Abstract
Damage to cerebral systems is frequently followed by the emergence of compensatory mechanisms, which serve to reduce the effects of brain damage and allow recovery of function. Intrinsic recovery, however, is rarely complete. Non-invasive brain stimulation technologies have the potential to actively shape neural circuits and enhance recovery from brain damage. In this study, a stable deficit for detecting and orienting to visual stimuli presented in the contralesional visual hemifield was generated by producing unilateral brain damage of the right posterior parietal and contiguous visual cortical areas. A long regimen of inhibitory non-invasive transcranial direct-current stimulation (cathodal 2mA, 20 min) was applied to the contralateral (intact) posterior parietal cortex over 14 weeks (total of 70 sessions, one per day, five days per week) and behavioral outcomes were periodically assessed. In three out of four stimulated cats, lasting recovery of visuospatial function was observed. Recovery started after 2–3 weeks of stimulation, and recovered targets were located first in the periphery, and moved to more central visual field locations with the accrual of stimulation sessions. Recovery for moving tasks followed a biphasic pattern before reaching plateau levels. Recovery did not occur for more difficult visual tasks. These findings highlight the ability of multiple sessions of transcranial direct-current stimulation to produce recovery of visuospatial function after unilateral brain damage.
Keywords: cathodal tDCS, brain damage, animal model, contralesional, neglect, cat
Introduction
Recovery from brain damage is limited in large part by the restricted ability of the central nervous system to structurally regenerate after injury. The recovery that does occur relies on functional reorganization to change function at the areal level or to promote the activity of secondary pathways that reroute function around the lesion. However, these intrinsic mechanisms rarely produce full recovery.
In the last decade, non-invasive brain stimulation technologies such as transcranial direct-current stimulation (tDCS) have been used to activate functional reorganization and promote higher levels of recovery after brain damage (Sparing et al., 2009). TDCS uses weak electric currents to penetrate extraneural tissues, polarize brain regions and influence the ability of neurons to fire. While the precise neural effects of tDCS are highly complex and likely to depend on factors such as the orientation of somatodendritic axis and axonal axis relative to the electric field as well as non-linear effects of stimulation intensity (Bikson et al., 2004; Radman et al., 2009; Kabakov et al., 2012; Batsikadze et al., 2013), placing the anodal tDCS electrode over a brain area is generally thought to induce a lasting increase in brain activity under the electrode, while cathodal tDCS generally reduces neural excitability (Bindman et al., 1964; Purpura & McMurtry, 1965; Nitsche & Paulus, 2000; Stagg & Nitsche, 2011). TDCS effects outlast the period of stimulation and, as with other neurostimulation techniques, a greater number of stimulation sessions is thought to increase the efficacy and size of the effect (Valero-Cabré et al., 2008; Reis et al., 2009; Afifi et al., 2013; Monte-Silva et al., 2013). This characteristic could be utilized to promote neuroplastic mechanisms and restore function after cerebral damage. However, the potential of multiple sessions of tDCS to restore function after large brain lesions remains to be fully explored.
To test the idea that repeated and regular sessions of tDCS promotes progressive and lasting recovery of function after brain damage, a well-characterized animal model previously validated for tDCS neurostimulation was used (Schweid et al., 2008). In the visual system of the cat, unilateral damage to the posterior parietal cortex and all contiguous visual areas produces an intractable visual deficit and animals are unable to respond to stimuli in the contralesional visual hemifield (Sprague & Meikle, 1965, Wallace et al., 1990). Backup circuits are available in the injured hemisphere but are blocked from use by the spared hemisphere. Altering activity in specific ipsi- and contralesional brain areas through temporary deactivation or lesion unmasks the backup circuits and restores visual function (Sprague, 1966; Wallace et al., 1990; Durmer & Rosenquist, 2001; Lomber et al., 2002). In particular, invasive cooling deactivation of the contralesional visuoparietal cortex produces recovery of function, but restoration of function is only observed during deactivation of the cortex; when the deactivation ceases, the recovery disappears (Lomber et al., 2002).
The current study applied cathodal tDCS to the contralesional visuoparietal cortex to reduce excitability and restore visual function. Unlike cooling deactivation, tDCS is non-invasive and exhibits lasting effects that may accrue with repeated application. Therefore, 70 sessions of cathodal tDCS were administered over the course of 14 weeks. The results support the utility of using multiple sessions to maximize the effect of tDCS on neural function, and represent the first demonstration that a large number of tDCS sessions can improve recovery from brain injury.
Materials and methods
Experimental subjects
Experiments were performed on four domestic short-haired female adult cats (>6 months old) obtained from a licensed USDA-approved cat breeder (Liberty Labs, Waverly, NY, USA). All procedures were performed in accord with the NIH guidelines governing laboratory animal use, and were approved by the Institutional Animal Care and Use Committee at the Boston University School of Medicine. The cats were housed together in an enriched environment and placed on a 12-h light–dark cycle. Data from this cohort were also compared to three control animals with equivalent unilateral lesions that did not undergo any form of tDCS.
Behavioral training
Over a 2-month period, cats (n=4) were trained and tested (~8,500 trials) on tasks designed to assess their ability to detect, orient to and approach moving visual targets (Lomber & Payne, 1996; Payne et al., 1996; Rushmore & Payne, 2004; Valero-Cabré et al., 2006). All testing and training was performed in an 88-cm-diameter semicircular white arena that was enclosed by 28-cm-high walls and that contained evenly spaced openings at the union of the floor and the wall (Figure 1). When the lateral canthi of the animal’s eyes were lined up with the most eccentric openings and the midline of the animal was in line with the cynosure of the semicircle, each of the holes then corresponded to 15° increments of visual angle, extending from left 90° to right 90°.
