Abstract
Traumatic brain injury (TBI) is characterized by histopathological damage and long-term sensorimotor and cognitive dysfunction. Recent studies have reported the discovery of the P7C3 class of aminopropyl carbazole agents with potent neuroprotective properties for both newborn neural precursor cells in the adult hippocampus and mature neurons in other regions of the central nervous system. This study tested, for the first time, whether the highly active P7C3-A20 compound would be neuroprotective, promote hippocampal neurogenesis, and improve functional outcomes after experimental TBI. Sprague-Dawley rats subjected to moderate fluid percussion brain injury were evaluated for quantitative immunohistochemical and behavioral changes after trauma. P7C3-A20 (10 mg/kg) or vehicle was initiated intraperitoneally 30 min postsurgery and twice per day every day thereafter for 7 days. Administration of P7C3-A20 significantly reduced overall contusion volume, preserved vulnerable anti-neuronal nuclei (NeuN)-positive pericontusional cortical neurons, and improved sensorimotor function 1 week after trauma. P7C3-A20 treatment also significantly increased both bromodeoxyuridine (BrdU)- and doublecortin (DCX)-positive cells within the subgranular zone of the ipsilateral dentate gyrus 1 week after TBI. Five weeks after TBI, animals treated with P7C3-A20 showed significantly increased BrdU/NeuN double-labeled neurons and improved cognitive function in the Morris water maze, compared to TBI-control animals. These results suggest that P7C3-A20 is neuroprotective and promotes endogenous reparative strategies after TBI. We propose that the chemical scaffold represented by P7C3-A20 provides a basis for optimizing and advancing new pharmacological agents for protecting patients against the early and chronic consequences of TBI.
Key words: : adult hippocampal neurogenesis, functional recovery, hippocampal-dependent memory, neuroprotection, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is a major cause of death and disability in the United States.1 Focal contusive injury, diffuse axonal damage, and selective patterns of neuronal loss contribute to long-term functional impairments.2 The hippocampus is highly vulnerable to TBI,3,4 and subsequent circuit disruption leads to hippocampal-dependent memory dysfunction.5,6 Thus, identification of novel therapeutic approaches that include both neuroprotective as well as reparative strategies is critically important.
New neurons are continuously generated throughout life in the subgranular zone (SGZ) of the hippocampal dentate gyrus (DG), where neural progenitor cells (NPCs) undergo distinct developmental stages of proliferation, fate specification, migration, maturation, and integration.7–15 Newly generated dentate granule cells (DGCs) integrate within hippocampal circuitry to participate in hippocampal-dependent memory.9,16–19 Stimuli that augment hippocampal neurogenesis are associated with memory improvement,20–22 whereas specific exposures that decrease hippocampal neurogenesis correlate with impairment of hippocampal-dependent learning and memory.17,21,23–25
Several studies have reported that NPC proliferation is significantly enhanced after TBI.26–29 This response is transient, however, because levels of doublecortin (DCX)-positive cells remain persistently depressed, indicating a chronic depletion of immature neurons and neurogenesis.30–34 The mechanisms responsible for the persistent loss of immature neurons are not clear, but studies have reported a unique vulnerability of the post-traumatic hippocampal progenitor pool to necrotic and apoptotic cell death processes. Gao and colleagues30 have observed degenerating cells in the DG, with 80% being immature neurons, after moderate TBI. Given that hippocampal neurogenesis is closely correlated with hippocampal-dependent memory,9,16,21,33,35,36 therapeutic interventions to augment the survival of newly generated hippocampal neurons and enhance neurogenesis may improve hippocampal-dependent functional outcomes after brain trauma.
Using a target-agnostic in vivo screen of 1000 compounds to identify small drug-like molecules with neurogenic efficacy in the hippocampus of living mice, Pieper and colleagues37 discovered and characterized a molecule they named P7C3. Both potency and efficacy, as well as toxicity profile, were further improved through synthesis of an analog (P7C3-A20), which differs from P7C3 chiefly by substitution of a fluorine for the hydroxyl group within the linker region of the molecule (Supplementary Fig. 1) (see online supplementary material at http://www.liebertpub.com).37,38 The P7C3 class of molecules, including P7C3-A20, enhances hippocampal neurogenesis by blocking apoptosis of newly generated hippocampal neurons, thus resulting in a greater survival of new neurons, and is thought to operate through stabilization of mitochondria under otherwise toxic conditions.37 Previously, the potent proneurogenic effects of P7C3-A20 (and its parent compound, P7C3) were demonstrated using two different rodent models of impaired adult neurogenesis: neuronal PAS domain protein 3 (NPAS3) transcription factor-deficit mice and aged rats.37–39 After prolonged administration of P7C3 and P7C3-A20 (hereafter referred to as A20), Pieper and colleagues showed that the intensified apoptosis of newly born DG neurons in npas3–/– mice was significantly attenuated, restoring hippocampal structure and function. Further, administration of P7C3 enhanced endogenous DG neurogenesis in aged rats, preventing neuronal death and preserving hippocampal-dependent learning that normally declines with aging.37 More recently, this group has also demonstrated the beneficial effects of the most active variants of P7C3 in adult models of neurodegeneration, including protection of dopaminergic neurons in the substantia nigra in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease40 and also of spinal cord motor neurons in the G93A-SOD1 transgenic mouse model of amyotrophic lateral sclerosis.41
Given the vulnerability of newly born DG neurons to brain injury and their important role in hippocampal-dependent memory, we hypothesized that administration of the A20 proneurogenic compound after TBI would enhance hippocampal neurogenesis and protect against hippocampal-dependent spatial memory deficits. Antiapoptotic activity of A20 is attributed to the stabilization of mitochondrial membrane potentials (MMPs), which are compromised in several vulnerable cell types after a traumatic insult. Thus, we also hypothesized that A20 would protect mature cortical neurons from degeneration after TBI and attenuate sensorimotor impairment.
To these hypotheses, we investigated whether administration of A20 would 1) reduce contusion volume, 2) protect vulnerable cortical neurons, 3) enhance SGZ NPC proliferation, 4) promote and prolong enhanced SGZ neurogenesis, and 5) protect against sensorimotor and hippocampal-dependent cognitive deficits after TBI.
Methods
Experimental design
When performing fluid percussion injury studies, there is a considerable amount of physiological and metabolic variations. Therefore, sufficient numbers of animals are required to obtain proper statistical significance. In accord with this, we performed a sample-size estimate based on our historical data from over the past 15 years using SigmaStat (Systat Software, Inc., San Jose, CA) with a power of 0.80, indicating that there is an 80% chance of detecting a difference between groups. Based on this analysis, we determined that 5–10 rats are needed per group for each proposed experiment.
Animals
Eighty adult male Sprague-Dawley rats were randomly assigned to a 1-, 3-, or 5-week group (1-week group: TBI-vehicle, n=10; TBI-A20, n=10; 3-week group: sham-vehicle, n=5; sham-A20, n=5; TBI-vehicle, n=10; TBI-A20, n=10; 5-week group: sham-vehicle, n=5; sham-A20, n=5; TBI-vehicle, n=10; TBI-A20, n=10). Animal care was in accord with the guidelines set forth by the University of Miami Animal Care and Use Committee and the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Animals were housed in a temperature-controlled room (22°C) with a 12-h light/dark cycle. They had at least 7 days of acclimation time before undergoing any experimentation. All animals had access to food and water ad libitum, except for a 24-h fast before the surgical procedure to maintain consistent glucose levels.
