NICU); and use of mechanical ventilation. Perinatal morbidity/mortality (secondary outcome measure) was assessed using a composite outcome, which was defined as the occurrence of any of the following events: RDS, intraventricular hemorrhage, necrotizing enterocolitis, proven neonatal sepsis, or neonatal death.78
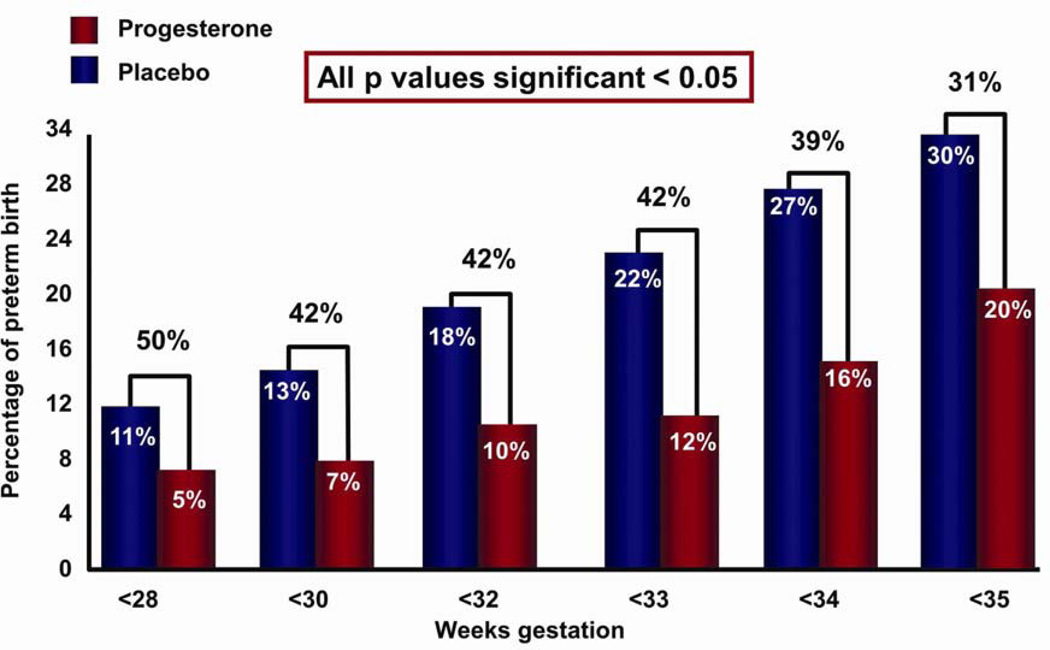
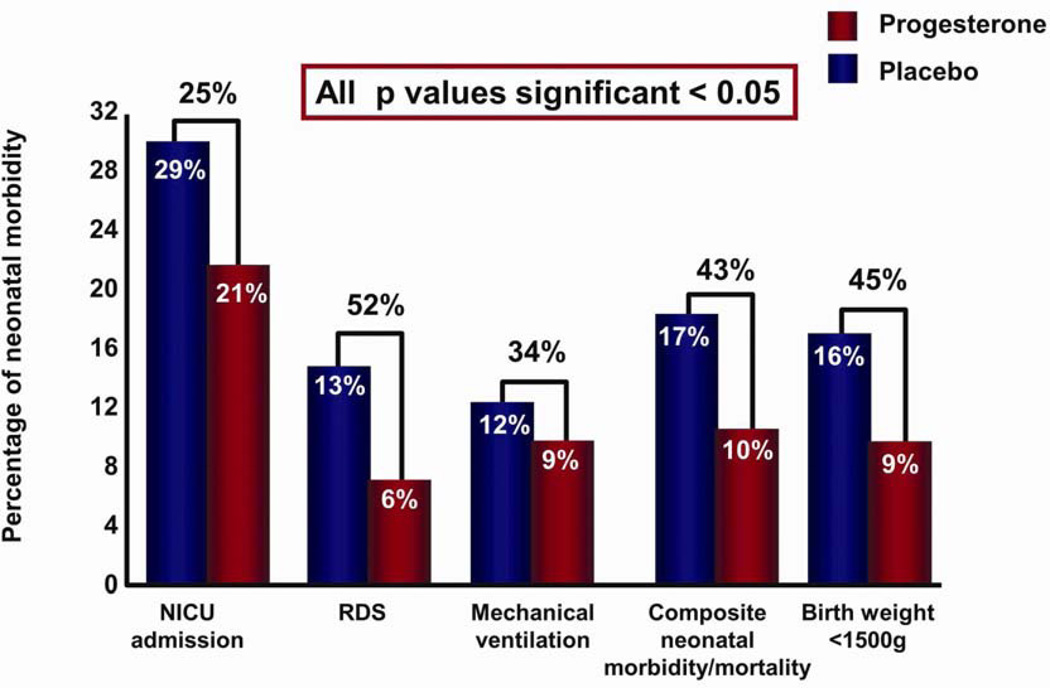
Five high-quality studies were included with a total of 775 women and 827 infants.74,75,79–81 Treatment with vaginal progesterone was associated with a significant reduction in the rate of preterm birth <33 weeks of gestation (RR: 0.58; 95% CI: 0.42–0.80).78 Vaginal progesterone treatment was also associated with a significant reduction in the rate of preterm birth <35 weeks of gestation (0.69; 0.55–0.88), <28 weeks of gestation (0.50; 0.30–0.81) (Fig. 6), RDS (0.48; 0.30–0.76), birth weight <1500 g (0.55; 0.38–0.80), admission to NICU (0.75; 0.59–0.94), requirement for mechanical ventilation (0.66; 0.44–0.98), and composite neonatal morbidity and mortality (0.57; 0.40–0.81) (Fig. 7).78
Figure 6.
Patients with a short cervix allocated to receive vaginal progesterone (vs placebo) had a significantly lower risk in the rate of preterm birth <28, <33, and <35 weeks of gestation. Reproduced with permission from Romero et al.78
Figure 7.
Infants whose mothers (with a short cervix) received vaginal progesterone (vs placebo) had a significantly lower risk of respiratory distress syndrome, composite neonatal morbidity and mortality, birthweight <1500 g, admission to neonatal intensive care unit, and requirement for mechanical ventilation. Reproduced with permission from Romero et al.78
Subgroup analysis on the effect of vaginal progesterone was only performed for the primary outcome of preterm birth <33 weeks of gestation, and for the secondary outcome of composite neonatal morbidity and mortality. The following results have clinical implications.78
A daily dosage of 90–100 mg of progesterone was equivalent to a daily dosage of 200 mg in both the reduction of preterm birth and composite neonatal morbidity and mortality.
Vaginal progesterone was equally effective in women having a short cervix both without a history of prior preterm birth and those with a history of prior preterm birth, in reducing preterm birth <33 weeks of gestation and composite neonatal morbidity and mortality.
No differences were shown in the effect of progesterone as a function of cervical length in women with a short cervix (<25 mm) for the prevention of preterm birth or reduction of neonatal morbidity and mortality (as determined by a test of interaction).
Altogether, the evidence suggests that vaginal progesterone prevents preterm delivery at <33 weeks of gestation in women with a midtrimester short cervix, and this is also associated with a reduction in neonatal morbidity. Based upon the results of the individual patient meta-analysis,78 the indication for vaginal progesterone can also be extended to women with a history of spontaneous preterm birth who have a short cervix.