Figure 1.
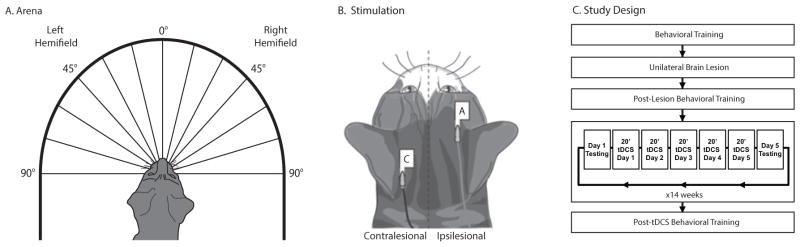
(A) Behavioral arena. Schematic drawing of the arena and testing procedures; subjects were placed in the center of the arena. The cat was trained to fixate on a low-contrast stimulus presented at the center of the arena (0°). A high-contrast secondary stimulus then appeared at one of 13 different positions. A response was scored as correct if the cat moved the head and eyes to fixate on the secondary stimulus, and the animal was given a wet food reward at the position of the secondary. If the animal did not respond, she would move toward the fixation stimulus and collect a dry food reward. The secondary stimulus could be also be a laser point (laser perimetry task), or could be in motion towards the fixation stimulus at the time of secondary stimulus presentation (runway perimetry task). For each of the three tasks, data was collected in blocks of 34 trials consisting of two samples from each of the 14 eccentricities along with six interspersed catch trials. (B) Stimulation placement. Electrode montage on the animal’s head. In the drawing, electrodes are labeled ‘C’ for cathode, and ‘A’ for anode (adapted from Schweid et al. 2008). (C) Study design. Participants were trained prior to the lesion in a series of visuospatial tasks until plateau levels were achieved. After receiving a unilateral lesion of the right cerebral hemisphere, participants were evaluated for 2 months post-lesion surgery to ensure no spontaneous recovery. They were then treated with a daily regimen cathodal tDCS for 14 weeks (5 days per week). Assessments were made on Mondays prior to stimulation, Fridays after stimulation, and after the entire tDCS stimulation session.
The standard moving perimetry task was designed to test the subject’s visual spatial performance on targets presented at the horizontal meridian representation of the left and right visual hemifields (Fig. 1A; Sprague, 1966; Lomber & Payne, 1996; Payne et al., 1996; Payne et al., 2003). Once the cat was positioned, a low-incentive stimulus associated with a dry food reward was presented at the 0° position. After a random interval (~1–2s), a high-contrast (black) high-incentive stimulus associated with a wet food reward was presented at a target location (0, 15, 30, 45, 60, 75 or 90°) to the left or right of the initial fixation point. The location of the second stimulus was determined based on a pseudorandom sequence of targets that was balanced for hemifield and for location. Each eccentricity was presented twice per column, and catch trials (in which the primary but not secondary stimulus was presented) were interleaved to make sure animals were not exhibiting non-stimulus cued orienting responses.
For the laser perimetry task, a small diameter laser point was used as the peripheral stimulus. The lighting in the room was brought from 85 cd/m2 to 1.3 cd/m2. After fixation, a laser was projected onto one of the 13 target eccentricities at the bottom of the arena and was moved (Afifi et al., 2013). If the cat redirected its attention to the laser they would receive a high-incentive food reward and the trial was scored as correct. If the cat did not approach the laser or did not orient correctly, the trial was scored as incorrect.
In the aforementioned tasks, the visual stimulus was presented when the animal was stationary. The runway perimetry task presented the visual stimuli when the animal was in motion. This task was based on the work of Hardy and Stein (1988). The background lighting was set at 85 cd/m2. The fixation stimulus was introduced through the 0° hole, and the cat began 140cm from the 0° position. After fixation, the animal was released and made its way towards the fixation stimulus. When the cat was 45cm away from the 0° position the peripheral target was then presented. Trials in which cats were able to disengage from the fixation stimulus and reorient to the peripheral target were scored as correct. Trials in which cats were unable to register the presentation of the peripheral target or oriented to the peripheral target but continued toward the central stimulus were scored as incorrect. All animals were trained to plateau performance levels prior to surgery.
Surgical procedures
All animals underwent unilateral resection of the posterior parietal regions and contiguous visual areas of the right hemisphere, as performed previously (Lomber et al., 2002; Rushmore et al., 2006). On the day prior to undergoing surgery, all cats were sedated with a ketamine and acepromazine mixture (10mg/kg ketamine and 0.1mg/kg acepromazine). Once the animal was sedated, catheters were implanted in the cephalic veins of the front legs and bound with surgical tape to prevent irritation and tampering with by the cats. Dexamethasone (1mg/kg, i.v.) was administered to minimize brain edema, and antibiotics (30mg/kg cefazolin, i.v.) were given to guard against infection. Ringer’s solution was administered (50–100ml, s.c.). Cats were then placed on a warming pad in individual housing and monitored until they completely recovered.