Traumatic brain injury
Rats were anesthetized with 3% isoflurane in 30% O2/70% N2O and placed in a stereotaxic frame. Animals received a 4.8-mm craniotomy over the right parietal cortex, 3.8 mm posterior to bregma and 2.5 mm lateral to the mid-line. A modified plastic injury hub (3.5 mm inner diameter) was bonded to the skull over the exposed dura with cyanoacrylic adhesive. Twenty-four hours after craniotomy, animals were reanesthetized, intubated, and mechanically ventilated with 0.5–0.75% isoflurane. Pancuronium bromide (1.0 mg/kg) was administered intravenously to facilitate ventilation. The tail artery was cannulated to ensure that blood gas, pH, glucose, and mean arterial blood pressure (MABP) measurements were consistent among animals. Rectal and temporalis muscle thermometers measured core and brain temperatures using self-adjusting feedback warming lamps. All variables were maintained within physiological ranges from 15 min preceding TBI and for up to 45 min postinjury.
A moderate (1.8–2.2 atmosphere) fluid percussion-induced TBI was produced over the right parietal cortex. Sham animals underwent identical procedures, except for the fluid percussion insult. Thirty minutes post-TBI, animals were administered 10 mg/kg of A20 or vehicle intraperitoneally (i.p.) using a randomized selection process. Beginning 24 h after surgery, animals received injections every day twice per day for 7 days postsurgery. This dosage was selected based on previous studies showing an optimal ceiling effect and favorable brain distribution in rodent models of neurodegeneration and impaired hippocampal neurogenesis.37,38,40 Animals also received injections of a concentrated aqueous solution of 5-bromo-2′-deoxyuridine (BrdU) and 5-fluoro-2′-deoxyuridine labeling reagent (10:1; 1 mL/100g of rat body weight administered i.p.; Invitrogen Life Technologies, Grand Island, NY) 24 h post-TBI and daily thereafter for 7 days.
Physiology
All physiological variables, including pH, pO2, pCO2, and MABP, were within normal ranges before and after fluid percussion insult (Supplementary Table 1) (see online supplementary material at http://www.liebertpub.com). Animals displayed normal activity within 24 h after recovery from anesthesia as detected by visual inspection of grooming behavior, posture, and locomotion. There was no significant weight loss between experimental groups at time of injury or at perfusion fixation.
Behavior analyses
Spontaneous forelimb task
Sensorimotor performance was evaluated by an investigator blinded to experimental groups using the spontaneous forelimb task. A transparent Plexiglas cylinder (20 cm diameter and 30 cm high) encourages use of forelimbs for vertical wall exploration. Animals were placed in the cylinder for 5 min per test and videotaped. The videotape was played in slow motion (one-fifth real-time speed), and blinded observers counted the number of times the right forelimb, left forelimb, or both forelimbs simultaneously made contact with the cylinder wall during a rearing movement. Asymmetry index was calculated by dividing the number of contralateral placements by the total placements of both paws.42,43 Both 3- and 5-week groups were tested 1 day before surgery (baseline) and 7 days after TBI. Identical experimental procedures were carried out for both groups. The 1-week group did not undergo any behavioral testing. Data presented are from the 3-week animals. Similar outcomes were observed in the 5-week group (data not shown).
Spatial memory
One week before sacrifice (2 or 4 weeks after TBI or sham surgery), spatial memory was evaluated by the Morris water maze (MWM), which consisted of a circular pool (122 cm diameter and 60 cm deep) filled with water rendered opaque by white, nontoxic paint. Submerged heaters maintained water temperature at 22–25°C. A round platform (10 cm diameter) was submerged 5 cm beneath the water surface. The pool was divided into four equal-sized quadrants, one of which included the hidden platform. Prominent extramaze cues served as visual reference points. Swim patterns were recorded and analyzed by Ethovision (Noldus Information Technology, Leesburg, VA). Parameters evaluated included escape latency (time to locate platform), swim speed, path length to platform (distance traveled), and percentage of total time spent in each quadrant.
Animals were tested for 4 consecutive days, each day consisting of four trials at 5-min intervals. For each trial, animals were released from the edge of the pool into a randomly selected quadrant. Release point order was identical for all animal groups. During each trial, animals were allowed 60 sec to find the hidden platform. If they failed to locate it, they were guided to the platform. Animals were allowed to remain on the platform for an additional 30 sec.
Histopathology and immunohistochemistry
At 1 or 5 weeks post-TBI or sham procedures, animals were anesthetized with 3% isoflurane in 30% O2/70% N2O and transcardially perfused with saline (80 mL for 2 min), followed by 4% paraformaldehyde (PFA; 4°C, 350 mL at a pressure of 100–120 mmHg for 28 min). Brains were immediately removed, placed in 4% PFA for 48 h, and then cryoprotected in 20% sucrose in phosphate-buffered saline. Brains sections (45-μm thickness) were cut with a frozen sliding microtome (LEICA SM200R; Leica Microsystems, Inc., Buffalo Grove, IL). Serial sections at 270-μm intervals were immunostained with hematoxylin and eosin (H&E), goat anti-DCX (1:200), rat anti-BrdU (1:100), mouse anti-BrdU (1:200), or mouse anti-neuronal nuclei (NeuN; 1:500), as previously described.31,44 Development of immunostaining was conducted with goat anti-rat immunoglobulin G (1:200), horse anti-mouse (1:200), goat anti-rat Alexa Fluor 488 (1:200), or goat anti-mouse Alexa Fluor 594 (1:200).
Volumetric and stereological analyses
Antibody penetration was verified in all sections using 100×magnification. Cortical contusion volumes were determined by tracing the contused areas in serial H&E sections with a 5×objective on an Axiophot 200 M microscope (Zeiss Microscopy, LLC, Thornwood, NY) using Neurolucida software (MicroBrightfield, Inc., Williston, VT). Cortical contusion boundaries were well demarcated by hemorrhage and shearing at the gray/white matter (GM/WM) interface between the cortex and external capsule on the ipsilateral hemisphere. Contusion areas were calculated for six coronal levels proximal and distal to the epicenter of injury at −3.8 bregma (−0.8, −1.8, −3.3, −4.3, −5.8, and −6.3 mm posterior to bregma). Separate serial sections from −3.0 to −5.8 mm posterior to bregma were chosen to determine neuron survival within vulnerable cortical regions. NeuN-immunoreactive cells were quantified by a blinded observer in an unbiased manner using Stereoinvestigator software (MicroBrightfield, Inc., Williston, VT).45 The parietal cortex overlying the contusion area was contoured at 5×, and a counting grid of 150×150 μm was placed over the contoured parietal cortex. Using a 35×35 μm counting frame, NeuN-positive cells were counted in 30–40 randomly placed sampling sites with a 63×1.4 numerical aperture objective. Stereoinvestigator software was also used to conduct quantitative assessment of BrdU-, DCX-, and BrdU/NeuN-immunoreactive cells.45 The DG cell layer was contoured using a BX51TRF Olympus microscope (Olympus America, Center Valley, PA) at 4×magnification. A blinded observer analyzed five sections between bregma levels −3.0 and −5.0 mm posterior to bregma using a 70×70 μm counting frame in 40–55 randomly placed sampling sites and a PlanApo N 60X/1.42 oil objective.