17α-Hydroxyprogesterone caproate (17OHP-C) in women with a short cervix
‘Progestogen’ is a compound with progesterone-like action (natural or synthetic),82–86 which has been defined as the ability of a chemical agent to transform a proliferative into a secretory endometrium to support pregnancy. The term ‘progestins’ refers to synthetic progestogens and, for the sake of clarity, should not be applied to natural progesterone (examples of progestins include medroxyprogesterone acetate, norethindrone, and levonorgestrel, which have been used as agents for contraception and hormone replacement).39 17OHP-C is a synthetic progestogen, and the human body does not produce the caproate molecule.39 The main reason for adding the caproate molecule is to prolong the half-life of the compound. However, this change alters the structure of the molecule, and could result in modifications of the pharmacologic or physiologic properties of the drug.25 Progesterone is not the same as 17OHP-C: the chemical differences and a comparison between the two agents in aspects relevant to the prevention of preterm birth have been recently reviewed.39
Is 17OHP-C effective in preventing preterm birth in women with a short cervix? A multicenter randomized controlled trial, conducted in nulliparous women, with a singleton gestation between 16 and 223/7 weeks of gestation, and a cervical length <30 mm (10th percentile in this population) was designed to examine this question and yielded negative results.87 Women were randomized to receive weekly 250 mg intramuscular injections of 17OHP-C or an identical-appearing placebo through 36 weeks. The primary outcome was preterm birth <37 weeks of gestation. Of 15 435 women screened, 1588 (10.3%) had a cervical length <30 mm. After 657 women had been randomized (n = 327 to 17OHP-C and n = 330 to placebo), the study was ended by the Data Safety Monitoring Board after a planned interim analysis had revealed that further enrollment was unlikely to demonstrate a significant difference between the study groups.87 There was no difference in the frequency of preterm birth <37 weeks between the 17OHP-C and placebo groups (25.1% vs 24.2%; RR: 1.03; 95% CI: 0.79–1.35). Moreover, there was no difference in the rate of preterm delivery <35 weeks (13.5% vs 16.1%; RR: 0.84, 95% CI: 0.58–1.21), or at <32 weeks of gestation (8.6% vs 9.7%; RR: 0.88; 95% CI: 0.54–1.43). Subgroup analysis showed no benefit from 17OHP-C in women with either a cervical length of <15 mm, or at 10–20 mm. Based upon such evidence, weekly 17OHP-C intramuscular administration cannot be recommended for nulliparous patients having a cervical length <30 mm. This observation is consistent with other studies indicating that 17OHP-C does not reduce the rate of cervical shortening when administered to women with a prior history of preterm birth.88
17α-Hydroxyprogesterone caproate to prevent preterm delivery in women with a prior history of preterm birth
Meis et al.89 conducted a double-blind, placebo-controlled trial of women with a history of prior spontaneous preterm birth in which women were enrolled at 16–20 weeks of gestation, and assigned to receive either weekly injections of 250 mg of 17OHP-C or an inert oil placebo. Injections were continued until delivery, or 36 weeks of gestation. The primary outcome was preterm delivery <37 weeks of gestation. Treatment with 17OHP-C significantly reduced the risk of delivery at <37 weeks of gestation (36.3% vs 54.9%; RR: 0.66; 95% CI: 0.54–0.81). There was also a significant reduction in the rate of preterm delivery <35 and <32 weeks of gestation. However, there are issues of efficacy and safety. Keirse90 questioned the results because of the unexpectedly high frequency of preterm birth in the placebo group (54.9%; 84/153). He suggested that 17OHP-C may not have been effective because the rate of preterm birth in this group was 36.3%,89 which was similar to the baseline rate of preterm birth (37%) for a similar population,91 and the placebo group in another trial by the same investigators. In fact, the power calculation of the Meis et al. trial was based on the observed rates of prematurity in a study by the Maternal–Fetal Medicine Units Network.91 The power calculation estimated that 37% of the women in the placebo group would deliver before 37 weeks of gestation.89–91 Similarly, US Food and Drug Administration (FDA) officials analyzing this trial also indicated that the rate of preterm birth in the 17OHP-C group (36.3%) was very similar to that in the placebo group of a similar study (http://www.fda.gov/ohrms/dockets/ac/06/slides/2006-4227S1-index.htm; see below).
It is not widely known that an initial randomized, placebo-controlled study (with a target enrollment of 500 women) known as ‘17P-IF-001’ was conducted by the Maternal–Fetal Medicine Units Network. The purpose of this study was to test the effectiveness and safety of 17OHP-C in the prevention of preterm birth <37 weeks of gestation. However, after 150 women had been enrolled and treated, the study was prematurely ended because of a study drug recall, secondary to quality control issues. In women allocated to receive placebo, the rate of preterm delivery was 38.5% (15/39), and the rate was 43.1% (28/65) in those allocated to receive 17OHP-C (non-significant results).89 Yet, 38.5% is much lower than the 54.9% in the trial of Meis et al.89 The high rate of preterm delivery in the control group (54.9%) has been the subject of debate. The investigators of the trial89 have argued that the participants were at very high risk for preterm delivery based upon obstetrical history, ethnicity, and willingness to be randomized to a painful weekly injection. It has been suggested that the latter would apply mainly to highly motivated patients at substantial risk for preterm delivery. Yet, if this is the actual explanation for the high rate of preterm delivery in the control group (54.9%), this argument goes against the trial’s external validity. For example, if the rationale is that 17OHP-C is only effective in African-American women with bacterial vaginosis and more than one preterm birth (which were allegedly overrepresented in the control group), and who are strongly motivated to receive weekly intramuscular injections, then it is valid to ask whether 17OHP-C should be administered to women with a prior preterm birth, but who do not have the other poor prognostic factors used to explain the high rate of preterm delivery in the control group.25
An important issue with the administration of any drug is one of safety. Meis et al. reported an excess of miscarriages and stillbirths in those receiving 17OHP-C.89 Yet, this was not statistically significant. This finding was not discussed in the paper, in the Editorial which followed,92 nor in subsequent articles and opinions of professional organizations.93–95 On 29 August 2006, this matter was first brought up by the medical officer of the FDA when reviewing the results of the trial at the Advisory Committee meeting.96 A slide was produced by the FDA which indicated that women receiving 17OHP-C in the midtrimester had a higher rate of fetal and neonatal death in the first 66 days of treatment, than in those receiving placebo. This observation is known as a ‘safety signal’ in pharmacovigilance. A safety signal is a non-statistically significant increase in the rate of adverse events during exposure to a drug.97 Data and Safety Monitoring Committees are appointed for several reasons; one is to monitor for adverse events or safety signals that may lead to termination of a trial for unexpected risks. It is noteworthy that the FDA approval of the commercial preparation of 17OHP-C includes a warning that administration of this agent may increase the frequency of gestational diabetes and other complications, and requires physicians to inform potential patients of the numerically non-significant increase in the rate of stillbirth and spontaneous abortions. (The package insert is available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021945s000lbl.pdf). Both the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine recommend that patients be counseled appropriately and sign an informed consent when receiving 17OHP-C (Letter to Members, Friday, 29 April 2011).
The FDA has approved administration of 17OHP-C to prevent preterm birth in women with a prior history under Subpart H of the Code of Federal Regulations, which is a regulatory pathway used when the decision is based on a surrogate endpoint (delivery <37 weeks of gestation), and further studies are necessary. Currently in the USA, another randomized clinical trial of 17OHP-C is underway; women with a prior history of preterm birth will be allocated to receive either placebo or 17OHP-C. The primary endpoint is preterm delivery <35 weeks of gestation. The originally predicted date for conclusion has been extended from October 2013 until 2016. One question to consider is that if professional organizations and regulatory agencies are truly convinced that 17OHP-C is effective, is it ethical to randomize women with a history of prior preterm delivery to receive a placebo? Another issue is a report that 17OHP-C may increase perinatal mortality. A recent double-blind, randomized clinical trial in mothers with triplet gestations who were randomly assigned to weekly injections of 250 mg 17OHP-C or placebo reported 13 midtrimester fetal losses in the treatment group (vs none in the placebo group).98 In France, a recent randomized controlled trial conducted in asymptomatic women with a twin gestation and short cervix (≤25 mm) reported that the rate of early preterm delivery (<32 weeks of gestation) was significantly greater in patients given 17OHP-C compared to the placebo group (29% vs 12%; P = 0.007).99 Moreover, neonatal morbidity was slightly higher in the 17OHP-C group (but not significant). If the randomized clinical trial currently taking place in the USA is completed and yields negative results, the FDA has the authority to change the approval status of 17OHP-C.