On the day of surgery, cats were anesthetized with intravenous injection of sodium pentobarbital (25mg/kg and to effect). Atropine sulfate (0.03mg/kg, s.c.) was administered to reduce alimentary secretions, and dexamethasone (1mg/kg, i.v.) and cephazolin (30mg/kg, i.v.) were readministered. The cat was intubated, placed in a stereotaxic apparatus and prepared for sterile and aseptic surgery. The hair was clipped and a depilatory cream was used to eliminate hair from the site. The site was cleaned with alcohol and with a betadine scrub, and the dorsum of the head draped. A skin incision was made along the midline, the temporalis and occipitalis muscles reflected, and a craniotomy made over the occipitoparietal and temporal neocortices. A durectomy revealed the brain, and mannitol (1.5gm/kg/min; 25% solution) was intravenously infused to harden the brain. All contiguous visual cortical areas were removed by subpial aspiration (Areas 17, 18, 19, 20a, 20b, 21a, 21b, 20b, 7, PS, PMLS, PLLS, AMLS, ALLS, CVA, SVA, DLS, VLS and ectosylvian fringe). An acrylic plug was placed in the bone over the contralesional PMLS/PLLS cortex for later localization of the stimulation site. Throughout the procedures heart and respiratory rates were monitored along with core body temperature, respiratory waveform shape, expired carbon dioxide concentration, and pedal reflexes. A change in any of these measures was countered by supplementary administration of sodium pentobarbital. Dura and bone were replaced, and the muscle and skin sutured in place.
Postsurgical recovery was closely monitored, especially respiratory rate, reflex tone, heart rate and body temperature. Postoperative fluids (50–100ml of Ringer’s solution, s.c.) were injected in addition to antibiotics (30mg/kg cefazolin every 8–12 h for 7 days, i.m.) and an analgesic (0.01mg/kg of buprenorphine, s.c.). Once conscious, cats were given soft food and water and closely monitored by research and veterinary staff over the next 3 days. Analgesics were administered for an additional 2 days, and discontinuation was made in consultation with attending veterinarians. Additional doses of dexamethasone were tapered over a 7- to 10-day period. Sutures were removed 2 weeks following surgery at which time cats returned to group housing. Recovery was uneventful in all cases.
Transcranial direct current stimulation (tDCS)
Cats were acclimated to sit quietly in a nylon veterinary cat bag, and periodically rewarded. Stimulation was performed as previously described (Figure 1; Schweid et al., 2008). The tDCS machine (ActivaDose; ActivaTek, Inc., Salt Lake City, UT, USA) was connected to two 2 × 2 cm electrodes (Uni-Tab Electrodes, Balego and Associates, Inc. Wabasha, MN, USA). Hair over the electrode sites was cut regularly to minimize electrical resistance. The anode was placed on the ipsilesional supraorbital location of the scalp and the cathode was positioned over the contralesional parietal cortex such that the center of the electrode was placed over the palpable surgically-placed acrylic plug. Each electrode was covered in electrode paste (EEG Paste, Elfex, Foothill Ranch, CA, USA) and secured to the head with minimally-adhesive cloth tape. After acclimation to electrode positioning, cats were not bothered by and did not tamper with the electrodes or the tape.
Once the electrodes were secure, the current was applied. The current ramped up to 2.0mA over an 18-s period. During this time, occasional muscle twitches were noted, but the cats did not appear to be bothered. The current remained on for 20 min, after which an 18-s ramping-down period brought the current back to zero. Each cat received tDCS five times weekly (Monday – Friday) for a total of 14 weeks (70 sessions in total).
Stimulation had no obvious effects on the behavior of the cats; in all cases they sat quietly in the veterinary bag. Redness over the supraorbital anode was noted after the first few sessions of tDCS but resolved thereafter. Even so, a surgical lubricant (Fougerd) was applied to electrode sites after tDCS to minimize any potential irritation.
Behavioral analysis
The behavioral effects of tDCS were assessed twice weekly. Following the completion of tDCS stimulation of Fridays, 2 h elapsed before the cats were tested. Following this recovery period each cat was tested on the standard, laser and runway perimetry tasks. The second behavioral assessment occurred on Monday mornings prior to the start of the week’s tDCS stimulation. At this time the cats were tested on one or more sets of trials for the standard, laser and runway perimetry tasks. This assessment was used to determine whether there was a reduction in behavioral recovery 48 h or more from the last tDCS stimulation. As no consistent differences were observed between Monday and Friday sessions (paired t-test, P=0.27), the data were pooled. No difference was noted when Monday’s performance was compared with the subsequent Friday (paired t-test, P=0.40). Data were analyzed for each hemifield using a one-way ANOVA design, with performance as the independent variable, and time points after lesion as the main factor. Pre-tDCS and post-tDCS performance were pooled for the purpose of analysis, but were kept separate for graphical illustrations. Post hoc comparisons were made with Tukey’s HSD tests. Data were statistically analyzed using JMP Pro v.10.
Terminal procedures
Cats were injected with an overdose of pentobarbital (120mg/kg, i.v.), then injected with sodium nitrite (1% w/v; 1.5 mL) and heparin (5000 units) and perfused with 2% paraformaldehyde in 15% sucrose and 0.1 M phosphate buffer (pH 7.4). Brains were removed, frozen in a bath of −30°C 2-methyl butane, and stored in the −80° freezer until cutting. Sections of 23μm were cut using a cryostat (Bright OTF); one of every 25 sections was mounted on a gelatin–chrome alum subbed slide and processed for Nissl substance.