Statistical analysis
Data are expressed as mean±standard error of the mean. Data were analyzed using the Student's t-test, one-way analysis of variance (ANOVA), or two-way repeated-measures ANOVA, depending on the outcome measure. Post-hoc analysis was performed using Bonferroni's corrections. Significance level was p<0.05 using two-tailed testing.
Results
A20 decreased contusion volume and blocked mature cortical neuron death after traumatic brain injury
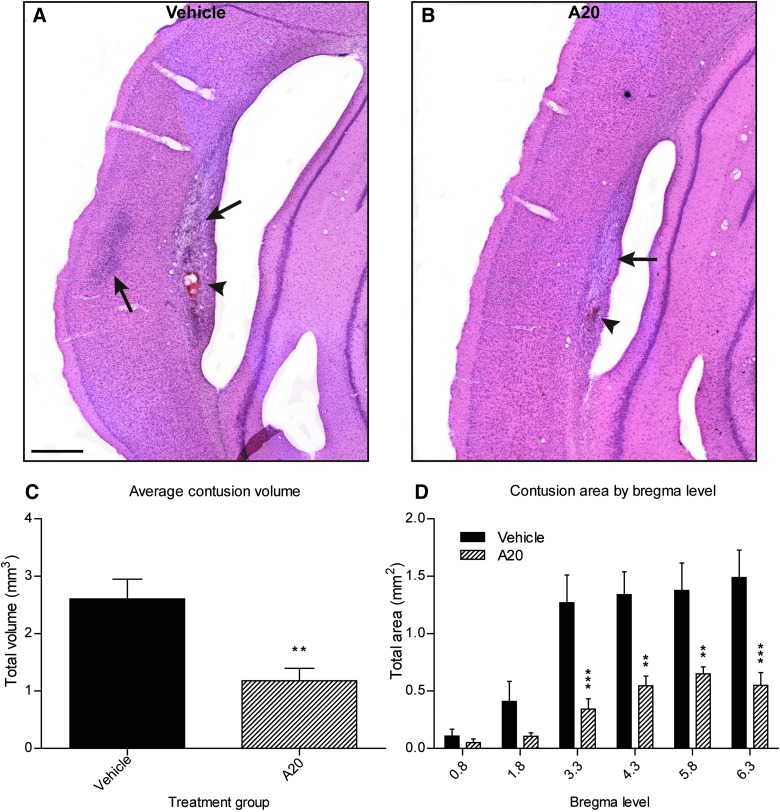
Consistent with our previous experience with the rat model of fluid percussion-induced moderate TBI, we observed well-demarcated contusions along the GM/WM interface between the cortex and external capsule on the ipsilateral, traumatized hemisphere (Fig. 1A,B). Volumetric analyses of contusion areas at 1 week post-TBI demonstrated that animals receiving A20 had significantly reduced contusions at multiple bregma levels surrounding the site of injury (Fig. 1D). A20 administration reduced average contusion volume 2-fold, compared to vehicle-treated control animals (Fig. 1C).
FIG. 1.
Administration of A20 significantly decreased contusion volumes 1 week post-TBI. (A and B) Representative images showing contusions at gray/white matter interface in vehicle and A20 animals, respectively. Black arrows show contusions at ipsilateral external capsule and parietal cortex; arrowheads indicate areas of intracerebral hemorrhage. (C) Contusions were contoured and volumetric analyses were conducted at six bregma levels stained with H&E. A20 animals exhibited greater than a 2-fold decrease in average contusion volumes, compared to vehicle control animals (Student's t-test: p=0.0025; t=3.504). (D) A20 exerted significant cytoprotective effects at four of six bregma levels (epicenter of injury, −3.8 mm). Two-way repeated-measures ANOVA established significance for bregma level (p<0.0001), treatment (p=0.0012), and treatment×bregma level interaction (p=0.0002). Bonferroni's post-hoc analysis showed significant differences in contusion area at the four most posterior bregma levels evaluated (**p<0.01; ***p<0.001; scale bar, 500 μm). Data are expressed as mean±standard error of the mean; n=20. TBI, traumatic brain injury; H&E, hematoxylin and eosin; ANOVA, analysis of variance.
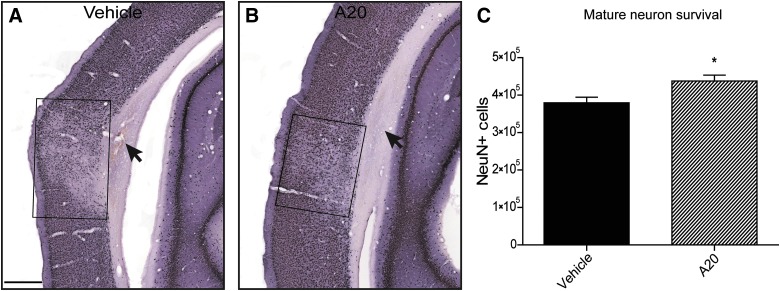
In addition to a subcortical contusion after TBI, we observed selective neuronal dropout within a reproducibly located pericontusional cortical region (Fig. 2A,B). NeuN-immunoreactive (a marker of mature neurons) cell survival in this susceptible cortical region was quantified. At 1 week postinjury, A20 administration resulted in significantly greater numbers of NeuN-positive cells in ipsilateral parietal cortical regions, compared to vehicle-treated TBI animals (Fig. 2C). Further, TBI control animals exhibited expansion of lateral ventricles and greater external capsule loss (Fig. 2A).
FIG. 2.
A20 administration was neuroprotective in vulnerable ipsilateral cortical regions 1 wk post TBI. (A and B) Representative NeuN-stained images of vehicle- (A) and A20-treated (B) animals. Selective neuronal loss was observed in the cortical region overlying the contusion site. Treatment with A20 significantly reduced NeuN-positive cell loss. Boxes in A, B enclose the areas of neuronal loss. Arrows indicate subcortical lesion. (C) Quantification of NeuN-positive cells between treatment groups revealed a significant neuroprotective effect of A20 1 wk post injury (Student's t-test: p=0.015, t=2.695; *p<0.05; scale bar, 500 μm); n=20. TBI, traumatic brain injury; NeuN, anti-neuronal nuclei.