An interesting clinical scenario is that of a patient undergoing weekly injections of 17OHP-C (for a history of preterm birth) who is diagnosed with a short cervix (<25 mm) in the midtrimester.25 The question is whether the patient should continue receiving 17OHP-C, or should this be discontinued and the patient switched to vaginal progesterone or undergo cerclage placement? 17OHP-C administration has not been shown to be effective in women having a short cervix, and therefore this agent should not be continued. Nor does evidence exist that 17OHP-C should be combined with vaginal progesterone; thus, this strategy cannot be recommended. Based upon the safety concerns of 17OHP-C, we recommend that the best strategy is to discontinue 17OHP-C, and begin treatment with vaginal progesterone, since this has proven to be effective in women with a short cervix and history of preterm birth.25,100
Vaginal progesterone to prevent preterm birth in twin gestations
Three randomized clinical trials have explored whether vaginal progesterone can prevent preterm birth in twin gestations (without considering cervical length); two used vaginal progesterone (90 mg daily, in a bioadhesive gel),101,102 whereas the third trial used 200 mg in the form of vaginal progesterone pessaries.81 All trials were negative. A logical question is whether a larger dose of vaginal progesterone for the prevention of preterm birth is required in twin gestations. Serra et al.103 reported a randomized, controlled, double-blind, multicenter trial in which women with dichorionic, diamniotic twin gestations were randomized at 20 weeks of gestation to either placebo, or two different doses of vaginal progesterone in daily pessaries (one group received 200 mg, and the other group received 400 mg). The primary end point of the study was preterm birth <37 weeks of gestation. The rate of preterm birth at <37, <34, <32, and <28 weeks of gestation was not significantly different among the three groups. The frequency of a sonographic short cervix (<25 mm) in this trial was low (1.7%; 5/290), and this may be one explanation for the negative results.
There are two noteworthy observations from the trial of Serra et al.103 First, a higher dosage (400 mg vs 200 mg) of vaginal progesterone did not yield efficacy. Second, higher doses of progesterone appeared to have side-effects. A dose-dependent trend (non-significant) was noted towards a higher incidence of intrahepatic cholestasis among women treated with progesterone.103 Therefore, it is prudent to use the lowest effective dose, even for a natural hormone that is present in high concentrations in the peripheral blood during pregnancy.
It is clear that regardless of the reason for lack of effectiveness of vaginal progesterone for the prevention of preterm delivery in twin gestations, further randomized clinical trials in unselected twin gestations do not seem justified.104 The question is whether randomized clinical trials have not focused on the specific population that could benefit from this treatment. A sonographic short cervix is also a powerful predictor of preterm birth in twin gestations.105,106 Indeed, the same cervical length confers a greater risk for preterm birth in twin than in singleton pregnancies. A cervical length of ≤15 mm in singletons confers a 50% risk for preterm delivery at <32 weeks of gestation,66 whereas the same risk is conferred to twin gestations by a cervical length of ≤25 mm.105 A systematic review and meta-analysis of twin gestations reported that among asymptomatic women, a cervical length ≤20 mm (at 20–24 weeks of gestation) was a major predictor of preterm birth <32 and <34 weeks of gestation (with pooled positive likelihood ratios of 10.1 and 9.0, respectively).106 Thus, the question arises as to whether vaginal progesterone administered to women with dichorionic twin gestations and a short cervix can prevent preterm birth.
In the individual patient meta-analysis described above,78 a subgroup analysis in twin gestations with a cervical length of ≤25 mm was performed. Vaginal progesterone was associated with a non-significant trend towards reduction in the rate of preterm birth <33 weeks of gestation (30.4% vs 44.8%; RR: 0.70; 95% CI: 0.34–1.44). Yet, vaginal progesterone did lead to a significant reduction in composite neonatal morbidity and mortality (23.9% vs 39.7%; RR: 0.52; 95% CI: 0.29–0.93). It is important to note that the observations are based upon a small number of patients; thus, the 30% decrease may not have reached statistical significance because of the small sample size. However, composite neonatal morbidity and mortality was significant when the sample size was larger. A properly designed randomized controlled trial in twin gestations is required to determine the efficacy of vaginal progesterone to prevent preterm birth and neonatal morbidity and mortality in women with a short cervix.25,78,104
Patients with a short cervix, prior preterm birth, and singleton gestation (cervical cerclage vs vaginal progesterone)
There is evidence that patients with a sonographically short cervix (<25 mm) and a prior history of preterm birth may benefit from placement of a cervical cerclage.107 Such evidence is derived from a meta-analysis of five randomized clinical trials of women diagnosed with a short cervix prior to 24 weeks of gestation, in which cerclage was compared to expectant management.108–112 Women receiving a cerclage had a lower rate of preterm birth <35 weeks of gestation (primary outcome) than the no-cerclage group [28.4% (71/250) vs 41.3% (105/254); RR: 0.70; 95% CI: 0.55–0.89]. Cerclage placement also reduced preterm birth <37, <32, <28, and <24 weeks of gestation. Regarding composite perinatal morbidity and mortality, this was significantly reduced in the cerclage vs no-cerclage group (15.6% vs 24.8%; RR: 0.64; 95% CI: 0.45–0.91). Recently, two professional organizations recommended that cerclage may be considered for the treatment of women with a singleton gestation, prior spontaneous preterm birth, and short cervical length (<25 mm) at <24 weeks of gestation.113,114
Hence, there are two interventions that may reduce the rate of preterm delivery in patients with a history of preterm birth and a short cervix (<25 mm): vaginal progesterone administration or cervical cerclage. Yet, this situation could create a dilemma for physicians and patients about the optimal choice of treatment. There are no randomized controlled trials comparing vaginal progesterone and cervical cerclage directly for the prevention of preterm birth in women with a midtrimester sonographic short cervix, singleton gestation, and history of prior spontaneous preterm birth.100 In the absence of such evidence, indirect meta-analysis has emerged as an accepted and valid method for the comparison of competing interventions with the use of a common comparator.115–118 Such indirect meta-analysis of randomized clinical trials comparing vaginal progesterone vs placebo, and cerclage vs expectant management in patients with a singleton gestation, history of preterm birth, and midtrimester cervical length <25 mm was recently performed.100 The conclusion was that the efficacy of both interventions (cervical cerclage or vaginal progesterone) is similar in the prevention of preterm birth or adverse perinatal outcomes, and patients can thus be treated with either intervention (Table 1). Consideration of patient/physician preference and costs should be taken into account. For example, vaginal progesterone administration requires patient compliance. Placement of a cervical cerclage requires anesthesia and surgery, and has been associated with complications (e.g. bleeding, rupture of membranes).25
Table 1.
Results of an indirect patient meta-analysis of randomized clinical trials comparing vaginal progesterone vs placebo and cerclage vs expectant management in women with singleton gestations, history of preterm birth, and midtrimester cervical length <25 mm
| Outcome | Vaginal progesterone vs cerclage | |
|---|---|---|
| RR (95% CI) | P-valuea | |
| Preterm birth | ||
| <32 weeks | 0.70 (0.33–1.50) | 0.88 |
| <28 weeks | 0.71 (0.27–1.88) | 0.88 |
| <35 weeks | 0.88 (0.51–1.52) | 0.96 |
| <37 weeks | 1.19 (0.82–1.74) | 0.94 |
| Perinatal mortality | 1.05 (0.30–3.64) | 0.98 |
RR, relative risk; CI, confidence interval.
For the test of association.