Results
Lesion reconstruction
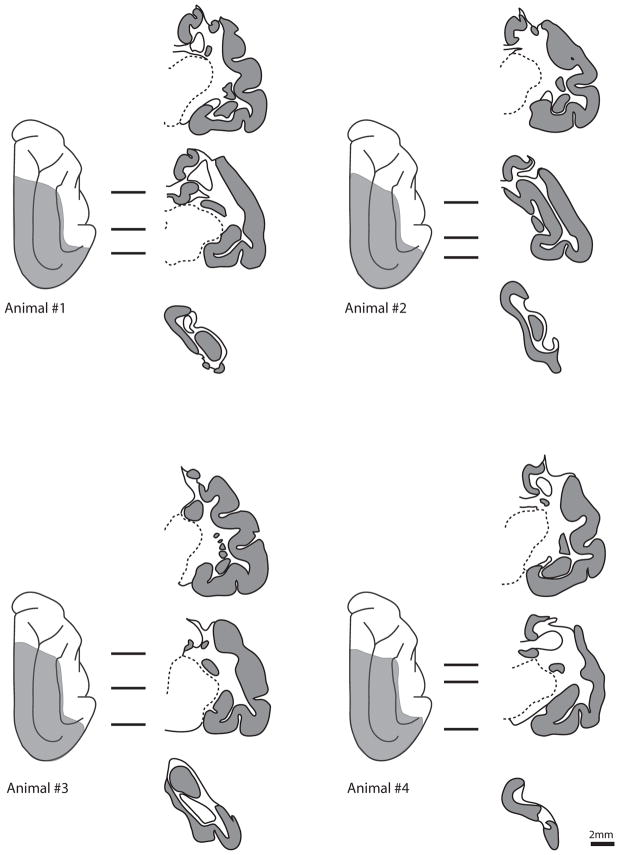
Evaluation of the lesion was made at the time of brain removal and subsequently using microscopic analysis of Nissl-stained sections. In all cases, the intended brain areas were removed (Figure 2). The lesion extended past the borders of the visual areas to include portions of the posterior ectosylvian gyrus, the ectosylvian fringe area lateral to the middle suprasylvian sulcus, and portions of area 5 anteriorly on the middle suprasylvian gyrus. These are all non-visual regions. In one animal (animal no. 3), the posterior cingulate cortex was also removed from the bend of the splenial sulcus posteriorly to ~ A13 anteriorly. Inclusion of this cortex in the lesion did not change the effect of lesion or the pattern of recovery, and we conclude that this cortex is probably unable to compensate for the effects of the lesion.
Figure 2.
Lesion reconstructions of the four animals in the study. On the left is a dorsal view of the cerebrum. The shaded region represents the extent of the lesion. The three lines to the right of each hemisphere represent sections corresponding to the drawings. In these coronal sections, grey represents intact cortex. Scale bar, 2mm.
A secondary evaluation was made by microscopic examination of the thalamus, which showed widespread gliosis and volumetric reduction in regions of the visual thalamus connected with the cerebrum. The laminae of the ipsilesional dorsal lateral geniculate nucleus had been reduced in volume and were filled with small cells consistent with glia. Large cells characteristic of geniculate relay neurons were not observed. The lateral posterior and pulvinar nuclei were similarly devoid of large neurons and showed a decrease in volume that altered the morphology of the thalamus. Overall, no regions of sparing were identified in any cats after primary or secondary analysis, and we conclude that the lesions were complete.
Behavioral data
All animals exhibited perfect (100%) performance in the standard moving perimetry task prior to lesion. After lesion, performance to targets presented in the contralesional visual hemifield fell to zero (Figure 3). Performance to targets in the ipsilesional visual hemifield was unaltered by lesion. Animals were evaluated 2 months after the lesion to account for any spontaneous recovery of function to contralesional targets; none was observed. Control animals did not show any recovery of function for any task for 2 years after lesion (data not shown).
Figure 3.
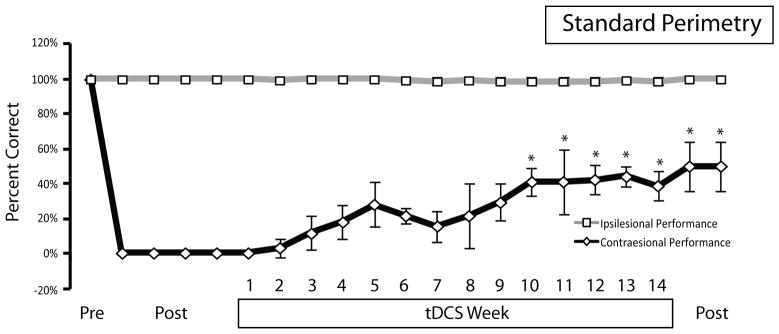
Average performance in the standard moving perimetry task for the three animals that responded to tDCS. All animals exhibited perfect performance before unilateral right hemisphere lesion (Pre). After lesion, animals were impaired in their responses to contralesional (diamonds) but not ipsilesional (squares) visual stimuli. Post-lesion assessments (post) were made at 6 days, 1 month, 2 months and immediately prior to tDCS. tDCS was applied 5 days per week over 14 weeks and performance progressively improved over that time. Performance was maintained at high levels after the cessation of tDCS (post). Data points illustrate average of three animals at each time point, and error bars represent SD of the mean. Note that data points represent differently spaced time points. Asterisks denote significant difference from post-lesion performance (Tukey’s HSD post hoc test).
Two months after lesion, a regimen of cathodal tDCS began. Stimulation was delivered to the intact hemisphere for 20 minutes per day for 5 days a week, and was centered on the posterior middle suprasylvian area. The stimulation strength was set at 2mA and the size of the electrodes was 4cm2 (2 × 2 cm), producing a current density of 0.5mA/cm2. Stimulation was performed for 14 consecutive weeks.