A20 increased generation of new hippocampal neurons in the dentate gyrus after traumatic brain injury
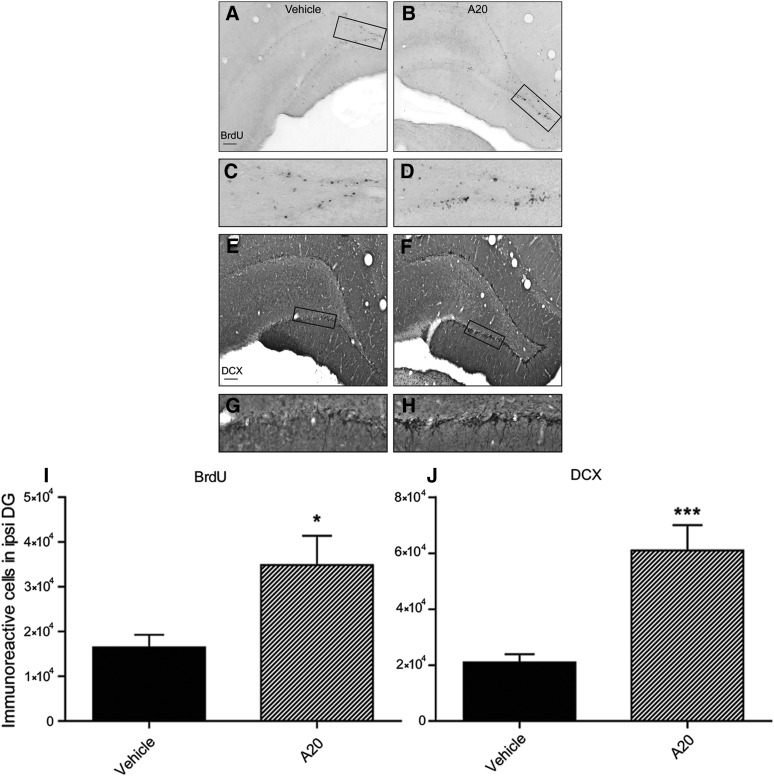
To determine whether A20 would significantly increase hippocampal neurogenesis in the DG, animals were injected with BrdU, a thymidine analog marker for dividing cells that is incorporated into newly replicated DNA during the s phase of the cell cycle. After TBI, A20 administration resulted in greater numbers of BrdU-positive cells observed throughout the full extent of the blades of the DG. In contrast, TBI-vehicle animals had less BrdU-positive cells, which were localized predominately to the apex of the DG (Fig. 3A–D,I).
FIG. 3.
Administration of A20 increased progenitor cell and immature neuron proliferation 1 week post-TBI. Representative images showing BrdU- (A–D) and DCX-immunoreactive cells (E–H) in TBI vehicle- (left) and A20- (right) treated groups. Images in (C and D) and (G and H) are magnified regions of boxed areas in (A), (B), (E), and (F). (I and J) Quantification of BrdU- and DCX- positive cells. A20 administration significantly increased both BrdU- and DCX-positive cells, indicative of early stage neurogenesis (Student's t-test: BrdU: p=0.0163, t=2.665; DCX: p=0.0006, t=4.174; *p<0.05; ***p<0.001; scale bars, 200 μm); n=20. TBI, traumatic brain injury; BrdU, bromodeoxyuridine; DCX, doublecortin.
After TBI, BrdU-positive cells have been shown to differentiate into astrocytes and microglia as well as neurons.26,46 Thus, to determine whether A20 enhanced the generation of new neurons, we performed immunohistochemical (IHC) staining with DCX, a marker of immature neurons at an early stage of neurogenesis. At 1 week post-TBI, there was a significant increase in DCX-positive cells in animals administered A20, compared to vehicle controls (Fig. 3J). As with BrdU staining, DCX-labeled cells in A20 animals were more widely dispersed throughout the DG than was observed in vehicle-treated animals after TBI (Fig. 3E–H).
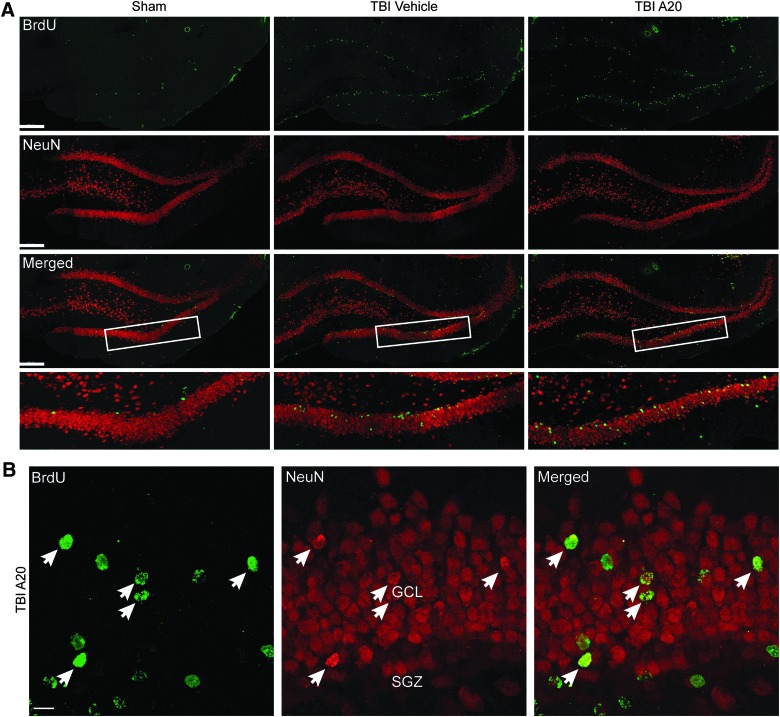
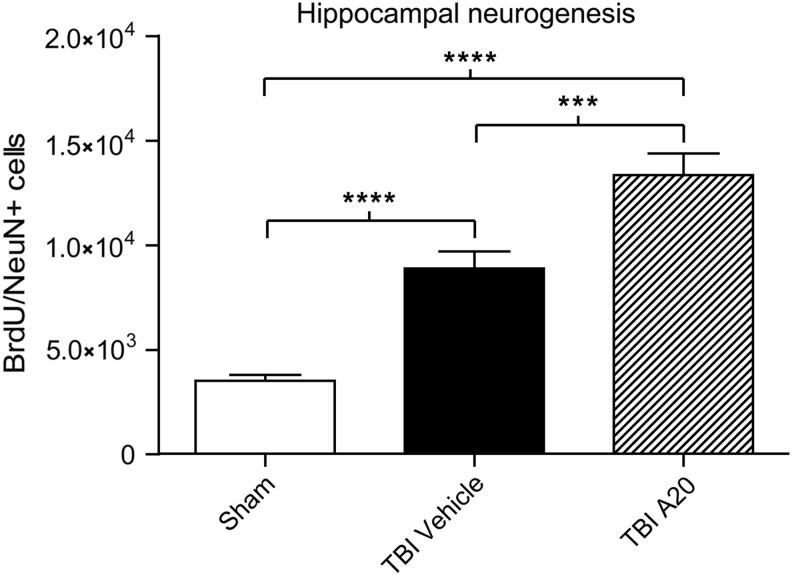
Under normal conditions, newly born DG neurons in rats take approximately 4 weeks to mature and integrate within the hippocampal circuitry.47 To determine whether proliferating immature neurons observed at 1 week also survived to this stage, we double stained cells in the SGZ for BrdU and NeuN 5 weeks after TBI. In these chronic experiments, animals were assigned to a sham-vehicle, sham-A20, TBI-vehicle, or TBI-A20 group. No differences were observed between sham-vehicle and sham-A20 groups, so they were collapsed into a single sham group for data analysis. Five weeks after TBI or sham surgery, TBI-vehicle and TBI-A20 groups both had BrdU/NeuN-positive cells in the ipsilateral DG (Fig. 4A,B). In sham animals, confocal micrographs revealed that BrdU/NeuN-positive cells were sparse and confined to the SGZ (Fig. 4A). Quantification of double-labeled cells supported these qualitative observations (Fig. 5). The TBI-vehicle group had a significantly greater number of double-labeled cells, relative to sham animals, which is consistent with acute proliferative responses induced by TBI. However, A20 administration after TBI further augmented hippocampal neurogenesis, as demonstrated by more-robust levels of BrdU/NeuN-positive cells in the ipsilateral DG. Cells coexpressing BrdU/NeuN in TBI-A20 animals appeared to migrate outside of the SGZ into the granular layer of the DG (Fig. 4B), consistent with maturation profiles of newborn neurons.