Source: modified with permission from Conde-Agudelo et al.100
Universal cervical screening, vaginal progesterone, and cost effectiveness
Cervical sonography is a powerful tool in performing risk assessment for spontaneous preterm birth. It is simple to perform, safe, acceptable, reproducible, informative, inexpensive (when performed at the time of the second trimester fetal anatomy survey), and can provide an estimate of risk in primigravid women. Because of the current availability of a treatment strategy for women with a short cervix (vaginal progesterone), the question arises whether we should actively search for this population of women, or if we should restrict offering vaginal progesterone to those women whose short cervix has been detected incidentally?119 As described in recent editorials,120,121 universal cervical length screening fulfills all of the general principles outlined by the World Health Organization for a good screening tool.114 It screens for an important adverse outcome (preterm birth), uses an acceptable and suitable screening test (transvaginal sonography), and there is an effective treatment (vaginal progesterone) available to those identified by screening.114,119
Therefore, we (and others) believe that measuring cervical length should be part of the standard sonographic examination in the midtrimester of pregnancy.114,120–123 For screening to be effective, however, sonographic examinations should be performed using the proper transvaginal technique to yield accurate results, and continuing quality control and monitoring should be implemented (Fig. 8).114,124 Transabdominal cervical length screening cannot be recommended for various reasons.125,126 Performing cervical sonography outside of the studied gestational age (18–24 weeks) and applying treatment to women outside studied cervical length ranges may potentially result in adverse unintended consequences.114
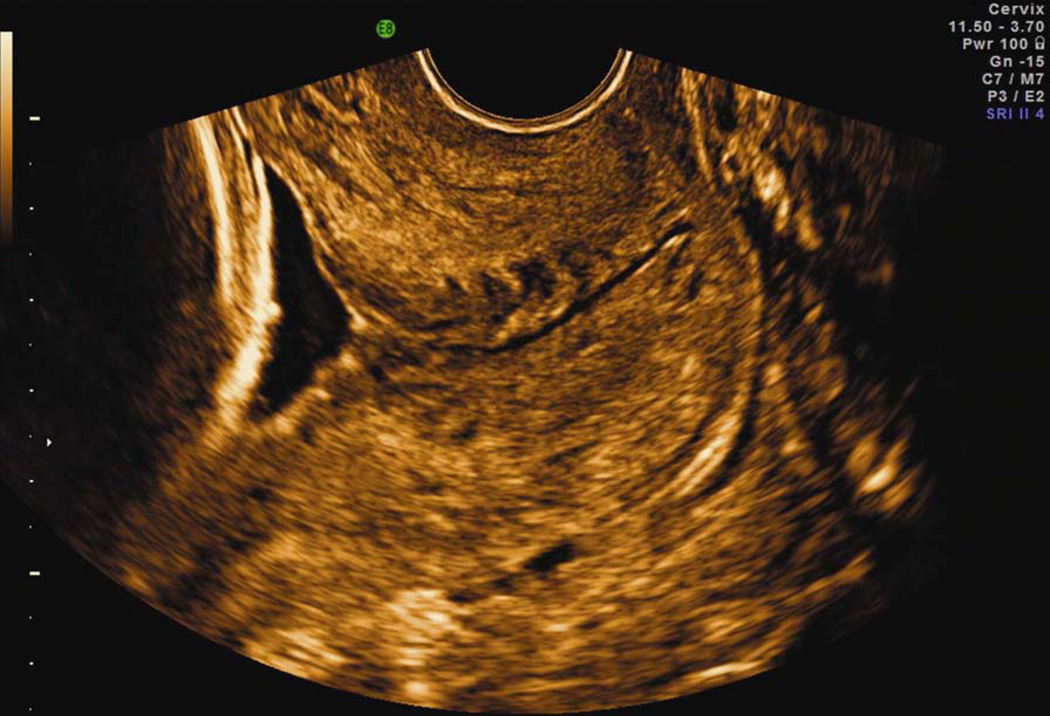
Figure 8.
Transvaginal ultrasound of the uterine cervix with a normal length. This is the ‘gold standard’ for the performance of cervical examinations during pregnancy. Note that the visualization of cervical anatomy is optimal.
Adoption of universal cervical length assessment is currently being considered as a preterm birth prevention strategy.127 Guidelines from the Society for Maternal–Fetal Medicine state that although universal cervical length screening remains controversial, ‘implementation of such a screening strategy should be viewed as reasonable, and can be considered by individual practitioners; third-party payers should not deny reimbursements for this screening’.114 An evidence-based algorithm for prediction and prevention of preterm birth based on transvaginal ultrasound cervical screening and selected interventions can be offered.114
Routine assessment of the risk for preterm birth using cervical ultrasound, along with vaginal progesterone for those with a short cervix, has been shown to be cost-effective and cost-saving.128,129 A recent economic analysis evaluated different strategies to reduce the rate of preterm delivery, including: (i) identifying patients at risk according to previous history; (ii) sonographic examination of the cervix; and (iii) treatment modalities, including cervical cerclage, 17α-hydroxyprogesterone caproate, and vaginal progesterone.128 The authors concluded that universal assessment of cervical length by transvaginal sonography, followed by vaginal progesterone administration, was the most cost-effective approach.128 Universal cervical ultrasound screening in singletons is predicted to result in a reduction of approximately 100 000 preterm births (<37 weeks) annually in the USA,128 or about 20% of all preterm births. Similarly, universal cervical screening and vaginal progesterone administration to those with a short cervix would lead to a cost saving of $19 million per 100 000 pregnant women, or $500–750 million per year in the USA alone.25,129 Recently, the cost-effectiveness of vaginal progesterone treatment for the prevention of preterm birth over a wide range of short cervical length measurements was determined.130 Vaginal progesterone was found to be an effective and inexpensive intervention, with the greatest reduction in preterm birth observed in the 10–14 mm cervical length group.
Conclusion
Patients with a short cervix (10–20 mm) should be offered vaginal progesterone to prevent preterm birth and to lead to an improvement in neonatal outcomes. Universal cervical screening by transvaginal ultrasound in the midtrimester followed by the use of vaginal progesterone appears to be cost-effective, and allows the prevention of preterm delivery in nulliparous women.128,129 However, progesterone treatment is only one of the solutions for the prevention of preterm birth. Interventions can only be expected to be successful if they interrupt the specific pathway leading to preterm delivery.25 Future clinical trials of preventive methods should be intelligently designed by keeping this concept in mind.
Practice points.
Preterm labor is a syndrome caused by multiple etiologies, one of which appears to be a suspension of progesterone action, which manifests as a sonographic short cervix.
A sonographic short cervix is the single most powerful predictor for spontaneous preterm birth and is more informative than a history of preterm birth.
In women with singleton gestations and a sonographic short cervix (<25 mm) in the midtrimester, vaginal progesterone can reduce the rate of spontaneous preterm birth by 45%, and also reduce the rate of neonatal morbidity.
An individual patient meta-analysis indicates that vaginal progesterone is effective in reducing the rate of spontaneous preterm deliveries in women with a midtrimester short cervix with or without a previous history of preterm birth.
Vaginal progesterone does not reduce the rate of preterm delivery in unselected twin gestations. However, an individual patient meta-analysis indicated that in patients with twin gestations and a sonographic cervix <25 mm, vaginal progesterone is associated with a significant reduction in composite neonatal morbidity and mortality and a 30% non-significant trend towards reduction in the rate of preterm birth <33 weeks of gestation. The latter probably reflects the small number of patients available for inclusion in the meta-analysis.
Patients with a history of preterm birth and a cervical length of <25 mm can be treated with either vaginal progesterone or cervical cerclage. This is based on the results of an individual patient meta-analysis.
Universal assessment of cervical length by transvaginal ultrasound in the midtrimester followed by the use of vaginal progesterone to women with a short cervix is a cost-effective means to prevent spontaneous preterm delivery.
Research directions.
Conduct a randomized clinical trial of vaginal progesterone vs placebo in women with twin gestations and a sonographic short cervix (<25 mm).
Identify biomarkers to predict the response to vaginal progesterone in women with a sonographic short cervix.
Determine the role of cervical cerclage in women with a sonographic short cervix treated with vaginal progesterone who experience further shortening of the cervix.
Determine the role of antibiotic and anti-inflammatory agents in the treatment of women with a sonographic short cervix and intra-amniotic infection or inflammation.
Explore the potential role of delivering progesterone using a cervical pessary for the prevention of spontaneous preterm birth.