Stimulation had a beneficial effect on contralesional performance in the standard perimetry task in three out of four animals. The fourth animal did not show recovery of any kind and was not considered in any group analysis. An ANOVA revealed a significant effect of time on performance to contralesional, but not ipsilesional, targets (contralesional, F17,36=7.610, P<0.0001; ipsilesional, F17,36=0.5210, P=0.9241). Tukey’s HSD post hoc tests between the pre-tDCS time point and subsequent points showed a significant improvement in contralesional performance at the week 10, 11, 12, 13 and 14 time points, and for the post-tDCS time points (assessed at post-tDCS days 5 and 11). Of the three animals that did recover, the recovery was biphasic in nature. Animals showed little to no recovery of function in the contralesional visual hemifield during the first 2–3 weeks. Thereafter, levels of performance increased to a maximum at week 5 (Figure 3). Performance then decreased, then increased again to a plateau level at around week 10. Recovery began at targets presented in the far periphery in the contralesional visual field and, as the number of stimulation sessions increased, recovery was observed at progressively more centrally-presented locations (Figure 4). Functional recovery was incomplete largely because performance to pericentral targets never recovered (Figure 4). Animals were tested 11 days after tDCS ended and performance was observed to be at levels similar to those of the final post-tDCS testing session, indicating no immediate decline in function.
Figure 4.
Eccentricity plots of performance for all subjects over the course of the experiment. Each plot represents correct performance to each of the 13 visual targets in the left and right visual hemifields. Line length indicates percentage correct performance, with the inner hemi-ring denoting 50% and the other hemi-ring denoting 100% correct performance. The performance for every other week of the stimulation period is not displayed for clarity. Note that the tDCS stimulation regimen induced a recovery of visuospatial abilities which was largely limited to peripheral visual field locations.
Two additional tasks were evaluated (Figure 5). One task was performed in low ambient light conditions and required animals orient to a small laser light stimulus at the same eccentricities as in the standard task (laser task; Afifi et al., 2013). The other task was a variant of the Hardy & Stein (1988) task in which targets were presented while the animal was in motion towards a central target (runway perimetry task). Both tasks were designed to be more difficult due to a requirement to disengage the fixation stimulus during transit (runway task) or a requirement to detect a smaller visual stimulus (laser task). In the laser perimetry task, performance to contralesional targets in the task fell to zero after lesion while performance to ipsilateral targets increased. While animals did respond to contralesional targets late in the tDCS phase, this performance was minor and did not persist after the cessation of tDCS. In the runway task, there was a similar pattern: some contralesional targets were identified during the later phase of tDCS but performance was inconsistent and was not maintained after tDCS. Anova of both tasks showed no effect of time point on performance (all P>0.05).
Figure 5.
Average performance in the laser and runway perimetry tasks for the animals that responded to tDCS. (A) Laser task. Pre-lesion performance in the laser task was not at ceiling levels. After lesion, performance to targets in the contralesional visual hemifield was reduced to zero and performance in the ipsilesional (intact) hemifield increased. Beginning at week 6, inconsistent and slight increases in correct orienting behavior to contralesional visual stimuli was noted but this increase subsequently disappeared. At week 7, a decrease in ipsilesional performance was noted but this returned to normal levels. (B) Pre-lesion performance in the runway perimetry task was perfect, and the lesion produced a deficit in responding to targets presented in the contralesional visual hemifield. Similar to the laser perimetry task, contralesional performance showed slight increases in week 9 but performance soon decayed back to baseline levels. Performance to ipsilesional targets was impaired starting largely at week 9, but later disappeared. Error bars represent SD of the mean, and statistical comparisons showed no significant findings.
Performance decrements were observed in the ipsilesional hemifield in both the runway and the laser tasks. These effects were not observed in the standard perimetry task, and were seen to principally begin at 5–7 weeks into the tDCS phase. All animals exhibited this effect in both tasks, but there was a large variation in the magnitude of the performance decrease. These impairments largely dissipated in subsequent weeks, and performance after the tDCS block was not significantly different than the post-lesion ipsilesional performance. The timing of these decrements in the ipsilesional field appeared to coincide with the second phase of recovery in the standard task.
Discussion
These data show that non-invasive brain stimulation can produce a restoration of function after brain damage, and are the first to demonstrate that a 70-session-long tDCS regimen produces extensive and lasting recovery. The recovery of function for the standard perimetry task exhibited a biphasic response prior to reaching plateau levels, and was only observed for peripheral visual stimuli. Restoration of function was incomplete for the standard perimetry task and no recovery was observed in more demanding tasks.
Removal of the posterior parietal cortex and contiguous visual areas produces an intractable deficit that is maintained so long as the lesion is complete (Wallace et al., 1990, Rushmore et al., 2006). Visual function returns after the contralesional superior colliculus is deactivated or damaged (Sprague, 1966; Lomber et al., 2002), or when afferents to the contralateral superior colliculus are damaged or deactivated (Wallace et al., 1990; Lomber et al., 2002; Durmer and Rosenquist, 2003; Payne and Rushmore, 2004). The approach in this study was modeled after previous results that demonstrated that invasive cooling deactivation of the intact posterior middle suprasylvian sulcus produced a restoration of function after unilateral lesion (Lomber et al., 2002). In this study, cathodal tDCS was used to produce a deactivation but, given the weak current strength, effects were not immediate. Instead, a large number of repeated stimulation sessions were required to produce restoration of function. In the three animals that recovered function, restoration only began after 10–20 sessions of tDCS. With an increasing number of tDCS sessions, performance to contralesional targets in the standard perimetry task progressively improved, reaching an initial peak at week 5 of stimulation. After week 5, performance dropped for another 1–2 weeks, after which performance began to climb to reach plateau levels by week 10.