FIG. 4.
Confocal micrographs showing BrdU- and NeuN-immunoreactive cells within the DG 5 weeks after TBI. (A) Representative fluorescent images showing cells that colabeled for BrdU and NeuN in the ipsilateral DG (scale bars, 200 μm). Boxed regions outline the areas of higher magnification shown in bottom row of (A). (B) Representative micrographs from a TBI-A20 animal showing several cells coexpressing markers for BrdU and NeuN (arrows) in the ipsilateral DG. Double-labeled cells in A20 animals appeared to migrate out of the SGZ into more superficial layers of the DG, consistent with neuronal maturation profiles (GCL, granular cell layer; SGZ, subgranular zone; scale bar, 10 μm). BrdU, bromodeoxyuridine; NeuN, anti-neuronal nuclei; DG, dentate gyrus; TBI, traumatic brain injury.
FIG. 5.
Quantitative assessment of hippocampal neurogenesis 5 weeks after TBI. A20-treated TBI animals demonstrated significant increases in numbers of BrdU/NeuN-positive cells, compared to vehicle-treated and sham-operated controls. TBI-vehicle controls had significantly more double-labeled cells than sham-operated animals. One-way analysis of variance showed significance (p<0.0001, F=46.16); Bonferroni's multiple comparison test showed significant differences among groups (***p<0.001; ****p<0.0001); n=27. BrdU, bromodeoxyuridine; NeuN, anti-neuronal nuclei; TBI, traumatic brain injury.
A20 improved functional neurological outcome 1 and 4 weeks after traumatic brain injury
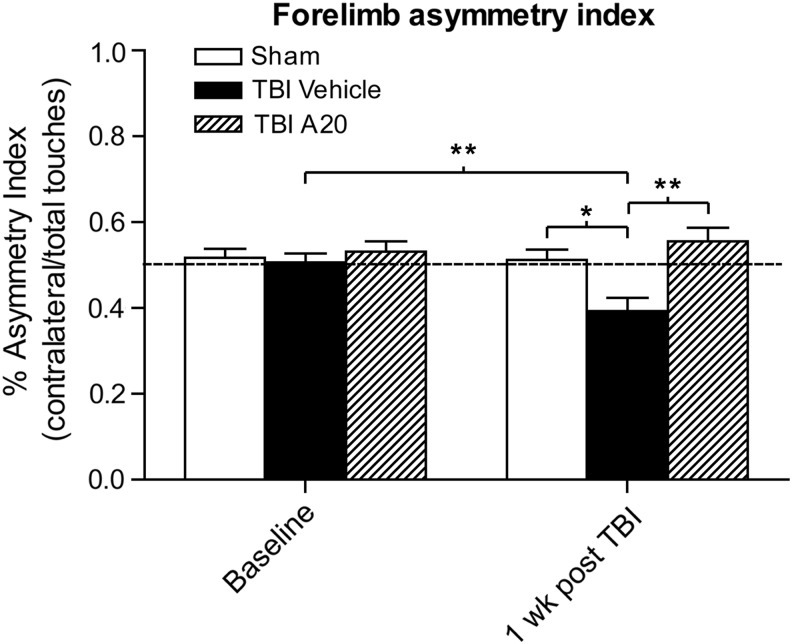
A significant functional deficit commonly observed in this model of moderate TBI is a contralateral sensorimotor deficit, in which a right-hemisphere traumatic insult results in a persistent left-forelimb deficit that can be quantified using the spontaneous forelimb task.42 This task encourages use of the forelimbs during vertical wall exploration, and animals with deficits do not use forelimbs equally. We evaluated baseline sensorimotor ability 1 day before TBI and then measured again 1 week after TBI (Fig. 6). Before surgery, all animal groups used both forelimbs equally. At 1 week post-TBI, however, TBI-vehicle control animals demonstrated a significant contralateral forelimb deficit. By contrast, TBI animals receiving A20 exhibited no contralateral forelimb sensorimotor deficit, performing equally well to sham-operated animals and significantly better than their TBI-control counterparts.
FIG. 6.
A20 protected from contralateral forelimb deficits 1 week post-TBI. An asymmetry index of less than 50% indicates a contralateral deficit. All animal groups performed similarly presurgery. One week after TBI, vehicle-treated controls exhibited a significant contralateral forelimb deficit, compared to their baseline time point (Student's t-test: p=0.0095; t=3.005). Contralateral forelimb usage in the TBI-vehicle control group 1 week postinjury was significantly decreased, compared to sham-uninjured animals, consistent with this injury model. In contrast, TBI animals treated with A20 showed no signs of a contralateral deficit and performed similar to sham-operated animals and significantly better than TBI-vehicle controls (one-way analysis of variance 1-week time point: p=0.0022, F=7.996; Bonferroni's multiple comparison test: sham vs. TBI-vehicle: *p<0.05; TBI-vehicle vs. TBI-A20: **p<0.01); n=27. TBI, traumatic brain injury.
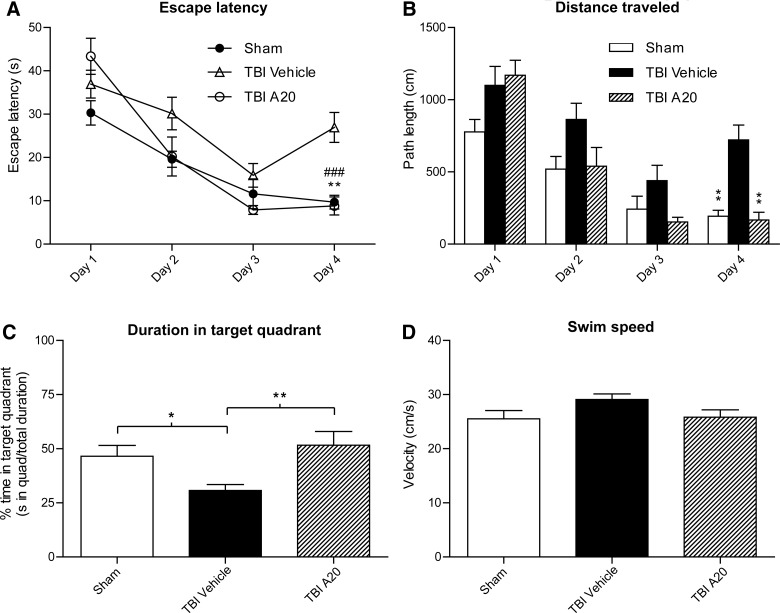
We next investigated whether A20 administration could improve the spatial memory deficits that are frequently observed in this model of TBI.5,42 Hippocampal-dependent cognitive capacity was evaluated using the MWM hidden platform task 4 weeks post-TBI. This interval was selected based on the temporal maturation and electrophysiological profiles of newly generated hippocampal neurons. After 16 trials over a period of 4 days, TBI-control animals exhibited significant spatial memory deficits. Injured animals receiving A20, however, performed significantly better than the TBI-vehicle control group in several parameters used to evaluate hippocampal-dependent spatial memory. These parameters included escape latency (Fig. 7A), path length (Fig. 7B), and time spent in the target quadrant (where the platform was submerged; Fig. 7C). TBI-vehicle animals did not exhibit a monotonic swim pattern during the hidden platform task (Fig. 7A,B). Swim speed was not significantly different among groups, indicating that the observed differences were not a result of an inability to execute the swim task (Fig. 7D).
