Abstract
Adipose tissue macrophage (ATM) plays a central role in obesity-associated inflammation and insulin resistance. Quercetin, a dietary flavonoid, possesses anti-inflammation and anti-insulin resistance properties. However, it is unclear whether quercetin can alleviate high-fat diet (HFD)-induced ATM infiltration and inflammation in mice. In this study, 5-week-old C57BL/6 mice were fed low-fat diet, HFD, or HFD with 0.l% quercetin for 12 weeks, respectively. Dietary quercetin reduced HFD-induced body weight gain and improved insulin sensitivity and glucose intolerance in mice. Meanwhile, dietary quercetin enhanced glucose transporter 4 translocation and protein kinase B signal in epididymis adipose tissues (EATs), suggesting that it heightened glucose uptake in adipose tissues. Histological and real-time PCR analysis revealed that quercetin attenuated mast cell and macrophage infiltration into EATs in HFD-fed mice. Dietary quercetin also modified the phenotype ratio of M1/M2 macrophages, lowered the levels of proinflammatory cytokines, and enhanced adenosine monophosphate-activated protein kinase (AMPK) α1 phosphorylation and silent information regulator 1 (SIRT1) expression in EATs. Further, using AMPK activator 5-aminoimidazole-4-carboxamide-1-β4-ribofuranoside and inhibitor Compound C, we found that quercetin inhibited polarization and inflammation of mouse bone marrow-derived macrophages through an AMPKα1/SIRT1-mediated mechanism. In conclusion, dietary quercetin might suppress ATM infiltration and inflammation through the AMPKα1/SIRT1 pathway in HFD-fed mice
Keywords: obesity, insulin resistance, adipose tissue macrophage, macrophages, adenosine monophosphate-activated protein kinase α1/silent information regulator 1
Obesity and type 2 diabetes, two worldwide epidemic metabolic diseases, are correlated with a chronic low-grade inflammation, especially in adipose tissue (1–3). During the development of diet-induced obesity, excess nutrition induces adipocyte hypertrophy and hyperplasia. Hypertrophic adipocytes secrete chemokines to activate and attract various inflammatory cells into white adipose tissues (WATs), promoting a state of chronic low-grade adipose tissue inflammation and systemic insulin resistance (2). Among these inflammatory cells, the recruitment of macrophages into adipose tissues has been regarded as an initial event in obesity-induced inflammation and insulin resistance (3). Moreover, the phenotypic alteration of adipose tissue macrophages (ATMs) from the anti-inflammatory “alternatively activated” M2 phenotype to the proinflammatory “classically activated” M1 phenotype also occurs in adipose tissues of obese individuals (4), and it is well correlated with obesity-associated insulin resistance (3, 5).
Quercetin is a major bioflavonoid and common in dietary plants, such as tea, onions, apples, and grapes (6, 7). A series of in vitro studies suggested a possible efficacy of quercetin on obesity and insulin resistance, including antiadipogenesis in preadipocytes (8, 9), anti-inflammation in macrophages (10–13) and inflammatory mediator-treated adipocytes (10, 14, 15), and anti-insulin resistance in adipocytes treated with TNF-α (15). While some animal studies have shown that quercetin failed to reduce high-fat diet (HFD)-induced body weight gain (6, 16–19) and insulin resistance (16), most recent studies have affirmed that quercetin ameliorated insulin sensitivity in diet-induced (17, 20, 21), genetic (22–24), and chemical-promoted (7, 22) diabetic model animals. Moreover, in HFD-fed mice, dietary quercetin could normalize the levels of proinflammatory TNF-α and interleukin-6 (IL-6) (20, 23, 25) and anti-inflammatory adiponectin (17, 20) in blood and/or WAT, suppress inflammatory cells’ infiltration into liver and heart (6), and alter hepatic inflammatory response gene expression (6, 19–21, 25, 26) and activity (26). However, more evidence is needed regarding whether quercetin alleviates ATM infiltration and inflammation in HFD-fed mice.
Adenosine monophosphate-activated protein kinase (AMPK) and silent information regulator 1 (SIRT1) are two important nutrient sensors and inflammatory regulators (27, 28). Relative to AMPKα2, AMPKα1 is preponderantly expressed in adipose tissues and macrophages (29, 30). In mice, HFD downregulates AMPKα1 signaling in WAT (29). AMPKα1 activation enhances the level of cellular SIRT1 activator NAD+, induces SIRT1 expression, and suppresses macrophage inflammation (29). Quercetin is a potent NAD+ase CD38 inhibitor (31, 32) and a direct activator of SIRT1 (33). Moreover, quercetin can activate AMPK signaling and attenuate adipogenesis in 3T3-L1 cells (8). Thus, we explored whether quercetin changed AMPKα1 and SIRT1 expression and activity in macrophages and adipose tissues in HFD-fed mice.
In this study, we investigated the effects of quercetin on HFD-induced fat accumulation, insulin resistance, inflammatory macrophage and mast cell infiltration, proinflammatory cytokines, and AMPKα1/SIRT1 signal in adipose tissues in mice. Furthermore, we affirmed the role of the AMPKα1/SIRT1 pathway in quercetin suppressing macrophage polarization and inflammation.
MATERIALS AND METHODS
Experimental animals and diets
Male 4-week-old C57BL/6 mice were purchased from Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). They were adapted for 1 week and then randomly divided into three dietary groups and fed on (1) low-fat diet (LFD) (based on Research Diets D12450B containing 3.85 kcal/g and 10% of energy from fat) (n = 8); (2) HFD (based on Research Diets D12451 containing 4.73 kcal/g and 45% of energy from fat) (n = 8); and (3) HFD supplemented with 0.1% (weight/weight) quercetin (≥98% purity; Sigma-Aldrich, St. Louis, MO) (n = 8).
All mice were housed in pathogen-free and ventilated cages with a 12 h-12 h light-dark cycle and allowed free access to autoclaved water and irradiated food. All research protocols were approved by Hefei University of Technology Standing Committee on Animals. Body weight and food intake were measured every week. After 12 weeks, mice were fasted overnight and then euthanized with CO2, and their blood and related adipose tissues were harvested. To obtain serum, blood stood overnight at 4°C and was then centrifuged at 5,000 g for 15 min. The serum was collected from the supernatant and stored at −80°C for serum analysis. The adipose tissues, including epididymis adipose tissues (EATs), subcutaneous adipose tissues (SATs), and brown adipose tissues (BATs), were rapidly dissected and weighed on ice. Then, the tissues were snap-frozen in liquid nitrogen and stored at −80°C.