Acknowledgments
Funding sources
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH); and, in part, with Federal funds from NICHD, NIH under Contract No. HSN275201300006C.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
None declared
References
- 1.Blencowe H, Cousens S, Oestergaard MZ, et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: a systematic analysis and implications. Lancet. 2012;379:2162–2172. doi: 10.1016/S0140-6736(12)60820-4. [DOI] [PubMed] [Google Scholar]
- 2.Beck S, Wojdyla D, Say L, et al. The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. Bull WHO. 2010;88:31–38. doi: 10.2471/BLT.08.062554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Romero R. Vaginal progesterone to reduce the rate of preterm birth and neonatal morbidity: a solution at last. Women’s Health. 2011;7:501–504. doi: 10.2217/whe.11.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L, Johnson HL, Cousens S, et al. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. doi: 10.1016/S0140-6736(12)60560-1. [DOI] [PubMed] [Google Scholar]
- 5.Jongbloed-Pereboom M, Janssen AJ, Steenbergen B, Nijhuis-Van Der Sanden MW. Motor learning and working memory in children born preterm: a systematic review. Neurosci Biobehav Rev. 2012;36:1314–1330. doi: 10.1016/j.neubiorev.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Mwaniki MK, Atieno M, Lawn JE, Newton CR. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet. 2012;379:445–452. doi: 10.1016/S0140-6736(11)61577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gravett MG, Rubens CE. A framework for strategic investments in research to reduce the global burden of preterm birth. Am J Obstet Gynecol. 2012;207:368–373. doi: 10.1016/j.ajog.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Grobman WA. Making sense of preterm birth. Am J Obstet Gynecol. 2012;206:99–100. doi: 10.1016/j.ajog.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Taylor HG. Outcomes of late preterm birth: who is at risk and for what? Am J Obstet Gynecol. 2012;206:181–182. doi: 10.1016/j.ajog.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 10.Bastek JA, Sammel MD, Pare E, Srinivas SK, Posencheg MA, Elovitz MA. Adverse neonatal outcomes: examining the risks between preterm, late preterm, and term infants. Am J Obstet Gynecol. 2008;199:367 e1–367 e8. doi: 10.1016/j.ajog.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 11.McCormick MC, Litt JS, Smith VC, Zupancic JA. Prematurity: an overview and public health implications. Annu Rev Public Health. 2011;32:367–379. doi: 10.1146/annurev-publhealth-090810-182459. [DOI] [PubMed] [Google Scholar]
- 12.Romero R, Sirtori M, Oyarzun E, et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol. 1989;161:817–824. doi: 10.1016/0002-9378(89)90409-2. [DOI] [PubMed] [Google Scholar]
- 13.Yoon BH, Romero R, Yang SH, et al. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174:1433–1440. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]
- 14.Yoon BH, Jun JK, Romero R, et al. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- 15.Yoon BH, Romero R, Park JS, et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000;182:675–681. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- 16.Yoon BH, Romero R, Moon JB, et al. Clinical significance of intra-amniotic inflammation in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2001;185:1130–1136. doi: 10.1067/mob.2001.117680. [DOI] [PubMed] [Google Scholar]
- 17.Romero R, Gotsch F, Pineles B, Kusanovic JP. Inflammation in pregnancy: its roles in reproductive physiology, obstetrical complications, and fetal injury. Nutr Rev. 2007;65:S194–S202. doi: 10.1111/j.1753-4887.2007.tb00362.x. [DOI] [PubMed] [Google Scholar]
- 18.Dessardo NS, Mustac E, Dessardo S, et al. Chorioamnionitis and chronic lung disease of prematurity: a path analysis of causality. Am J Perinatol. 2012;29:133–140. doi: 10.1055/s-0031-1295654. [DOI] [PubMed] [Google Scholar]
- 19.Sato M, Nishimaki S, Yokota S, et al. Severity of chorioamnionitis and neonatal outcome. J Obstet Gynaecol Res. 2011;37:1313–1319. doi: 10.1111/j.1447-0756.2010.01519.x. [DOI] [PubMed] [Google Scholar]
- 20.Gantert M, Been JV, Gavilanes AW, Garnier Y, Zimmermann LJ, Kramer BW. Chorioamnionitis: a multiorgan disease of the fetus? J Perinatol. 2010;30(Suppl):S21–S30. doi: 10.1038/jp.2010.96. [DOI] [PubMed] [Google Scholar]
- 21.Romero R, Espinoza J, Kusanovic JP, et al. The preterm parturition syndrome. Br J Obstet Gynecol. 2006;113(Suppl)(3):17–42. doi: 10.1111/j.1471-0528.2006.01120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romero R. Prenatal medicine: the child is the father of the man. Prenatal Neonatal Med. 1996;1:8–11. doi: 10.1080/14767050902784171. [DOI] [PubMed] [Google Scholar]
- 23.Romero R. Prenatal medicine: the child is the father of the man. 1996. J Maternal-Fetal Neonatal Med. 2009;22:636–639. doi: 10.1080/14767050902784171. [DOI] [PubMed] [Google Scholar]
- 24.Romero R, Gomez R, Mazor M, Ghezzi F, Yoon BH. The preterm labor syndrome. In: Elder M, Romero R, Lamont R, editors. Preterm labor. New York: Churchill Livingstone; 1997. pp. 29–49. [Google Scholar]
- 25.Romero R, Yeo L, Miranda J, Hassan SS, Conde-Agudelo A, Chaiworapongsa T. A blueprint for the prevention of preterm birth: vaginal progesterone in women with a short cervix. J Perinat Med. 2013;41:27–44. doi: 10.1515/jpm-2012-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann NY Acad Sci. 1994;734:414–429. doi: 10.1111/j.1749-6632.1994.tb21771.x. [DOI] [PubMed] [Google Scholar]
- 27.Romero R, Chaiworapongsa T, Gotsch F, Yeo L, Madan I, Hassan SS. The diagnosis and management of preterm labor with intact membranes. In: Winn HN, Chervenak FA, Romero R, editors. Clinical maternal–fetal medicine online. 2nd ed. London: Informa Healthcare; 2011. [Google Scholar]
- 28.Iams JD, Johnson FF, Sonek J, Sachs L, Gebauer C, Samuels P. Cervical competence as a continuum: a study of ultrasonographic cervical length and obstetric performance. Am J Obstet Gynecol. 1995;172:1097–1103. doi: 10.1016/0002-9378(95)91469-2. [DOI] [PubMed] [Google Scholar]
- 29.Althuisius S, Dekker G, Hummel P, Bekedam D, Kuik D, Van Geijn H. Cervical Incompetence Prevention Randomized Cerclage Trial (CIPRACT): effect of therapeutic cerclage with bed rest vs bed rest only on cervical length. Ultrasound Obstet Gynecol. 2002;20:163–167. doi: 10.1046/j.1469-0705.2002.00770.x. [DOI] [PubMed] [Google Scholar]
- 30.Romero R, Espinoza J, Erez O, Hassan S. The role of cervical cerclage in obstetric practice: can the patient who could benefit from this procedure be identified? Am J Obstet Gynecol. 2006;194:1–9. doi: 10.1016/j.ajog.2005.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berghella V. Cerclage decreases preterm birth: finally the level I evidence is here. Am J Obstet Gynecol. 2011;205:89–90. doi: 10.1016/j.ajog.2011.02.079. [DOI] [PubMed] [Google Scholar]
- 32.Romero R, Espinoza J, Mazor M, Chaiworapongsa T. In: The preterm parturition syndrome. Critchely H, Bennett P, Thornton S, editors. London: RCOG Press; 2004. Preterm Birth. [Google Scholar]
- 33.Csapo AI, Knobil E, Van Der Molen HJ, Wiest WG. Peripheral plasma progesterone levels during human pregnancy and labor. Am J Obstet Gynecol. 1971;110:630–632. doi: 10.1016/0002-9378(71)90242-0. [DOI] [PubMed] [Google Scholar]