FIG. 7.
A20 administration significantly improved Morris water maze performance 4 weeks after TBI. (A) Post-hoc analysis revealed that TBI-A20 and sham animals had significantly reduced escape latencies on the hidden platform task, relative to TBI-vehicle controls, on the fourth day of testing (Bonferroni's multiple comparison test: TBI-A20 vs. TBI-vehicle, **p<0.01; sham vs. TBI-vehicle, ###p<0.001). Two-way repeated measures analysis of variance (ANOVA) was significant for trial day (p<0.0001), treatment (p=0.007), and treatment×trial day interaction (p=0.0005). (B) By day 4, sham and TBI-A20 animals exhibited significantly shorter path lengths to reach the platform, compared to TBI-vehicle controls (Bonferroni's multiple comparison test: **p<0.01). Two-way repeated-measures ANOVA was significant for trial day (p<0.0001), treatment (p=0.0013), and treatment×trial day interaction (p<0.016). (C) On day 4, sham and TBI-A20 animals spent a greater percentage of time in the target quadrant, compared to TBI-vehicle control animals (Bonferroni's multiple comparison test: *p<0.05; **p<0.01). Two-way repeated-measures ANOVA showed significance for trial day (p<0.0001) and treatment (0.0168), but not for treatment×trial day interaction (p=0.131). (D) There were no significant differences in swim speeds among groups, suggesting that the injury did not affect swimming skills (one-way ANOVA: p=0.129); n=27. TBI, traumatic brain injury.
Discussion
Results of this study demonstrate that treatment with the proneurogenic/neuroprotective compound, A20, after TBI reduced overall contusion volume and improved pericontusional neuronal survival. A20 treatment also increased the magnitude of hippocampal neurogenesis in the SGZ 5 weeks postinjury. These morphological changes correlated with improved sensorimotor and cognitive function, which are significant clinical problems experienced by patients after TBI. This study is the first to show both neuroprotective and neurogenic effects of A20, one of the new P7C3 class of neuroprotective agents, in an established model of TBI.
The proneurogenic capacity of A20 is thought to be a result of the protection of newly generated hippocampal neurons from apoptotic cell death.37 In a previous study, A20 preserved mitochondrial membrane integrity after a calcium challenge in a dose-dependent manner.37 Preservation of MMPs reduces activation of intrinsic proapoptotic pathways,48,49 one of the most detrimental and pervasive secondary injury mechanisms after TBI.48–55 Cortical and hippocampal neurons are particularly vulnerable to trauma and apoptotic mechanisms, and their survival has been targeted for various therapeutic interventions with only limited success.55 Currently, the cellular and molecular mechanisms underlying the benefits of A20 treatment after TBI are unknown. Ongoing studies in the laboratory are investigating whether A20 treatment after TBI targets molecular indicators of apoptosis related to mitochondrial dysfunction or other injury cascades. In the present study, animals administered A20 had significant preservation of vulnerable NeuN-positive mature cortical neurons in the surrounding cytoarchitecture of the traumatized hemisphere. Using the spontaneous forelimb task to evaluate sensorimotor capacity, we observed that A20-treated animals performed at similar levels to sham-operated animals, and significantly better than vehicle controls, 1 week after TBI. The beneficial effects of A20 treatment on significantly reducing contusion volume and protecting against cortical neuron vulnerability may participate in this clinically relevant sensorimotor improvement.
In previous TBI studies, the vulnerability of SGZ immature neurons has been identified as a prominent feature of damage.30–34 Several studies have shown that hippocampal proliferation is acutely increased after TBI; however, this cellular response appears to be transient and the chronic loss of immature neurons has been reported to persist up to 4 months.30–32 Here, we report that A20 administration after injury increased the magnitude of hippocampal neurogenesis in the ipsilateral DG. The ability of A20 treatment after TBI to enhance survival of newborn hippocampal neurons may be a significant and new therapeutic treatment strategy targeting endogenous reparative processes.
Newly generated hippocampal neurons take approximately 4 weeks to functionally integrate within the hippocampal circuitry.56 Animals receiving A20 performed better overall than TBI vehicle controls on the MWM at 4 weeks after TBI. Several specific measureable parameters, including escape latency, total distance traveled, and percentage of time spent in the target quadrant, supported the conclusion that administration of A20 in the early post-traumatic period improved cognitive outcome. Consistent with the notion of time-dependent neuronal maturation profiles, we also tested a separate group of animals on spatial memory capacity 2 weeks post-TBI, preceding when the majority of newly generated neurons are functionally incorporated into hippocampal circuitry. At this earlier time point, A20-treated animals performed no better than TBI-vehicle controls (Supplementary Fig 2) (see online supplementary material at http://www.liebertpub.com). Thus, based on the established time-dependent progression of neurogenesis and the integration of new neurons into the hippocampal circuitry, our IHC and behavioral findings support the view that augmentation of hippocampal neurogenesis by administration of A20 after TBI was a significant factor underlying improved cognitive function.
In terms of the potential to translate this discovery to the clinic, there are many advantages of this therapeutic compound, including desirable toxicity profiles, good blood/brain distribution, sufficient oral availability, and excellent metabolic stability in rodents.38 Further, the effects of P7C3 compounds have been demonstrated in other models of central nervous system neurodegeneration,40,41 including models of reduced adult hippocampal neurogenesis.37 Additional experiments evaluating dose response, therapeutic window, and the testing of efficacy in other TBI models would be warranted for future clinical consideration. These findings suggest that the newly discovered P7C3 class of neuroprotective agents might promote clinically relevant functional recovery in patients after TBI by preserving mature brain structures and helping to promote endogenous reparative processes within the hippocampus.
Supplementary Material
Acknowledgments
The authors thank Mr. David Sequeira, Mrs. Ofelia Furones-Alonso, and Ms. Jessie Truettner for their technical contributions to this study. The authors also thank Dr. Pantelis Tsoulfas for his critical review of the manuscript. The studies were funded by the National Institutes of Health (NS030291; to W.D.D.), Veterans Affairs (1 I01 BX000521; to H.M.B.), a Transformative RO1 grant (NIMH 1RO1MH087986) awarded to A.A.P. and Steven L. McKnight, and unrestricted endowment funds provided to Steven L. McKnight from an anonymous donor.