Glucose tolerance tests and insulin tolerance tests
Glucose tolerance tests and insulin tolerance tests were performed before mice were euthanized at 17 weeks old as previously described (34). Generally, mice were fasted overnight and intraperitoneally injected with D-glucose (1 g/kg body weight, Sigma-Aldrich) for the glucose tolerance test and with insulin (1.5 IU/kg body weight; WanBang BioPharma, Xuzhou, China) for the insulin tolerance test. The blood glucose level was measured from tail veins using a blood glucose meter (Omnitest Plus; B. Braun, Melsungen, Germany) at 0, 15, 30, 45, 60, 90, and 120 min after injection.
Histology and immunohistochemistry
For histopathology studies, adipose tissues were rapidly fixed in 4% formalin at room temperature for 24 h. Then, the tissues were embedded in paraffin and serially sliced at 5 μm thickness. The histological characterizations, including adipocyte sizes (hematoxylin-eosin staining, Sigma-Aldrich) and ATM (Mac-2 monoclonal antibody, 1:800; BioLegend, San Diego, CA) and mast cell (toluidine blue staining, Sigma-Aldrich) levels were carried out as described previously (34, 35). Five random fields from each section were examined, and Image-Pro Plus Version 6.0 (Media Cybernetics) was used to measure adipocyte average diameters and Mac-2 positive staining areas. Mast cell numbers, which presented as cell numbers per square millimeter, were counted under a light microscope (OPTEC).
Total RNA isolation and quantitative real-time PCR
Total RNA was isolated from adipose tissues or cultured cells using RNAiso Plus reagent (TaKaRa, Dalian, China). cDNA was synthesized from total RNA using Oligo d(T)18 (TaKaRa) and M-MLV reverse transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. The cDNA was used as a template for real-time PCR (Bio-Rad MyiQ2 Real-time PCR System) in the presence of SYBR® Premix Ex Taq™ II (TaKaRa). All primer sequences are listed in supplementary Table I. Data were processed using the ΔΔCT method. GAPDH was used as reference gene.
Protein extraction and Western blot analysis
For total protein extraction, adipose tissues or macrophages were lysed in RIPA lysis buffer (50 mM Tris HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) with total protease inhibitor and phosphatase inhibitor (Sangon, Shanghai, China) and centrifuged at 4°C and 12,000 g for 15 min. Plasma membrane (PM) proteins were extracted from adipose tissues and 3T3-L1 adipocytes as described previously (36). The protein concentration was determined with a BCA assay kit (Sangon). Samples of equal amounts were separated on SDS-PAGE and transferred to polyvinylidene difluoride membranes (Millipore, Bedford, MA). Immunoblot analysis was performed with the corresponding primary antibodies, which are listed in supplementary Table II. HRP-labeled goat anti-rabbit IgG (1:4,000; KPL, Gaithersburg, MD) was used as secondary antibody, and the reactive bands were detected using an ECL kit (Thermo, Rockford, IL) in the ImageQuant LAS 4000 mini (GE Healthcare). Immunoblots were quantified using ImageQuant TL 7.0 software (GE Healthcare) and expressed as the ratio of specific protein phosphorylation to the corresponding protein or specific protein to β-actin.
Serum analysis
Serum quercetin was determined using HPLC (1260 Infinity, Agilent Technologies). Briefly, 100 μl of serum was diluted with 100 μl of water and then incubated in 250 μl of 0.1 M acetate buffer (pH 5.0) containing 10,000 U β-glucuronidase (Sigma-Aldrich) solution and 10 μl of 0.1 M ascorbic acid at 37°C for 6 h. Hydrolyzed metabolites were extracted with 2 ml of ethyl acetate three times, dried under a nitrogen flow, and reconstituted in 80 μl of methanol. Finally, 20 μl of sample was applied to a Zorbax SB-C18 reversed-phase column (Agilent).
The serum levels of IL-6, TNF-α, and monocyte chemotactic protein-1 (MCP-1) (BD Biosciences, San Diego, CA); insulin (ALPCO, Salem, NH); and leptin and adiponectin (Peprotech, Rocky Hill, NJ) were respectively assayed by mouse ELISA kits according to manufacturer protocols.
Cell culture and treatment
Bone marrow was isolated from the femurs and tibias of C57BL/6 mice. The cells were cultured in Dulbecco's modified Eagle's medium (Gibco, Auckland, New Zealand) containing 20% fetal bovine serum (Gibco) and 30% L929 conditional medium for 6 days to differentiate into bone marrow-derived macrophages (BMDMs) (29, 37). After differentiation, the cells were planted in a 24-well plate overnight. Then, the cells were stimulated with 100 ng/ml lipopolysaccharide (LPS) (Sigma-Aldrich) for 6 h. To assess the inhibitory effects of quercetin on cytokine expression and the inflammatory signaling pathways in BMDMs, vehicle or 20 μM quercetin was added during BMDM differentiation and LPS treatment; 2 mM 5-aminoimidazole-4-carboxamide-1-β4-ribofuranoside (AICAR) (Sigma-Aldrich) as positive control and 1 μM Compound C as AMPK inhibitor (Santa Cruz) were added 2 h before LPS treatment. Adenine nucleotide levels of BMDMs were detected by HPLC with a Zorbax SB-C18 reversed-phase column as previously described (38).
The 3T3-L1 preadipocytes were purchased from Cell Culture Center of Peking Union Medical College, and cells were differentiated into adipocytes as previously described (39). For the glucose transporter 4 (GLUT4) translocation assay, differentiated 3T3-L1 adipocytes were starved for 2 h in serum-free medium before starting the experiment. Thereafter, 2 mM AICAR or 20 μM quercetin was added into medium with or without 1 μM Compound C for 30 min. After treatment, cell membrane proteins and total proteins were extracted for Western blot analysis. All aforementioned cell assays were performed in quadruplicate.
Statistical analysis
All data were presented as the mean ± SEM. The statistical differences among various animal groups were assessed with the Mann-Whitney test owing to our small data size and nonnormal distribution data. The difference in significance of various cell assay groups was tested using Student's t-test. P < 0.05 was considered as statistical significance.