- 34.Csapo AI, Pulkkinen MO, Ruttner B, Sauvage JP, Wiest WG. The significance of the human corpus luteum in pregnancy maintenance. I. Preliminary studies. Am J Obstet Gynecol. 1972;112:1061–1067. doi: 10.1016/0002-9378(72)90181-0. [DOI] [PubMed] [Google Scholar]
- 35.Csapo AI, Pulkkinen MO, Kaihola HL. The effect of luteectomy-induced progesterone-withdrawal on the oxytocin and prostaglandin response of the first trimester pregnant human uterus. Prostaglandins. 1973;4:421–429. doi: 10.1016/0090-6980(73)90030-0. [DOI] [PubMed] [Google Scholar]
- 36.Csapo AI, Erdos T. The critical control of progesterone levels and pregnancy by antiprogesterone. Am J Obstet Gynecol. 1976;126:598–601. doi: 10.1016/0002-9378(76)90759-6. [DOI] [PubMed] [Google Scholar]
- 37.Csapo AI, Erdos T. Prevention of the abortifacient action of antiprogesterone serum by progesterone. Am J Obstet Gynecol. 1977;128:212–214. doi: 10.1016/0002-9378(77)90691-3. [DOI] [PubMed] [Google Scholar]
- 38.Kerenyi T. Forgotten “father of progesterone”. Am J Obstet Gynecol. 2010;202:e10–e11. doi: 10.1016/j.ajog.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 39.Romero R, Stanczyk FZ. Progesterone is not the same as 17alpha-hydroxyprogesterone caproate: implications for obstetrical practice. Am J Obstet Gynecol. 2013;208:421–426. doi: 10.1016/j.ajog.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murthy YS, Arronet GH, Parekh MC. Luteal phase inadequacy. Its significance in infertility. Obstet Gynecol. 1970;36:758–761. [PubMed] [Google Scholar]
- 41.Stites DP, Siiteri PK. Steroids as immunosuppressants in pregnancy. Immunol Rev. 1983;75:117–138. doi: 10.1111/j.1600-065x.1983.tb01093.x. [DOI] [PubMed] [Google Scholar]
- 42.Fidel PI, Jr, Romero R, Maymon E, Hertelendy F. Bacteria-induced or bacterial product-induced preterm parturition in mice and rabbits is preceded by a significant fall in serum progesterone concentrations. J Matern Fetal Med. 1998;7:222–226. doi: 10.1002/(SICI)1520-6661(199809/10)7:5<222::AID-MFM2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 43.Chwalisz K. The use of progesterone antagonists for cervical ripening and as an adjunct to labour and delivery. Hum Reprod. 1994;9(Suppl 1):131–161. doi: 10.1093/humrep/9.suppl_1.131. [DOI] [PubMed] [Google Scholar]
- 44.Pepe GJ, Albrecht ED. Actions of placental and fetal adrenal steroid hormones in primate pregnancy. Endocr Rev. 1995;16:608–648. doi: 10.1210/edrv-16-5-608. [DOI] [PubMed] [Google Scholar]
- 45.Henson MC. Pregnancy maintenance and the regulation of placental progesterone biosynthesis in the baboon. Hum Reprod Update. 1998;4:389–405. doi: 10.1093/humupd/4.4.389. [DOI] [PubMed] [Google Scholar]
- 46.Bernal AL. Overview of current research in parturition. Exp Physiol. 2001;86:213–222. doi: 10.1113/eph8602178. [DOI] [PubMed] [Google Scholar]
- 47.Young IR. The comparative physiology of parturition in mammals. In: Smith R, editor. The endocrinology of parturition. Basel: Reinhardt Druck; 2001. [DOI] [PubMed] [Google Scholar]
- 48.Mesiano S. Roles of estrogen and progesterone in human parturition. Front Horm Res. 2001;27:86–104. doi: 10.1159/000061038. [DOI] [PubMed] [Google Scholar]
- 49.Mesiano S. Myometrial progesterone responsiveness and the control of human parturition. J Soc Gynecol Invest. 2004;11:193–202. doi: 10.1016/j.jsgi.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Lye SJ. The initiation and inhibition of labor: towards a molecular understanding. Semin Reprod Endocrinol. 1994;12:284–294. [Google Scholar]
- 51.Stjernholm Y, Sahlin L, Akerberg S, et al. Cervical ripening in humans: potential roles of estrogen, progesterone, and insulin-like growth factor-I. Am J Obstet Gynecol. 1996;174:1065–1071. doi: 10.1016/s0002-9378(96)70352-6. [DOI] [PubMed] [Google Scholar]
- 52.Lye SJ, Ou CW, Tech TG, et al. The molecular basis of labour and tocolysis. Fetal Maternal Med Rev. 1998;10:121–136. [Google Scholar]
- 53.Smith R, Mesiano S, Mcgrath S. Hormone trajectories leading to human birth. Regul Pept. 2002;108:159–164. doi: 10.1016/s0167-0115(02)00105-2. [DOI] [PubMed] [Google Scholar]
- 54.Oh SY, Kim CJ, Park I, et al. Progesterone receptor isoform (A/B) ratio of human fetal membranes increases during term parturition. Am J Obstet Gynecol. 2005;193:1156–1160. doi: 10.1016/j.ajog.2005.05.071. [DOI] [PubMed] [Google Scholar]
- 55.Smith R. Parturition. N Engl J Med. 2007;356:271–283. doi: 10.1056/NEJMra061360. [DOI] [PubMed] [Google Scholar]
- 56.Chwalisz K, Shi Shao O, Neff G, Elger J. The effect of antigestagen ZK 98, 199 on the uterine cervix. Acta Endocrinol. 1987;283:113. [Google Scholar]
- 57.Norman J. Antiprogesterones. Br J Hosp Med. 1991;45:372–375. [PubMed] [Google Scholar]
- 58.Elliott CL, Brennand JE, Calder AA. The effects of mifepristone on cervical ripening and labor induction in primigravidae. Obstet Gynecol. 1998;92:804–809. doi: 10.1016/s0029-7844(98)00284-1. [DOI] [PubMed] [Google Scholar]
- 59.Stenlund PM, Ekman G, Aedo AR, Bygdeman M. Induction of labor with mifepristone – a randomized, double-blind study versus placebo. Acta Obstet Gynecol Scand. 1999;78:793–798. [PubMed] [Google Scholar]
- 60.Giacalone PL, Daures JP, Faure JM, Boulot P, Hedon B, Laffargue F. The effects of mifepristone on uterine sensitivity to oxytocin and on fetal heart rate patterns. Eur J Obstet Gynecol Reprod Biol. 2001;97:30–34. doi: 10.1016/s0301-2115(00)00506-6. [DOI] [PubMed] [Google Scholar]
- 61.Word RA, Li XH, Hnat M, Carrick K. Dynamics of cervical remodeling during pregnancy and parturition: mechanisms and current concepts. Semin Reprod Med. 2007;25:69–79. doi: 10.1055/s-2006-956777. [DOI] [PubMed] [Google Scholar]
- 62.Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med. 1996;334:567–572. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 63.Berghella V, Kuhlman K, Weiner S, Texeira L, Wapner RJ. Cervical funneling: sonographic criteria predictive of preterm delivery. Ultrasound Obstet Gynecol. 1997;10:161–166. doi: 10.1046/j.1469-0705.1997.10030161.x. [DOI] [PubMed] [Google Scholar]
- 64.Heath VC, Southall TR, Souka AP, Elisseou A, Nicolaides KH. Cervical length at 23 weeks of gestation: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol. 1998;12:312–317. doi: 10.1046/j.1469-0705.1998.12050312.x. [DOI] [PubMed] [Google Scholar]
- 65.Taipale P, Hiilesmaa V. Sonographic measurement of uterine cervix at 18–22 weeks’ gestation and the risk of preterm delivery. Obstet Gynecol. 1998;92:902–907. doi: 10.1016/s0029-7844(98)00346-9. [DOI] [PubMed] [Google Scholar]
- 66.Hassan SS, Romero R, Berry SM, et al. Patients with an ultrasonographic cervical length < or = 15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol. 2000;182:1458–1467. doi: 10.1067/mob.2000.106851. [DOI] [PubMed] [Google Scholar]
- 67.Botsis D, Papagianni V, Vitoratos N, Makrakis E, Aravantinos L, Creatsas G. Prediction of preterm delivery by sonographic estimation of cervical length. Biol Neonate. 2005;88:42–45. doi: 10.1159/000084457. [DOI] [PubMed] [Google Scholar]
- 68.De Carvalho MH, Bittar RE, Brizot ML, Bicudo C, Zugaib M. Prediction of preterm delivery in the second trimester. Obstet Gynecol. 2005;105:532–536. doi: 10.1097/01.AOG.0000154157.22500.1d. [DOI] [PubMed] [Google Scholar]
- 69.Grimes-Dennis J, Berghella V. Cervical length and prediction of preterm delivery. Curr Opin Obstet Gynecol. 2007;19:191–195. doi: 10.1097/GCO.0b013e3280895dd3. [DOI] [PubMed] [Google Scholar]