The importance of multiple sessions on the efficacy and magnitude of non-invasive neurostimulation effects have been noted in intact animals and human participants (Valero-Cabré et al. 2008; Reis et al., 2009, Monte-Silva et al., 2013), in human subjects with depression (Alonzo et al., 2012, Loo et al., 2012, Brunoni et al., 2011, Boggio et al., 2008), and in similar animals models of focal brain damage (Afifi et al. 2013). Increasing sessions of cathodal tDCS also progressively elevates the number of neural stem cells labeled by bromodeoxyuridine and Hes3 antibodies (Rueger et al., 2012). However, in humans cautionary measures have generally limited duration of stimulation to a maximum of 15 days (5 days a week; Loo et al., 2012), which is considerably less than the number of sessions applied in the current tDCS report and other similar animal repetitive transcranial magnetic stimulation (rTMS) studies (Valero-Cabré et al. 2008, Afifi et al. 2013). Overall, these data support the contention that, as for rTMS, the effectiveness of cathodal tDCS is related to the number of sessions, and that effects seen when tDCS is applied to clinical populations could be improved by increasing the number of stimulation sessions.
The recalcitrance of cortical circuits in undergoing change is well established. Long-term potentiation and long-term depression, long held as the principal means of producing lasting change in cerebral circuits, are easily induced in the hippocampus (Bliss & Lomo, 1973; Dudek & Bear, 1992) but are more difficult to produce in the cortex (Trepel & Racine, 1998). Induction of synaptic plasticity in the cortex requires multiple sessions of tetanizing trains to be effective and reflects the relative stability of neocortical circuits. While the mechanisms underlying the ability of tDCS to produce lasting neural changes in these circuits have not yet been fully established (see Stagg & Nitsche, 2011, Márquez-Ruiz et al., 2012), the number of sessions required for recovery is probably due to tDCS overcoming cortical resistance to synaptic plasticity, a delay period in the accumulation of critical neuromodulators or growth factors (e.g., brain-derived neurotrophic factor; Fritsch et al., 2010), or both.
Recovery was only observed to more peripherally located visual targets, and this finding may reflect a limited capacity of the tDCS to penetrate into the depths of the cortex. The targeted cortex is retinotopically organized: the representation of the contralateral peripheral visual field is located near the skull on the crest of each gyrus, and the neurons in the fundus of the sulcus represent central and pericentral locations (Palmer et al., 1978). The behavioral results, therefore, may reflect a selective reduction in activity or in the firing probability of the neurons that represent peripheral targets and that are located closer to the skull. The results also may reflect selective activation of neurons in this cortex whose somatodendritic axis or axonal axis is optimally oriented to the electric field (e.g., Bikson et al., 2004; Radman et al., 2009; Kabakov et al., 2012). The behavioral results also indicate that the resting membrane potential of neurons near the depth of the sulcus, which correspond to central visual field locations (Palmer et al., 1978), may not be sufficiently modulated by tDCS to produce a behavioral change. In as much as functional alterations in these neurons are the basis for the recovery, this result runs counter to predictions of modeling studies that show a preferential effect of tDCS on neurons at the bases of sulci (Miranda et al., 2013) and also suggests that the tDCS-mediated reduction in activity also does not feed down to neurons in the depth of the sulcus through substantial intra-areal circuits demonstrated to fill this region (Norita et al., 1996). Further modeling of tDCS currents and biological study is required to provide a definitive answer to the mechanisms and the precise neuronal elements underlying the present results.
It is notable that one animal did not respond to tDCS treatment. Examination of the lesion showed no identifiable differences in terms of size or extent of lesion, a finding that has precedence in the animal neurostimulation literature (Afifi et al., 2013). There were no obvious differences in the sulcal or gyral pattern of the intact hemisphere that would predispose the tDCS from being less effective. This result is consistent with analogous findings in non-invasive brain stimulation studies in animals and humans that suggest that the response to transcranial stimulation is highly variable. In one recent lesion study using a feline model (Afifi et al., 2013), half the subjects positively responded to transcranial magnetic stimulation and half the subjects responded negatively, and the dichotomy of the response was not reflected in the extent or the size of lesion. In humans, the response of the motor evoked potential amplitude to 1-Hz rTMS was similarly split: 75% of the participants displayed a decrease in the signal while 25% showed no change or an increase (Gangitano et al., 2002). Similar findings have been seen in studies of tDCS and depression (Loo et al., 2012). The biological basis of responsivity to transcranial stimulation is an open question, and a question in need of resolution to achieve maximum efficacy.
It is interesting to note that the recovery of contralesional targets occurred in two phases. The basis of this recovery and whether each phase represents a different mechanism is unclear, although the time period between the two phases of recovery in the standard task is accompanied by a decrease in performance to targets in the ipsilesional hemifield in the more demanding laser and runway tasks. This finding suggests that tDCS may have done more than simply reduce aberrant hyperexcitability in the contralesional cerebral hemisphere. The posterior parietal cortex is critical for performance in the runway and laser tasks (Hardy & Stein, 1988; Afifi et al., 2013), and these data are consistent with the notion that tDCS is deactivating this cortex. This effect may best be considered a cost of this ultra-long stimulation paradigm, and in this system the cost ultimately dissipated. However, this effect should be carefully considered during similar applications in the human, both as a potential side effect and also as an early signature of treatment response and a mechanism which the lesioned hemisphere might require in order to adopt function.