Author Disclosure Statement
The authors declare no competing financial interests exist.
References
- 1.Langlois J.A., and Rutland-Brown W. (2005) Traumatic Brain Injury in the United States: The Future of Registries and Data Systems. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA [Google Scholar]
- 2.Bramlett H.M., and Dietrich W.D. (2007). Progressive damage after brain and spinal cord injury: pathomechanisms and treatment strategies. Prog. Brain Res. 161, 125–141 [DOI] [PubMed] [Google Scholar]
- 3.Lowenstein D.H., Thomas M.J., Smith D.H., and McIntosh T.K. (1992). Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J. Neurosci. 12, 4846–4853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kotapka M.J., Gennarelli T.A., Graham D.I., Adams J.H., Thibault L.E., Ross D.T., and Ford I. (1991). Selective vulnerability of hippocampal neurons in acceleration-induced experimental head injury. J. Neurotrauma 8, 247–258 [DOI] [PubMed] [Google Scholar]
- 5.Bramlett H.M., Green E.J., and Dietrich W.D. (1997). Hippocampally dependent and independent chronic spatial navigational deficits following parasagittal fluid percussion brain injury in the rat. Brain Res. 762, 195–202 [DOI] [PubMed] [Google Scholar]
- 6.Witgen B.M., Lifshitz J., Smith M.L., Schwarzbach E., Liang S.L., Grady M.S., and Cohen A.S. (2005). Regional hippocampal alteration associated with cognitive deficit following experimental brain injury: a systems, network and cellular evaluation. Neuroscience 133, 1–15 [DOI] [PubMed] [Google Scholar]
- 7.Altman J., and Das G.D. (1965). Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 124, 319–335 [DOI] [PubMed] [Google Scholar]
- 8.Zhao C., Teng E.M., Summers R.G., , Jr., Ming G.L., and Gage F.H. (2006). Distinct morphological stages of dentate granule neuron maturation in the adult mouse hippocampus. J. Neurosci. 26, 3–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao C., Deng W., and Gage F.H. (2008). Mechanisms and functional implications of adult neurogenesis. Cell 132, 645–660 [DOI] [PubMed] [Google Scholar]
- 10.Gross C.G. (2000). Neurogenesis in the adult brain: death of a dogma. Nat. Rev. Neurosci. 1, 67–73 [DOI] [PubMed] [Google Scholar]
- 11.Abrous D.N., Koehl M., and Le Moal M. (2005). Adult neurogenesis: from precursors to network and physiology. Physiol. Rev. 85, 523–569 [DOI] [PubMed] [Google Scholar]
- 12.Gage F.H. (2002). Neurogenesis in the adult brain. J. Neurosci. 22, 612–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Emery D.L., Fulp C.T., Saatman K.E., Schutz C., Neugebauer E., and McIntosh T.K. (2005). Newly born granule cells in the dentate gyrus rapidly extend axons into the hippocampal CA3 region following experimental brain injury. J. Neurotrauma 22, 978–988 [DOI] [PubMed] [Google Scholar]
- 14.Toni N., Laplagne D.A., Zhao C., Lombardi G., Ribak C.E., Gage F.H., and Schinder A.F. (2008). Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat. Neurosci. 11, 901–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Praag H., Schinder A.F., Christie B.R., Toni N., Palmer T.D., and Gage F.H. (2002). Functional neurogenesis in the adult hippocampus. Nature 415, 1030–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng W., Aimone J.B., and Gage F.H. (2010). New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 11, 339–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Drapeau E., Mayo W., Aurousseau C., Le Moal M., Piazza P.V., and Abrous D.N. (2003). Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc. Natl. Acad. Sci. U. S. A. 100, 14385–14390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dupret D., Revest J.M., Koehl M., Ichas F., De Giorgi F., Costet P., Abrous D.N., and Piazza P.V. (2008). Spatial relational memory requires hippocampal adult neurogenesis. PLoS One 3, e1959, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun D., McGinn M.J., Zhou Z., Harvey H.B., Bullock M.R., and Colello R.J. (2007). Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Exp. Neurol. 204, 264–272 [DOI] [PubMed] [Google Scholar]
- 20.van Praag H., Kempermann G., and Gage F.H. (1999). Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 2, 266–270 [DOI] [PubMed] [Google Scholar]
- 21.Gould E., Beylin A., Tanapat P., Reeves A., and Shors T.J. (1999). Learning enhances adult neurogenesis in the hippocampal formation. Nat. Neurosci. 2, 260–265 [DOI] [PubMed] [Google Scholar]
- 22.Gaulke L.J., Horner P.J., Fink A.J., McNamara C.L., and Hicks R.R. (2005). Environmental enrichment increases progenitor cell survival in the dentate gyrus following lateral fluid percussion injury. Brain Res. Mol. Brain Res. 141, 138–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuhn H.G., Dickinson-Anson H., and Gage F.H. (1996). Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 16, 2027–2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McDonald H.Y., and Wojtowicz J.M. (2005). Dynamics of neurogenesis in the dentate gyrus of adult rats. Neurosci. Lett. 385, 70–75 [DOI] [PubMed] [Google Scholar]
- 25.Lazarov O., Mattson M.P., Peterson D.A., Pimplikar S.W., and van Praag H. (2010). When neurogenesis encounters aging and disease. Trends Neurosci. 33, 569–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chirumamilla S., Sun D., Bullock M.R., and Colello R.J. (2002). Traumatic brain injury induced cell proliferation in the adult mammalian central nervous system. J. Neurotrauma 19, 693–703 [DOI] [PubMed] [Google Scholar]
- 27.Dash P.K., Mach S.A., and Moore A.N. (2001). Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J. Neurosci. Res. 63, 313–319 [DOI] [PubMed] [Google Scholar]
- 28.Gao X., Enikolopov G., and Chen J. (2009). Moderate traumatic brain injury promotes proliferation of quiescent neural progenitors in the adult hippocampus. Exp. Neurol. 219, 516–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urrea C., Castellanos D.A., Sagen J., Tsoulfas P., Bramlett H.M., and Dietrich W.D. (2007). Widespread cellular proliferation and focal neurogenesis after traumatic brain injury in the rat. Restor. Neurol. Neurosci. 25, 65–76 [PubMed] [Google Scholar]
- 30.Gao X., Deng-Bryant Y., Cho W., Carrico K.M., Hall E.D., and Chen J. (2008). Selective death of newborn neurons in hippocampal dentate gyrus following moderate experimental traumatic brain injury. J. Neurosci. Res. 86, 2258–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Atkins C.M., Truettner J.S., Lotocki L., Sanchez-Molano J., Kang Y., Alonso O.F., Sick T.J., Dietrich W.D., and Bramlett H.M. (2010). Post-traumatic seizure susceptibility is attenuated by hypothermia therapy. Eur. J. Neurosci. 28, 35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X.H., Iwata A., Nonaka M., Browne K.D., and Smith D.H. (2003). Neurogenesis and glial proliferation persist for at least one year in the subventricular zone following brain trauma in rats. J. Neurotrauma 20, 623–631 [DOI] [PubMed] [Google Scholar]