- 70.Celik E, To M, Gajewska K, Smith GC, Nicolaides KH. Cervical length and obstetric history predict spontaneous preterm birth: development and validation of a model to provide individualized risk assessment. Ultrasound Obstet Gynecol. 2008;31:549–554. doi: 10.1002/uog.5333. [DOI] [PubMed] [Google Scholar]
- 71.Tekesin I, Eberhart LH, Schaefer V, Wallwiener D, Schmidt S. Evaluation and validation of a new risk score (CLEOPATRA score) to predict the probability of premature delivery for patients with threatened preterm labor. Ultrasound Obstet Gynecol. 2005;26:699–706. doi: 10.1002/uog.2633. [DOI] [PubMed] [Google Scholar]
- 72.Owen J, Yost N, Berghella V, et al. Mid-trimester endovaginal sonography in women at high risk for spontaneous preterm birth. JAMA. 2001;286:1340–1348. doi: 10.1001/jama.286.11.1340. [DOI] [PubMed] [Google Scholar]
- 73.To MS, Skentou CA, Royston P, Yu CK, Nicolaides KH. Prediction of patient-specific risk of early preterm delivery using maternal history and sonographic measurement of cervical length: a population-based prospective study. Ultrasound Obstet Gynecol. 2006;27:362–367. doi: 10.1002/uog.2773. [DOI] [PubMed] [Google Scholar]
- 74.Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH. Progesterone and the risk of preterm birth among women with a short cervix. N Engl J Med. 2007;357:462–469. doi: 10.1056/NEJMoa067815. [DOI] [PubMed] [Google Scholar]
- 75.Hassan SS, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2011;38:18–31. doi: 10.1002/uog.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions version 510. Cochrane Collaboration; 2011. [updated March 2011]. [Google Scholar]
- 77.Stewart LA, Parmar MK. Meta-analysis of the literature or of individual patient data: is there a difference? Lancet. 1993;341:418–422. doi: 10.1016/0140-6736(93)93004-k. [DOI] [PubMed] [Google Scholar]
- 78.Romero R, Nicolaides K, Conde-Agudelo A, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and metaanalysis of individual patient data. Am J Obstet Gynecol. 2012;206:124 e1–124 e19. doi: 10.1016/j.ajog.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.O’Brien JM, Adair CD, Lewis DF, et al. Progesterone vaginal gel for the reduction of recurrent preterm birth: primary results from a randomized, double-blind, placebo-controlled trial. Ultrasound Obstet Gynecol. 2007;30:687–696. doi: 10.1002/uog.5158. [DOI] [PubMed] [Google Scholar]
- 80.Cetingoz E, Cam C, Sakalli M, Karateke A, Celik C, Sancak A. Progesterone effects on preterm birth in high-risk pregnancies: a randomized placebo-controlled trial. Arch Gynecol Obstet. 2011;283:423–429. doi: 10.1007/s00404-009-1351-2. [DOI] [PubMed] [Google Scholar]
- 81.Rode L, Klein K, Nicolaides K, Krampl-Bettelheim E, Tabor A. Prevention of preterm delivery in twin gestations (PREDICT): a multicentre randomised placebo-controlled trial on the effect of vaginal micronised progesterone. Ultrasound Obstet Gynecol. 2011;38:272–280. doi: 10.1002/uog.9093. [DOI] [PubMed] [Google Scholar]
- 82.Stanczyk FZ. All progestins are not created equal. Steroids. 2003;68:879–890. doi: 10.1016/j.steroids.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 83.Role of progestogen in hormone therapy for postmenopausal women: position statement of North American Menopause Society. Menopause. 2003;10:113–132. doi: 10.1097/00042192-200310020-00003. [DOI] [PubMed] [Google Scholar]
- 84.Schindler AE, Campagnoli C, Druckmann R, et al. Classification and pharmacology of progestins. Maturitas. 2008;61:171–180. doi: 10.1016/j.maturitas.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 85.Stanczyk FZ, Hapgood JP, Winer S, Mishell DR., Jr Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev. 2013;34:171–208. doi: 10.1210/er.2012-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Romero R. Prevention of spontaneous preterm birth: the role of sonographic cervical length in identifying patients who may benefit from progesterone treatment. Ultrasound Obstet Gynecol. 2007;30:675–686. doi: 10.1002/uog.5174. [DOI] [PubMed] [Google Scholar]
- 87.Grobman WA, Thom EA, Spong CY, et al. 17 alpha-hydroxyprogesterone caproate to prevent prematurity in nulliparas with cervical length less than 30 mm. Am J Obstet Gynecol. 2012;207:390 e1–390 e8. doi: 10.1016/j.ajog.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Durnwald CP, Lynch CD, Walker H, Iams JD. The effect of treatment with 17 alpha-hydroxyprogesterone caproate on changes in cervical length over time. Am J Obstet Gynecol. 2009;201:410 e1–410 e5. doi: 10.1016/j.ajog.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meis PJ, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate. N Engl J Med. 2003;348:2379–2385. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 90.Keirse MJ. Progesterone and preterm: seventy years of “deja vu” or “still to be seen”? Birth. 2004;31:230–235. doi: 10.1111/j.0730-7659.2004.00315.x. [DOI] [PubMed] [Google Scholar]
- 91.Iams JD, Newman RB, Thom EA, et al. Frequency of uterine contractions and the risk of spontaneous preterm delivery. N Engl J Med. 2002;346:250–255. doi: 10.1056/NEJMoa002868. [DOI] [PubMed] [Google Scholar]
- 92.Greene MF. Progesterone and preterm delivery – deja vu all over again. N Engl J Med. 2003;348:2453–2455. doi: 10.1056/NEJMe030081. [DOI] [PubMed] [Google Scholar]
- 93.Meis PJ. 17 hydroxyprogesterone for the prevention of preterm delivery. Obstet Gynecol. 2005;105:1128–1135. doi: 10.1097/01.AOG.0000160432.95395.8f. [DOI] [PubMed] [Google Scholar]
- 94.American College of Obstetricians and Gynecologists Committee Opinion number 419 October 2008 (replaces no. 291, November 2003). Use of progesterone to reduce preterm birth. Obstet Gynecol. 2008;112:963–965. doi: 10.1097/AOG.0b013e31818b1ff6. [DOI] [PubMed] [Google Scholar]
- 95.Farine D, Mundle WR, Dodd J, et al. The use of progesterone for prevention of preterm birth. J Obstet Gynaecol Can. 2008;30:67–77. doi: 10.1016/S1701-2163(16)32716-5. [DOI] [PubMed] [Google Scholar]
- 96.Food and Drug Administration Review by the Division of Reproductive and Urologic Products. 17α-Alpha hydroxyprogesterone caproate for prevention of preterm birth. 2006;vol. 2013 Overview of FDA Background Document. [Google Scholar]
- 97.United States Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (Cder) Center for Biologics Evaluation and Research (Cber) Guidance for industry: good pharmacovigilance practices and pharmacoepidemiologic assessment. 2005;vol. 2013 [Google Scholar]
- 98.Combs CA, Garite T, Maurel K, Das A, Porto M. Failure of 17-hydroxyprogesterone to reduce neonatal morbidity or prolong triplet pregnancy: a double-blind, randomized clinical trial. Am J Obstet Gynecol. 2010;203:248 e1–248 e9. doi: 10.1016/j.ajog.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 99.Senat MV, Porcher R, Winer N, et al. Prevention of preterm delivery by 17 alpha-hydroxyprogesterone caproate in asymptomatic twin pregnancies with a short cervix: a randomized controlled trial. Am J Obstet Gynecol. 2013;208:194 e1–194 e8. doi: 10.1016/j.ajog.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 100.Conde-Agudelo A, Romero R, Nicolaides K, et al. Vaginal progesterone vs cervical cerclage for the prevention of preterm birth in women with a sonographic short cervix, previous preterm birth, and singleton gestation: a systematic review and indirect comparison metaanalysis. Am J Obstet Gynecol. 2013;208:42 e1–42 e42. doi: 10.1016/j.ajog.2012.10.877. e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Norman JE, Mackenzie F, Owen P, et al. Progesterone for the prevention of preterm birth in twin pregnancy (STOPPIT): a randomised, double-blind, placebo-controlled study and meta-analysis. Lancet. 2009;373:2034–2040. doi: 10.1016/S0140-6736(09)60947-8. [DOI] [PubMed] [Google Scholar]