RESULTS
Dietary quercetin reduced HFD-induced body weight gain and fat deposition
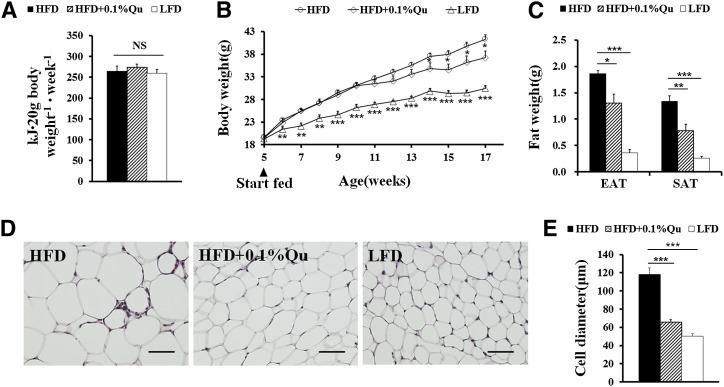
To reconfirm the effect of quercetin on diet-induced obesity, 5-week-old C57BL/6 mice were fed LFD, HFD, or HFD supplemented with 0.1% quercetin for 12 weeks, respectively. There were no differences in food intake among all three groups (Fig. 1A). During weeks 1 to 7, dietary quercetin did not markedly affect HFD-induced body weight gain (Fig. 1B). However, after 8 weeks, dietary quercetin led to significant body weight difference in HFD-fed mice (Fig. 1B). At 17 weeks old, the mice fed quercetin-containing HFD also had reduced adipose tissue weight (Fig. 1C, supplementary Table III) and adipocyte sizes (Fig. 1D, E) compared with the mice only fed HFD. The results indicate that quercetin gently inhibits diet-induced obesity. Additionally, we used HPLC to determine serum quercetin concentration after hydrolysis of quercetin metabolites. The mice fed quercetin-containing HFD possessed significantly higher serum quercetin levels (Table 1).
Fig. 1.
Quercetin-containing diet reduces body weight gain and fat weight in HFD-induced obese mice. Male 5-week-old C57BL/6 mice were randomly divided into three dietary groups fed on LFD, HFD, or HFD supplemented with 0.1% quercetin (HFD+0.1%Qu) for 12 weeks (n = 8 per group). (A) The energy intake per 20 g body weight of mice during 12-week treatment. (B) Body weight gain of the mice. (C) Weights of EAT and SAT. (D) Hematoxylin-eosin staining and (E) adipocyte diameters in EAT of the mice; scale bars in (D), 100 μm in the length (×200). Statistical difference between groups was shown using a nonparametric Mann-Whitney test; n = 8 per group. * P < 0.05, ** P < 0.01, *** P < 0.001. All data in (A), (B), (C), and (E) are mean ± SEM.
TABLE 1.
Quercetin normalizes concentrations of serum constituents in HFD-fed mice
| Diet | |||
| Parameter | HFD | HFD + 0.1% quercetin | LFD |
| Serum quercetin (μmol/l) | n.d. | 9.19 ± 0.46 | n.d. |
| Serum insulin (mIU/l) | 6.07 ± 0.10 | 5.74 ± 0.08a | 5.49 ± 0.13a |
| Serum leptin (pg/ml) | 7,904 ± 391.92 | 4,463 ± 840.14b | 1,760 ± 432.76c |
| Serum adiponectin (pg/ml) | 1,897.41 ± 119.05 | 2,387.31 ± 146.10a | 2,298.54 ± 129.71a |
| Serum TNF-α (pg/ml) | 36.92 ± 4.42 | 20.66 ± 5.82a | 19.86 ± 3.00a |
| Serum IL-6 (pg/ml) | 2.75 ± 0.83 | 1.27 ± 0.21a | 0.62 ± 0.10a |
| Serum MCP-1 (pg/ml) | 58.60 ± 2.90 | 43.03 ± 3.12a | 40.55 ± 2.66a |
C57BL/6 mice were randomly divided into three dietary groups fed on LFD, HFD, or HFD supplemented with 0.1% quercetin for 12 weeks (n = 8 per group). Serum concentrations of quercetin, insulin, and cytokines were detected at the end of the 12-week dietary intervention period. n.d. represented not detectable. Statistical difference between groups was shown using a nonparametric Mann-Whitney test; n = 8 per group. All data in the table are mean ± SEM.
P < 0.05.
P < 0.01.
P < 0.001.
Dietary quercetin ameliorated HFD-induced insulin resistance and enhanced glucose uptake in adipose tissues
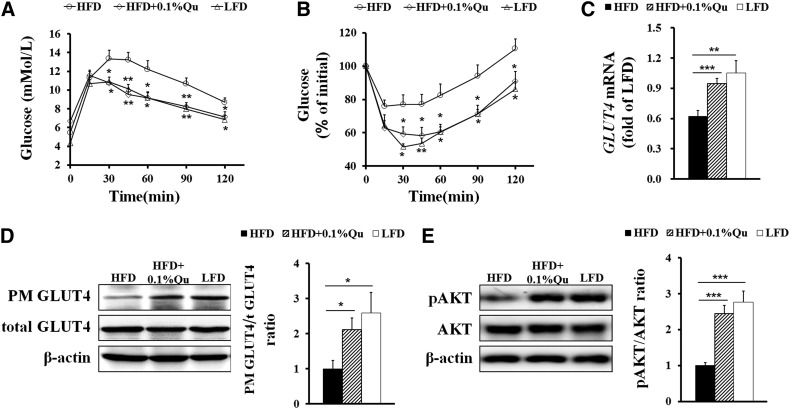
We next detected the glucose metabolism and insulin sensitivity in these mice. Along with their lower body weight gain, the mice fed quercetin-containing HFD had improved glucose tolerance (Fig. 2A) and insulin sensitivity (Fig. 2B) compared with the mice only fed HFD. Moreover, dietary quercetin also lowered the levels of insulin and leptin and enhanced the level of adiponectin in sera in HFD-fed mice (Table 1).
Fig. 2.