- 33.Blaiss C.A., Yu T.S., Zhang G., Chen J., Dimchev G., Parada L.F., Powell C.M., and Kernie S.G. (2011). Temporally specified genetic ablation of neurogenesis impairs cognitive recovery after traumatic brain injury. J. Neurosci. 31, 4906–4916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rola R., Mizumatsu S., Otsuka S., Morhardt D.R., Noble-Haeusslein L.J., Fishman K., Potts M.B., and Fike J.R. (2006). Alterations in hippocampal neurogenesis following traumatic brain injury in mice. Exp. Neurol. 202, 189–199 [DOI] [PubMed] [Google Scholar]
- 35.Kernie S.G., and Parent J.M. (2010). Forebrain neurogenesis after focal ischemic and traumatic brain injury. Neurobiol. Dis. 37, 267–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Snyder J.S., Hong N.S., McDonald R.J., and Wojtowicz J.M. (2005). A role for adult neurogenesis in spatial long-term memory. Neuroscience 130, 843–852 [DOI] [PubMed] [Google Scholar]
- 37.Pieper A.A., Xie S., Capota E., Estill S.J., Zhong J., Long J.M., Becker G.L., Huntington P., Goldman S.E., Shen C., Capota M., Britt J.K., Kotti T., Ure K., Brat D.J., Williams N.S., MacMillian K.S., Naidoo J., Melito L., Hsieh J., De Brabander J., Ready J.M., and McKnight S.L. (2010). Discovery of a proneurogenic, neuroprotective chemical. Cell 142, 39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacMillan K.S., Naidoo J., Liang J., Melito L., Williams N.S., Morlock L., Huntington P.J., Estill S.J., Longgood J., Becker G.L., McKnight S.L., Pieper A.A., De Brabander J.K., and Ready J.M. (2011). Development of proneurogenic, neuroprotective small molecules. J. Am. Chem. Soc. 133, 1428–1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pieper A.A., Wu X., Han T.W., Estill S.J., Dang Q., Wu L.C., Reece-Fincanon S., Dudley C.A., Richardson J.A., Brat D.J., and McKnight S.L. (2005). The neuronal PAS domain protein 3 transcription factor controls FGF-mediated adult hippocampal neurogenesis in mice. Proc. Natl. Acad. Sci. U. S. A. 102, 14052–14057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Jesús-Cortés H., Xu P., Drawbridge J., Estill S.J., Huntington P., Tran S., Britt J., Tesla R., Morlock L., Naidoo J., Melito L.M., Wang G., Williams N.S., Ready J.M., McKnight S.L., and Pieper A.A. (2012). Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of Parkinson disease. Proc. Natl. Acad. Sci. U. S. A. 109, 17010–17015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tesla R., Wolf H.P., Xu P., Drawbridge J., Estill S.J., Huntington P., McDaniel L., Knobbe W., Burket A., Tran S., Starwalt R., Morlock L., Naidoo J., Williams N.S., Ready J.M., McKnight S.L., and Pieper A.A. (2012). Neuroprotective efficacy of aminopropyl carbazoles in a mouse model of amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. U. S. A. 109, 17016–17021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bramlett H.M., Green E.J., Dietrich W.D., Busto R., Globus M.Y.T., and Ginsberg M.D. (1995). Posttraumatic brain hypothermia provides protection from sensorimotor and cognitive behavioral deficits. J. Neurotrauma 12, 289–298 [DOI] [PubMed] [Google Scholar]
- 43.Baskin Y.K., Dietrich W.D., and Green E.J. (2003). Two effective behavioral tasks for evaluating sensorimotor dysfunction following traumatic brain injury in mice. J. Neurosci. Methods 129, 87–93 [DOI] [PubMed] [Google Scholar]
- 44.Bregy A., Nixon R., Lotocki G., Alonso O.F., Atkins C.M., Tsoulfas P., Bramlett H.M., and Dietrich W.D. (2012). Posttraumatic hypothermia increases doublecortin expressing neurons in the dentate gyrus after traumatic brain injury in the rat. Exp. Neurol. 233, 821–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gundersen H.J.G., Bagger P., Bendtsen T.F., Evans S.M., Korbo L., Marcussen N., Møller A., Nielsen K., Nyengaard J.R., Pakkenberg B., Sørensen F.B., Vesterby A., and West M.J. (1988). The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS 96, 857–881 [DOI] [PubMed] [Google Scholar]
- 46.Di Giovanni S., Movsesyan V., Ahmed F., Cernak I., Schinelli S., Stoica B., and Faden A.I. (2005). Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proc. Natl. Acad. Sci. U. S. A. 102, 8333–8338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ming G.L., and Song H. (2005). Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 28, 223–250 [DOI] [PubMed] [Google Scholar]
- 48.Gottlieb E., Armour S.M., Harris M.H., and Thompson C.B. (2003). Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. 10, 709–717 [DOI] [PubMed] [Google Scholar]
- 49.Kroemer G., Dallaporta B., and Resche-Rigon M. (1998). The mitochondrial death/life regulator in apoptosis and necrosis. Annu. Rev. Physiol. 60, 619–642 [DOI] [PubMed] [Google Scholar]
- 50.Keane R.W., Kraydieh S., Lotocki G., Alonso O.F., Aldana P., and Dietrich W.D. (2001). Apoptotic and antiapoptotic mechanisms after traumatic brain injury. J. Cereb. Blood Flow Metab. 21, 1189–1198 [DOI] [PubMed] [Google Scholar]
- 51.Newcomb J.K., Zhao X., Pike B.R., and Hayes R.L. (1999). Temporal profile of apoptotic-like changes in neurons and astrocytes following controlled cortical impact injury in the rat. Exp. Neurol. 158, 76–88 [DOI] [PubMed] [Google Scholar]
- 52.Raghupathi R., Conti A.C., Graham D.I., Krajewski S., Reed J.C., Grady M.S., Trojanowski J.Q., and McIntosh T.K. (2002). Mild traumatic brain injury induces apoptotic cell death in the cortex that is preceded by decreases in cellular Bcl-2 immunoreactivity. Neuroscience 110, 605–616 [DOI] [PubMed] [Google Scholar]
- 53.Raghupathi R., Graham D.I., and McIntosh T.K. (2000). Apoptosis after traumatic brain injury. J. Neurotrauma 17, 927–938 [DOI] [PubMed] [Google Scholar]
- 54.Conti A.C., Raghupathi R., Trojanowski J.Q., and McIntosh T.K. (1998). Experimental brain injury induces regionally distinct apoptosis during the acute and delayed post-traumatic period. J. Neurosci. 18, 5663–5672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yakovlev A.G., Knoblach S.M., Fan L., Fox G.B., Goodnight R., and Faden A.I. (1997). Activation of CPP32-like caspases contributes to neuronal apoptosis and neurological dysfunction after traumatic brain injury. J. Neurosci. 17, 7415–7424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Y., Mu Y., and Gage F.H. (2009). Development of neural circuits in the adult hippocampus. Curr. Top. Dev. Biol. 87, 149–174 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.