- 102.Wood S, Ross S, Tang S, Miller L, Sauve R, Brant R. Vaginal progesterone to prevent preterm birth in multiple pregnancy: a randomized controlled trial. J Perinat Med. 2012 Jul 14; doi: 10.1515/jpm-2012-0057. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 103.Serra V, Perales A, Meseguer J, et al. Increased doses of vaginal progesterone for the prevention of preterm birth in twin pregnancies: a randomised controlled double-blind multicentre trial. Br J Obstet Gynecol. 2013;120:50–57. doi: 10.1111/j.1471-0528.2012.03448.x. [DOI] [PubMed] [Google Scholar]
- 104.Romero R. Progesterone to prevent preterm birth in twin gestations: what is the next step forward? Br J Obstet Gynecol. 2013;120:1–4. doi: 10.1111/1471-0528.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Souka AP, Heath V, Flint S, Sevastopoulou I, Nicolaides KH. Cervical length at 23 weeks in twins in predicting spontaneous preterm delivery. Obstet Gynecol. 1999;94:450–454. doi: 10.1016/s0029-7844(99)00277-x. [DOI] [PubMed] [Google Scholar]
- 106.Conde-Agudelo A, Romero R, Hassan SS, Yeo L. Transvaginal sonographic cervical length for the prediction of spontaneous preterm birth in twin pregnancies: a systematic review and metaanalysis. Am J Obstet Gynecol. 2010;203:128 e1–128 e12. doi: 10.1016/j.ajog.2010.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Berghella V, Rafael TJ, Szychowski JM, Rust OA, Owen J. Cerclage for short cervix on ultrasonography in women with singleton gestations and previous preterm birth: a meta-analysis. Obstet Gynecol. 2011;117:663–671. doi: 10.1097/AOG.0b013e31820ca847. [DOI] [PubMed] [Google Scholar]
- 108.Althuisius SM, Dekker GA, Hummel P, Bekedam DJ, Van Geijn HP. Final results of the Cervical Incompetence Prevention Randomized Cerclage Trial (CIPRACT): therapeutic cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol. 2001;185:1106–1112. doi: 10.1067/mob.2001.118655. [DOI] [PubMed] [Google Scholar]
- 109.Rust OA, Atlas RO, Reed J, Van Gaalen J, Balducci J. Revisiting the short cervix detected by transvaginal ultrasound in the second trimester: why cerclage therapy may not help. Am J Obstet Gynecol. 2001;185:1098–1105. doi: 10.1067/mob.2001.118163. [DOI] [PubMed] [Google Scholar]
- 110.Berghella V, Odibo AO, Tolosa JE. Cerclage for prevention of preterm birth in women with a short cervix found on transvaginal ultrasound examination: a randomized trial. Am J Obstet Gynecol. 2004;191:1311–1317. doi: 10.1016/j.ajog.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 111.To MS, Alfirevic Z, Heath VC, et al. Cervical cerclage for prevention of preterm delivery in women with short cervix: randomised controlled trial. Lancet. 2004;363:1849–1853. doi: 10.1016/S0140-6736(04)16351-4. [DOI] [PubMed] [Google Scholar]
- 112.Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201:375. e1–375. e8. doi: 10.1016/j.ajog.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Committee opinion no: 522: incidentally detected short cervical length. Obstet Gynecol. 2012;119:879–882. doi: 10.1097/AOG.0b013e3182538e64. [DOI] [PubMed] [Google Scholar]
- 114.Progesterone and preterm birth prevention: translating clinical trials data into clinical practice. Am J Obstet Gynecol. 2012;206:376–386. doi: 10.1016/j.ajog.2012.03.010. [DOI] [PubMed] [Google Scholar]
- 115.Benichou J, Bellissant E, Chastang C. Analysis of phase II clinical trials in haematology and oncology: comparison of the triangular test to the usual methods. Stat Med. 1991;10:989. doi: 10.1002/sim.4780100620. discussion 89–90. [DOI] [PubMed] [Google Scholar]
- 116.Song F, Altman DG, Glenny AM, Deeks JJ. Validity of indirect comparison for estimating efficacy of competing interventions: empirical evidence from published meta-analyses. BMJ Clin Res Ed. 2003;326:472. doi: 10.1136/bmj.326.7387.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Edwards SJ, Clarke MJ, Wordsworth S, Borrill J. Indirect comparisons of treatments based on systematic reviews of randomised controlled trials. Int J Clin Pract. 2009;63:841–854. doi: 10.1111/j.1742-1241.2009.02072.x. [DOI] [PubMed] [Google Scholar]
- 118.Wells GA, Sultan A, Chen L, Khan M, Coyle D. Indirect evidence: indirect treatment comparisons in meta-analysis. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2009. [Google Scholar]
- 119.Hutcheon JA, Skoll MA, Eastabrook GD, Lim KI. The case for universal cervical length screening to prevent preterm birth: is it strong enough to change practice in Canada? J Obstet Gynaecol Can. 2012;34:1184–1187. doi: 10.1016/S1701-2163(16)35467-6. [DOI] [PubMed] [Google Scholar]
- 120.Campbell S. Universal cervical-length screening and vaginal progesterone prevents early preterm births, reduces neonatal morbidity and is cost saving: doing nothing is no longer an option. Ultrasound Obstet Gynecol. 2011;38:1–9. doi: 10.1002/uog.9073. [DOI] [PubMed] [Google Scholar]
- 121.Combs CA. Vaginal progesterone for asymptomatic cervical shortening and the case for universal screening of cervical length. Am J Obstet Gynecol. 2012;206:101–103. doi: 10.1016/j.ajog.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 122.Lockwood CJ. The real progesterone story. Contemp Obstet Gynecol. 2011;5:10–15. [Google Scholar]
- 123.Khandelwal M. Vaginal progesterone in risk reduction of preterm birth in women with short cervix in the midtrimester of pregnancy. Int J Women’s Health. 2012;4:481–490. doi: 10.2147/IJWH.S28944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Berghella V. Universal cervical length screening for prediction and prevention of preterm birth. Obstet Gynecol Surv. 2012;67:653–658. doi: 10.1097/OGX.0b013e318270d5b2. [DOI] [PubMed] [Google Scholar]
- 125.Podobnik M, Bulic M, Smiljanic N, Bistricki J. Ultrasonography in the detection of cervical incompetency. J Clin Ultrasound. 1988;16:383–391. doi: 10.1002/jcu.1870160604. [DOI] [PubMed] [Google Scholar]
- 126.Hernandez-Andrade E, Romero R, Ahn H, et al. Transabdominal evaluation of uterine cervical length during pregnancy fails to identify a substantial number of women with a short cervix. J Maternal–Fetal Neonatal Med. 2012;25:1682–1689. doi: 10.3109/14767058.2012.657278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Parry S, Simhan H, Elovitz M, Iams J. Universal maternal cervical length screening during the second trimester: pros and cons of a strategy to identify women at risk of spontaneous preterm delivery. Am J Obstet Gynecol. 2012;207:101–106. doi: 10.1016/j.ajog.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 128.Cahill AG, Odibo AO, Caughey AB, et al. Universal cervical length screening and treatment with vaginal progesterone to prevent preterm birth: a decision and economic analysis. Am J Obstet Gynecol. 2010;202:548 e1–548 e8. doi: 10.1016/j.ajog.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Werner EF, Han CS, Pettker CM, et al. Universal cervical-length screening to prevent preterm birth: a cost-effectiveness analysis. Ultrasound Obstet Gynecol. 2011;38:32–37. doi: 10.1002/uog.8911. [DOI] [PubMed] [Google Scholar]
- 130.Page J, Emerson J, Cahill AG, et al. The impact of cervical length on the cost-effectness of vaginal progesterone as a preterm birth intervention. Am J Obstet Gynecol. 2013;208(Suppl 1):S66. [Google Scholar]