Quercetin improves insulin resistance and glucose uptake in HFD-induced obese mice. At 17 weeks old, blood glucose was measured in mice injected with 1 g/kg body weight D-glucose for glucose tolerance test (A) or 1.5 IU/kg body weight insulin for insulin tolerance test (B). (C) The mRNA level of GLUT4 in EAT. (D) The GLUT4 translocation in EAT. (E) Immunoblots for phospho-protein kinase B (AKT) (Ser 473) and AKT in EAT. GAPDH was used as reference gene in real-time PCR analysis. Quantification of GLUT4 and AKT was described as the ratio of PM GLUT4 to total GLUT4 and phosphorylated AKT to total AKT, respectively. Statistical difference between groups was shown using a nonparametric Mann-Whitney test; n = 8 per group in (A)–(E). * P < 0.05, ** P < 0.01, *** P < 0.001. All data in (A)–(E) are mean ± SEM.
The expression and translocation of insulin-dependent GLUT4, which is predominantly expressed in adipocytes and muscle cells, functionally regulates glucose uptake into adipose tissues in response to elevated levels of insulin in the circulation (40, 41). Using real-time PCR and Western blotting, we revealed that dietary quercetin enhanced mRNA expression (Fig. 2C) and translocation to the PM (Fig. 2D) of GLUT4 in EAT. AKT is a central adaptor connecting insulin signaling with GLUT4 downstream trafficking, and its activation regulates the translocation, orientation, and fusion of GLUT4 storage vesicles (41). Therefore, we further examined the expression and phosphorylation levels of AKT in EAT in mice after fasting overnight. Dietary quercetin reversed HFD-induced decline of the AKT phosphorylation level in EAT (Fig. 2E). Taken together, these results demonstrate that quercetin enhances glucose uptake in adipose tissues and may thereby protect HFD-fed mice against insulin resistance.
Quercetin inhibited inflammatory cell recruitment into adipose tissues
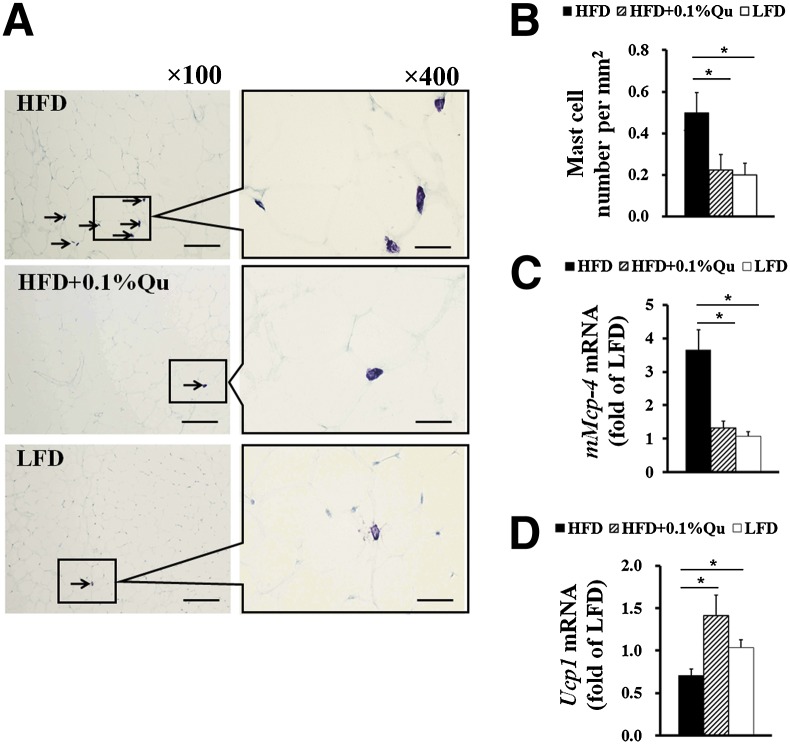
A large number of inflammatory cells, such as mast cells and macrophages, infiltrate into adipose tissue during diet-induced obesity, aggravating adipose tissue inflammation and insulin resistance (1, 3). Our recent study revealed that Western diets induced mast cell recruitment in WAT and promoted diet-induced obesity and insulin resistance (34). As a mast cell stabilizer, quercetin can inhibit the mediator releases of mast cells in vitro (42, 43). To test the effects of quercetin on adipose tissue mast cells in HFD-fed mice, we examined the number of mast cells in EAT (Fig. 3A, B) and the expression of the mast cell marker gene mouse mast cell protease-4 (mMcp-4) (Fig. 3C). The results showed that dietary quercetin dramatically reduced mast cell recruitment in EAT in HFD-fed mice. In addition, our previous study also showed that deficiency and stabilization of mast cells improved energy expenditure in HFD-fed mice (34). Here, along with lower mast cell levels in EAT, the mice receiving quercetin-containing HFD possessed higher BAT uncoupling protein 1 (Ucp1) mRNA expression (Fig. 3D), which is an important thermogenetic factor and energy expenditure marker in BAT. This implies that quercetin may enhance energy expenditure through suppressing mast cell recruitment and degranulation, a hypothesis that merits further investigation.
Fig. 3.
Quercetin reduces mast cell recruitments into EAT. (A) Toluidine blue staining of mast cells and (B) quantification of the numbers of mast cells in the indicated groups of mice; arrows indicate toluidine blue staining mast cells; scale bars in (A), 200 μm in length in low-power views (×100) and 50 μm in length in high-power views (×400). (C) The expression of mast cell marker gene mMcp-4 in EAT. (D) The mRNA level of Ucp1 in BAT. GAPDH was used as reference gene in real-time PCR analysis. Statistical difference between groups was shown using a nonparametric Mann-Whitney test; n = 8 per group. * P < 0.05. All data in (B)–(D) are mean ± SEM.