Conclusions
This is the first study to demonstrate that a 70-session tDCS regime to the contralesional (intact) cerebrum partially reverses lesion-induced deficits. The recovery was limited to moving stimuli located in the periphery of the contralateral visual hemifield, and occurred in two phases. A potential cost of the stimulation to intact targets was noted, but was minor and disappeared during the later phases of the stimulation regimen. These data indicate that increasing the number of tDCS sessions may improve the efficacy of non-invasive brain stimulation.
Acknowledgments
This study was supported by NIH NS062317 and FP68 ANR project eraNET-NEURONS BEYOND VIS. We thank Dr Linda Afifi for assisting with surgeries and behavioral training.
Abbreviations
- tDCS
transcranial direct-current stimulation
- rTMS
repetitive transcranial magnetic stimulation
References
- Afifi LM, Rushmore RJ, Valero-Cabré A. Benefits of multiple sessions of repetitive transcranial magnetic stimulation for an effective rehabilitation of visuo-spatial function. Eur J Neurosci. 2013;37:441–54. doi: 10.1111/ejn.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonzo A, Brassil J, Taylor JL, Martin D, Loo CK. Daily transcranial direct current stimulation (tDCS) leads to greater increases in cortical excitability than second daily transcranial direct current stimulation. Brain Stimul. 2012;5:208–213. doi: 10.1016/j.brs.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Batsikadze G, Moliadze V, Paulus W, Kuo MF, Nitsche MA. Partially non-linear stimulation intensity-dependent effects of direct current stimulation on motor cortex excitability in humans. J Physiol (Lond) 2013;591:1987–2000. doi: 10.1113/jphysiol.2012.249730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindman LJ, Lippold OC, Redfearn JW. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after effects. J Physiol (Lond) 1964;172:369–382. doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Inoue M, Akiyama H, Deans JK, Fox JE, Miyakawa H, Jefferys JG. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J Physiol (Lond) 2004;557:175–190. doi: 10.1113/jphysiol.2003.055772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol (Lond) 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggio PS, Rigonatti SP, Ribeiro RB, Myczkowski ML, Nitsche MA, Pascual-Leone A, Fregni F. A randomized, double-blind clinical trial on the efficacy of cortical direct current stimulation for the treatment of major depression. Int J Neuropsychopharmacol. 2008;11:249–254. doi: 10.1017/S1461145707007833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunoni AR, Ferrucci R, Bortolomasi M, Vergari M, Tadini L, Boggio PS, Giacopuzzi M, Barbieri S, Priori A. Transcranial direct current stimulation (tDCS) in unipolar vs. bipolar depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:96–101. doi: 10.1016/j.pnpbp.2010.09.010. [DOI] [PubMed] [Google Scholar]
- Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, Edwards DJ, Valero-Cabré A, Rotenberg A, Pascual-Leone A, Ferrucci R, Priori A, Boggio PS, Fregni F. Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimul. 2012;5:175–195. doi: 10.1016/j.brs.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci USA. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durmer JS, Rosenquist AC. Ibotenic acid lesions in the pedunculopontine region result in recovery of visual orienting in the hemianopic cat. Neuroscience. 2001;106:765–781. doi: 10.1016/s0306-4522(01)00321-9. [DOI] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, Lu B. Direct current stimulation promotes BDNF-dependent synaptic plasticity: potential implications for motor learning. Neuron. 2010;66:198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangitano M, Valero-Cabré A, Tormos JM, Mottaghy FM, Romero JR, Pascual-Leone A. Modulation of input-output curves by low and high frequency repetitive transcranial magnetic stimulation of the motor cortex. Clin Neurophysiol. 2002;113:1249–1257. doi: 10.1016/s1388-2457(02)00109-8. [DOI] [PubMed] [Google Scholar]
- Hardy SC, Stein BE. Small lateral suprasylvian cortex lesions produce visual neglect and decreased visual activity in the superior colliculus. J Comp Neurol. 1988;273:527–542. doi: 10.1002/cne.902730408. [DOI] [PubMed] [Google Scholar]
- Kabakov AY, Muller PA, Pascual-Leone A, Jensen FE, Rotenberg A. Contribution of axonal orientation to pathway-dependent modulation of excitatory transmission by direct current stimulation in isolated rat hippocampus. J Neurophysiol. 2012;107:1881–1889. doi: 10.1152/jn.00715.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomber SG, Payne BR. Removal of two halves restores the whole: reversal of visual hemineglect during bilateral cortical or collicular inactivation in the cat. Vis Neurosci. 1996;13:1143–1156. doi: 10.1017/s0952523800007781. [DOI] [PubMed] [Google Scholar]
- Lomber SG, Payne BR, Hilgetag CC, Rushmore J. Restoration of visual orienting into a cortically blind hemifield by reversible deactivation of posterior parietal cortex or the superior colliculus. Exp Brain Res. 2002;142:463–474. doi: 10.1007/s00221-001-0957-9. [DOI] [PubMed] [Google Scholar]
- Loo CK, Alonzo A, Martin D, Mitchell PB, Galvez V, Sachdev P. Transcranial direct current stimulation for depression: 3-week, randomised, sham-controlled trial. Br J Psychiatry. 2012;200:52–59. doi: 10.1192/bjp.bp.111.097634. [DOI] [PubMed] [Google Scholar]