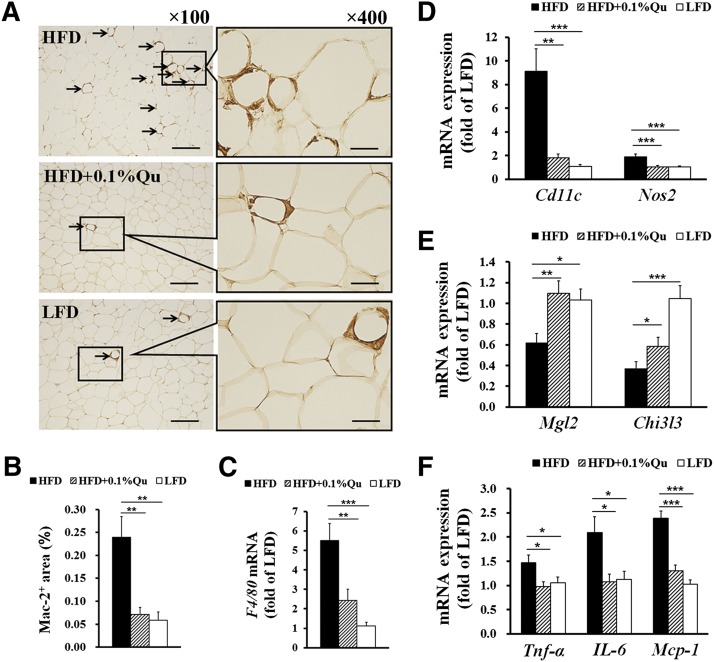
ATM plays a critical role in obesity-induced inflammation and insulin resistance (3), and we previously found that genetic deficiency and pharmacological stabilization of mast cells reduce ATM infiltration into WAT in HFD-fed mice (34). Therefore, the number of macrophages was investigated in EAT. Immunostaining with a macrophage-specific Mac-2 monoclonal antibody revealed that the mice fed with quercetin-containing HFD were similar to the mice fed with LFD in the number of EAT macrophages, which was significantly less than in the mice only fed with HFD (Fig. 4A, B). The real-time PCR results of macrophage-specific marker F4/80 supported the above-mentioned action of dietary quercetin against HFD-induced macrophage infiltration into EAT (Fig. 4C). To explore the subset levels of ATMs, we further analyzed the mRNA expression of some macrophage subtype-specific markers in EAT. Strikingly, dietary quercetin suppressed the expression of Cd11c and Nos2, which are markers of M1 macrophages, in EAT of HFD-fed mice (Fig. 4D). In contrast, the mRNA level of M2 macrophage markers Mgl2 and Chil3l was significantly increased by quercetin in EAT of the mice (Fig. 4E). These results indicate that dietary quercetin reduces the M1 macrophage number and increases the M2 macrophage number and thereby modifies the M1/M2 subtype ratio in EAT.
Fig. 4.
Quercetin decreases macrophage recruitments, alters subset phenotype, and inhibits inflammation levels in EAT. (A) Macrophage-specific Mac-2 immunostaining and (B) quantification of Mac-2+ macrophage area in the indicated groups of mice; arrows indicate Mac-2+ macrophages; scale bars in (A), 200 μm in length in low-power views (×100) and 50 μm in length in high-power views (×400). (C) Real-time PCR quantitative mRNA expression of macrophage marker gene F4/80, (D) M1 marker genes of macrophages Cd11c and Nos2, (E) M2 marker genes of macrophages Mgl2 and Chi3l3, and (F) inflammatory cytokines Tnf-α, IL-6, and Mcp-1 in EAT. GAPDH was used as reference gene in real-time PCR analysis. Statistical difference between groups was shown using a nonparametric Mann-Whitney test; n = 8 per group. * P < 0.05, ** P < 0.01, *** P < 0.001. All data in (B)–(E) are mean ± SEM.
Quercetin reduced inflammatory cytokine levels in adipose tissues and sera
During the development and progression of obesity, various proinflammatory cytokines can be secreted by hypertrophic adipocytes and infiltrated inflammatory cells (2). Here, we examined the expression levels of several important proinflammatory cytokines involved in fat deposition and insulin resistance. Real-time PCR and ELISA results showed that dietary quercetin significantly suppressed the levels of proinflammatory cytokines TNF-α, IL-6, and MCP-1 in EAT (Fig. 4F) and sera (Table 1). These results suggest that dietary quercetin reduces the obesity-induced adipose tissue and systematic inflammation.
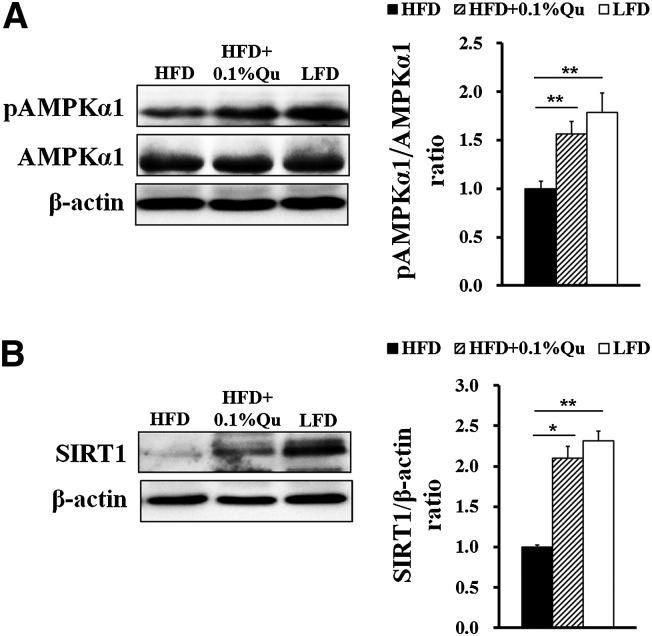
Quercetin enhanced AMPKα1 and SIRT1 activity in adipose tissues
AMPK and SIRT1 are two important nutrient sensors and inflammatory regulators (27, 28). Quercetin can activate AMPK signaling to attenuate adipogenesis in 3T3-L1 cells (8). As an activator of SIRT1, quercetin extends the life span of Saccharomyces cerevisiae (33). Therefore, we detected AMPKα1 and SIRT1 signaling in EAT in mice. Consistent with previous reports (29, 44), HFD suppressed AMPKα1 phosphorylation (Fig. 5A) and SIRT1 expression (Fig. 5B) in EAT. Interestingly, dietary quercetin distinctly resisted the inhibition (Fig. 5A, B).
Fig. 5.
Quercetin increases AMPKα1 activity and SIRT1 expression in EAT in HFD-fed mice. (A) Protein and phosphorylation levels of AMPKα1 in EAT. (B) Protein expression of SIRT1 in EAT. Quantification of AMPKα1 activity and SIRT1 expression was described as the ratio of phosphorylated AMPKα1 to total AMPKα1 and SIRT1 to β-actin, respectively. Statistical difference between groups was shown using a nonparametric Mann-Whitney test; n = 8 per group in (A) and (B). * P < 0.05, ** P < 0.01. All data in (A) and (B) are mean ± SEM.
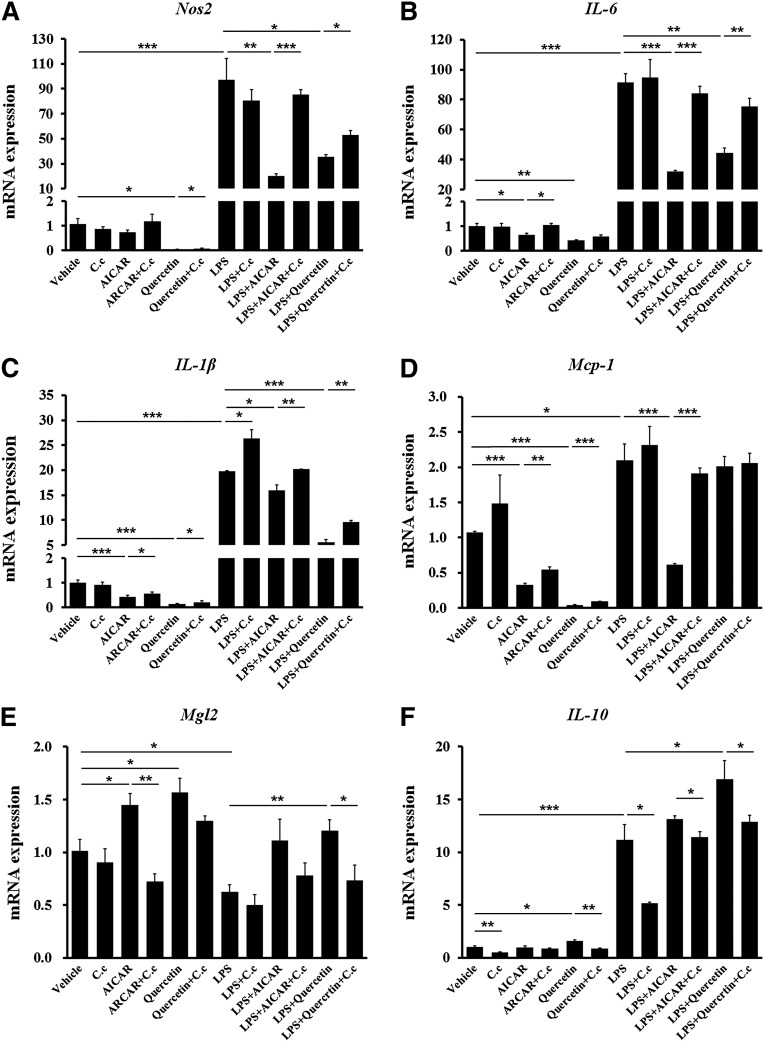
Quercetin regulated BMDM polarization and inflammation through the AMPKα1/ SIRT1 pathway
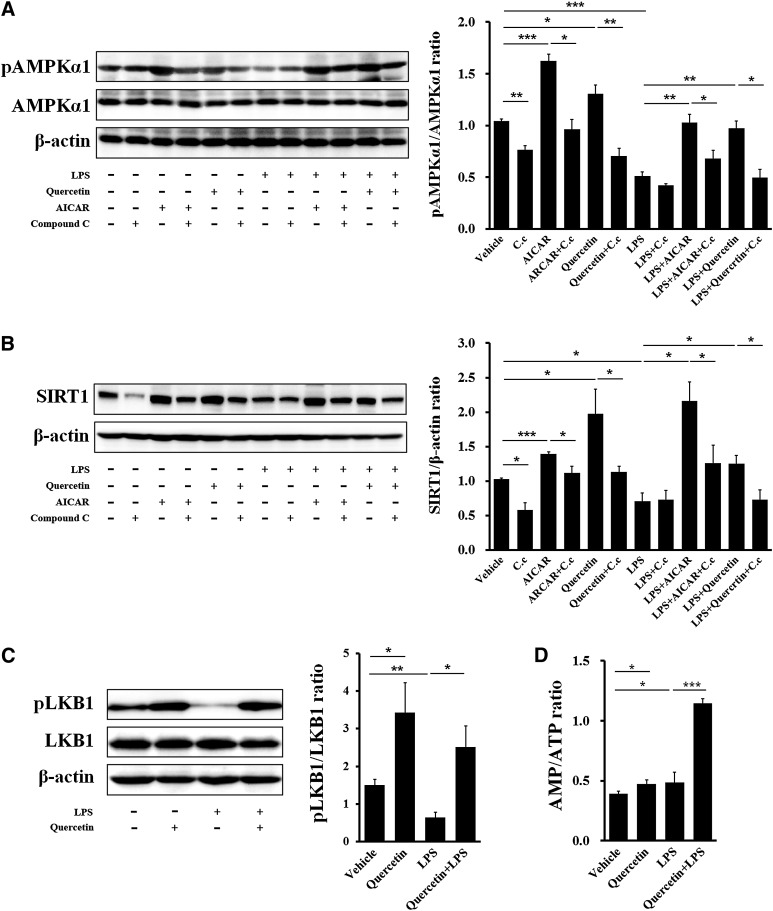
To explore the cellular mechanism of quercetin-antagonized inflammation and quercetin-promoted anti-inflammatory macrophage phenotype in adipose tissue, we investigated whether quercetin directly resisted the polarization and inflammation of differentiated BMDMs. Quercetin significantly reduced the expression of Nos2, the marker of M1 macrophage, in both basal and LPS-stimulated conditions (Fig. 6A). Subsequently, the expression of proinflammatory cytokines, including IL-6, IL-1β, and Mcp-1, was also reduced by quercetin treatment (Fig. 6B–D). On the contrary, M2 macrophage marker Mgl2 (Fig. 6E) and anti-inflammatory cytokine IL-10 (Fig. 6F) expression was enhanced by quercetin in BMDMs. Compound C, a selective AMPK inhibitor, blocked these inhibitory efficiencies of quercetin on BMDM inflammation (Fig. 6A–F). Further, the AMPKα1 and SIRT1 signal was detected in BMDMs. Similar to the AMPK activator AICAR, quercetin dramatically induced phosphorylation of AMPKα1 and expression of SIRT1 in both basal and LPS-stimulated conditions. Compound C could reverse the efficacies of quercetin and AICAR (Fig. 7A, B).