- Márquez-Ruiz J, Leal-Campanario R, Sánchez-Campusano R, Molaee-Ardekani B, Wendling F, Miranda PC, Ruffini G, Gruart A, Delgado-García JM. Transcranial direct-current stimulation modulates synaptic mechanisms involved in associative learning in behaving rabbits. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:6710–6715. doi: 10.1073/pnas.1121147109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda PC, Mekonnen A, Salvador R, Ruffini G. The electric field in the cortex during transcranial current stimulation. Neuroimage. 2013;70:48–58. doi: 10.1016/j.neuroimage.2012.12.034. [DOI] [PubMed] [Google Scholar]
- Monte-Silva K, Kuo M-F, Hessenthaler S, Fresnoza S, Liebetanz D, Paulus W, Nitsche MA. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul. 2013;6:424–32. doi: 10.1016/j.brs.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;15:633–9. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norita M, Kase M, Hoshino K, Meguro R, Funaki S, Hirano S, McHaffie JG. Extrinsic and intrinsic connections of the cat’s lateral suprasylvian visual area. Prog Brain Res. 1996;112:231–250. doi: 10.1016/s0079-6123(08)63333-6. [DOI] [PubMed] [Google Scholar]
- Palmer LA, Rosenquist AC, Tusa RJ. The retinotopic organization of lateral suprasylvian visual areas in the cat. J Comp Neurol. 1978;177:237–256. doi: 10.1002/cne.901770205. [DOI] [PubMed] [Google Scholar]
- Payne BR, Lomber SG, Geeraerts S, van der Gucht E, Vandenbussche E. Reversible visual hemineglect. Proc Natl Acad Sci USA. 1996;93:290–294. doi: 10.1073/pnas.93.1.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne BR, Lomber SG, Rushmore RJ, Pascual-Leone A. Cancellation of visuoparietal lesioninduced spatial neglect. Exp Brain Res. 2003;150:395–398. doi: 10.1007/s00221-003-1473-x. [DOI] [PubMed] [Google Scholar]
- Payne BR, Rushmore RJ. Functional circuitry underlying natural and interventional cancellation of visual neglect. Exp Brain Res. 2004;154:127–153. doi: 10.1007/s00221-003-1660-9. [DOI] [PubMed] [Google Scholar]
- Purpura DP, McMurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol. 1965;28:166–185. doi: 10.1152/jn.1965.28.1.166. [DOI] [PubMed] [Google Scholar]
- Radman T, Ramos RL, Brumberg JC, Bikson M. Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimul. 2009;2:215–228. doi: 10.1016/j.brs.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik PA, Krakauer JW. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci USA. 2009;106:1590–1595. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueger MA, Keuters MH, Walberer M, Braun R, Klein R, Sparing R, Fink GR, Graf R, Schroeter M. Multi-session transcranial direct current stimulation (tDCS) elicits inflammatory and regenerative processes in the rat brain. PloS One. 2012;7:e43776. doi: 10.1371/journal.pone.0043776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushmore RJ, Payne BR. Neuroplasticity after unilateral visual cortex damage in the newborn cat. Behav Brain Res. 2004;153:557–565. doi: 10.1016/j.bbr.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Rushmore RJ, Valero-Cabré A, Lomber SG, Hilgetag CC, Payne BR. Functional circuitry underlying visual neglect. Brain. 2006;129:1803–1821. doi: 10.1093/brain/awl140. [DOI] [PubMed] [Google Scholar]
- Schweid L, Rushmore RJ, Valero-Cabré A. Cathodal transcranial direct current stimulation on posterior parietal cortex disrupts visuo-spatial processing in the contralateral visual field. Exp Brain Res. 2008;186:409–417. doi: 10.1007/s00221-007-1245-0. [DOI] [PubMed] [Google Scholar]
- Sparing R, Thimm M, Hesse MD, Kust J, Karbe H, Fink GR. Bidirectional alterations of interhemipseric parietal balance by non-invasive cortical stimulation. Brain. 2009;132:3011–20. doi: 10.1093/brain/awp154. [DOI] [PubMed] [Google Scholar]
- Sprague JM. Interaction of cortex and superior colliculus in mediation of visually guided behavior in the cat. Science. 1966;153:1544–1547. doi: 10.1126/science.153.3743.1544. [DOI] [PubMed] [Google Scholar]
- Sprague JM, Meikle TH. The role of the superior colliculus in visually-guided behavior. Exp Neurol. 1965;11:115–146. doi: 10.1016/0014-4886(65)90026-9. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37–53. doi: 10.1177/1073858410386614. [DOI] [PubMed] [Google Scholar]
- Trepel C, Racine RJ. Long-term potentiation in the neocortex of the adult, freely moving rat. Cereb Cortex. 1998;8:719–729. doi: 10.1093/cercor/8.8.719. [DOI] [PubMed] [Google Scholar]
- Valero-Cabré A, Pascual-Leone A, Rushmore RJ. Cumulative sessions of repetitive transcranial magnetic stimulation (rTMS) build up facilitation to subsequent TMS-mediated behavioural disruptions. Eur J Neurosci. 2008;27:765–774. doi: 10.1111/j.1460-9568.2008.06045.x. [DOI] [PubMed] [Google Scholar]
- Valero-Cabré A, Rushmore RJ, Payne BR. Low frequency transcranial magnetic stimulation on the posterior parietal cortex induces visuotopically specific neglect-like syndrome. Exp Brain Res. 2006;172:14–21. doi: 10.1007/s00221-005-0307-4. [DOI] [PubMed] [Google Scholar]
- Wallace SF, Rosenquist AC, Sprague JM. Ibotenic acid lesions of the lateral substantia nigra restore visual orientation behavior in the hemianopic cat. J Comp Neurol. 1990;296:222–252. doi: 10.1002/cne.902960204. [DOI] [PubMed] [Google Scholar]