Fig. 6.
Quercetin reduces inflammation and polarization of BMDMs through an AMPK-associated signaling pathway. Quercetin (20 μM) was used during differentiation of BMDMs. Then, BMDMs were treated with 2 mM AICAR and/or 1 μM Compound C for 2 h before 100 ng/ml LPS stimulation. The mRNA levels of some macrophage inflammation-related genes in BMDMs. (A) M1 marker gene of macrophage Nos2; proinflammatory cytokines (B) IL-6, (C) IL-1β, and (D) Mcp-1; (E) M2 marker gene of macrophage Mgl2; and (F) anti-inflammatory cytokine IL-10. GAPDH was used as reference gene in real-time PCR analysis. Data are represented as fold changes compared with vehicle. C.c represented Compound C. Statistical difference between groups was shown using a Student's t-test; n = 4 per group (A–F). * P < 0.05, ** P < 0.01, *** P < 0.001. All data in (A)–(F) are mean ± SEM.
Fig. 7.
Quercetin affects activation of the AMPKα1/SIRT1 pathway in BMDMs. (A) Protein and phosphorylation levels of AMPKα1 in BMDMs. (B) Protein expression of SIRT1 in BMDMs. (C) Protein and phosphorylation levels of LKB1 in BMDMs. Quantification of AMPKα1 activity, SIRT1 expression, and LKB1 activity was described as the ratio of phosphorylated AMPKα1 to total AMPKα1, SIRT1 to β-actin, and phosphorylated LKB1 to total LKB1, respectively. (D) Changes of cellular AMP/ATP ratio by quercetin in BMDMs. Data are represented as fold changes compared with vehicle. C.c represented Compound C. Statistical difference between groups was shown using a Student's t-test; n = 4 per group (A–D). * P < 0.05, ** P < 0.01, *** P < 0.001. All data in (A)–(D) are mean ± SEM.
It has been reported that AMPK could be activated by an AMP-dependent pathway and liver kinase B1 (LKB1) is involved in activation of AMPK in response to an elevated AMP/ATP ratio (45). Therefore, we detected effects of quercetin treatment on LKB1 and adenine nucleotide levels in basal and LPS-stimulated BMDMs. Quercetin treatment increased LKB1 phosphorylation (Fig. 7C) and elevated the cellular AMP/ATP ratio (Fig. 7D).
Galic et al. (46) recently reported that activation of AMPK attenuated the inflammatory response induced by palmitate or LPS through activation of acetyl-CoA carboxylase (ACC) and carnitine palmitoyltransferase 1a (Cpt1a) in macrophages. To examine the effects of quercetin on the AMPK/ACC pathway in BMDMs, we detected ACC expression and phosphorylation and Cpt1a mRNA expression. In the basal condition, although quercetin reduced BMDM inflammation (Fig. 6) and activated the AMPK/SIRT1 signal (Fig. 7A, B), quercetin did not affect ACC phosphorylation and Cpt1a expression in the same condition (supplementary Fig. I), suggesting that ACC phosphorylation and associated FA oxidation may not be involved in the inhibition of macrophage basal inflammation by quercetin. By contrast, in the LPS-stimulated condition, both quercetin and AICAR increased ACC phosphorylation and Cpt1a expression (supplementary Fig. I). Compound C abolished the efficacies of quercetin and AICAR (supplementary Fig. I).
Quercetin increased GLUT4 translocation through AMPK activation in 3T3-L1 adipocytes
Because quercetin increased GLUT4 mRNA expression (Fig. 2C) and protein translocation (Fig. 2D) in EAT in HFD-fed mice and AMPK activation can promote GLUT4 translocation (40), we used Western blotting to detect the role of AMPK in GLUT4 translocation by quercetin in 3T3-L1 adipocytes. The results showed that either AICAR or quercetin strikingly increased the GLUT4 level in the cell membrane fraction and that Compound C reversed the action of quercetin and AICAR (supplementary Fig. II).
DISCUSSION
In this study, we demonstrate that dietary quercetin ameliorates obesity-associated insulin resistance and adipose tissue inflammation. Meanwhile, quercetin antagonizes ATM infiltration and inflammation and normalizes AMPKα1 activity and SIRT1 expression in adipose tissues. Further, in vitro studies show that quercetin suppresses BMDM polarization and inflammation through activating AMPKα1/SIRT1 signaling. These findings suggest that dietary quercetin can suppress inflammatory cell recruitment and regulate ATM polarization and inflammation through a mechanism including AMPKα1/SIRT1.
Quercetin, an important dietary flavonoid, has been widely investigated because of its beneficial effects on diabetes (7, 22, 24), hepatic fat accumulation (20), and metabolic syndromes (6, 23). Adipose tissue inflammation, especially ATM recruitment, has been regarded as an important central event in obesity-associated insulin resistance (3). Therefore, we designed this study to test whether quercetin antagonizes adipose tissue inflammation in HFD-fed mice. Moreover, although both visceral and SATs are white adipose depots, visceral adipose tissues have greater inflammatory cell recruitment and secrete more abundant proinflammatory mediators than SAT responding to HFD (3). In visceral adipose tissues, mast cell and macrophage infiltration of EAT is most severe during diet-induced obesity (35). Therefore, we mainly focus on the inflammatory cells and signaling in EAT in this study.
Previous studies showed that 0.05% or 0.4% quercetin significantly reduced body weight gain and blood glucose levels in HFD-fed mice (20, 21), but 0.8% or 1.2% quercetin did not affect diet-induced obesity and insulin resistance (16). Moreover, 0.5% or 1% quercetin diets, but not 0.1% quercetin diets, remarkably decreased the expression of ubiquitin C in healthy control mice (7). Therefore, to avoid the negative effects of excessive quercetin intake, we used 0.1% as the dose of dietary quercetin intervention in this study. After 12 weeks of feeding, the serum concentration of quercetin was 9.19 ± 0.46 μmol/l in mice fed quercetin-containing HFD. The concentration was at a comparative level with those in other related topic studies (7, 17, 20).
Until now, the effect of quercetin on HFD-induced body weight gain has been debated, and several different groups have reported that dietary quercetin could not impact the body weight of HFD-fed animals (6, 16–19). Similar to two other reports (20, 21), we found here that the mice fed dietary quercetin had significantly lower body weight gain than the mice fed only HFD during 8–12 weeks after diet interventions. Moreover, although dietary quercetin reduced EAT and SAT weights (Fig. 1C, supplementary Table III), it did not affect liver and BAT weights (supplementary Table III). It seems that quercetin is a mild weight-reducing and antilipid accumulation component; thus the efficiency of quercetin on HFD-induced body weight gain may greatly depend on the experimental animal strain, the fat and glucose ratio in HFD, and the dose, age at start of feeding, and duration of dietary quercetin intervention.
Besides reducing body weight, antiadipogenesis (Fig. 1C, E) and anti-insulin resistance (Fig. 2A, B) efficiency of dietary quercetin were also identified in our study. Correspondingly, dietary quercetin also increased GLUT4 mRNA expression (Fig. 2C) and protein translocation (Fig. 2D) and AKT phosphorylation (Ser473) (Fig. 2E) in EAT in HFD-fed mice, suggesting that it improves glucose uptake in adipose tissues. Moreover, several recent studies have shown that dietary quercetin could improve insulin sensitivity in liver (6, 20) and skeletal muscle (47), indicating that quercetin could improve systemic insulin resistance in HFD-fed mice.
Because a chronic low-grade adipose tissue inflammation participates in the development of diet-induced obesity and insulin resistance (1–3), we speculated that quercetin could ameliorate HFD-induced adipose tissue inflammation. Our next studies confirmed this hypothesis. Dietary quercetin attenuated mast cell (Fig. 3A–C) and macrophage (Fig. 4A–C) recruitment into adipose tissues, modified the M1/M2 macrophage ratio in EAT (Fig. 4D, E), and normalized the levels of proinflammatory cytokines TNF-α, IL-6, and MCP-1 in adipose tissues (Fig. 4F) and sera (Table 1). Similar to our results, some previous studies reported that dietary quercetin inhibited inflammatory cell infiltration into liver and the left ventricle (6) and altered hepatic inflammatory response gene expression (6, 19–21, 25, 26) and circulating inflammatory cytokine levels (18, 19). Therefore, as a potent inflammation inhibitor, quercetin ameliorates obesity-associated adipose tissue and systemic inflammation.
To explore the molecular mechanisms of quercetin improving HFD-induced adipose tissue inflammation and insulin resistance, we further focused on two important nutrient sensors and inflammatory regulators, AMPKα1 and SIRT1 (27, 28), in EAT. Dietary quercetin restored HFD-suppressed AMPKα1 activity (Fig. 5A) and SIRT1 expression (Fig. 5B) in EAT. This suggests that quercetin may impact AMPKα1/SIRT1 signaling in adipose tissues. Yang et al. (29) recently reported that AMPKα1 could activate SIRT1 and inhibit inflammation in macrophages. Moreover, quercetin inhibited adipogenesis through upregulating the phosphorylation of AMPKα and AMPKβ1 in 3T3-L1 preadipocytes (8), and quercetin could directly or indirectly activate SIRT1 in vitro (31, 33). Therefore, we asked whether quercetin could regulate macrophage polarization and inhibit macrophage inflammation through activating AMPKα1/SIRT1 signaling. To answer this question, we studied in vitro the effects of quercetin on BMDM inflammation.
As important tissue inflammatory cells, macrophages undergo several stages of differentiation, including bone marrow hematopoietic stem cell, blood monocyte, and tissue macrophage (48). In diet-induced obesity, excess nutrition promotes more ATM recruitment and M1 polarization than in the normal diet state (3). Our study shows that dietary quercetin inhibits at least two stages of macrophage differentiation and polarization: macrophage infiltration (from monocyte to macrophage) (Fig. 4A–C) and macrophage subtype transformation (from M2 to M1 subtype) (Fig. 4D, E). To mimic comprehensively the possible role of quercetin in macrophage differentiation and polarization, vehicle or 20 μM quercetin was added during BMDM differentiation and LPS treatment. In type 2 diabetic patients and humans after a high-fat meal, circulating LPS levels were increased (49, 50). In experimental animals, HFD enhanced circulating LPS levels through altering intestinal permeability, and subcutaneous infusion of LPS could initiate obesity and insulin resistance (50). Moreover, LPS can induce macrophage M1 activation and proinflammatory gene expression (51). Therefore, in this study, LPS was used to induce obesity-associated macrophage M1 polarization and inflammation.
In basal and LPS-stimulated conditions, quercetin downregulated M1 macrophage marker and proinflammatory cytokine expression and upregulated M2 macrophage marker and anti-inflammatory cytokine expression in BMDMs (Fig. 6). Further, quercetin significantly increased the phosphorylation level of AMPKα1 (Fig. 7A) and SIRT1 expression (Fig. 7B) in basal and LPS-exposed BMDMs. Meanwhile, the effects of quercetin were similar to those of AMPK activator AICAR and could be blocked by AMPK inhibitor Compound C (Fig. 6; Fig. 7A, B). LKB1 is an upstream kinase, which directly phosphorylates and activates AMPK in response to an elevated AMP/ATP ratio (46). To determine how quercetin activates AMPK, we detected the effect of quercetin on an AMP-dependent pathway including the AMP/ATP ratio, LKB1 expression, and phosphorylation. Quercetin treatment increased LKB1 activity (Fig. 7C) and the cellular AMP/ATP ratio (Fig. 7D) in BMDMs. Because LPS treatment promotes M1 activation of macrophages (51), the results suggest that quercetin can upregulate the AMP/ATP ratio and LKB1 activity and further activate AMPKα1/SIRT1 signaling to inhibit M1 polarization and inflammation of macrophages.
In diet-induced obesity, adipose tissues possess hypertrophic adipocytes and more inflammatory cells, such as mast cells and macrophages. The cells secrete more proinflammatory mediators and produce adipose tissue inflammation (1, 2). AMPK has been considered a central inhibitor of adipose tissue inflammation (28, 52). Activating AMPK may suppress c-Jun activation (52, 53) and stimulate SIRT1 (28, 29) to inhibit nuclear factor-κB (NF-κB). In macrophages, AMPKβ activation, which can mainly determine AMPKα1 activity and expression, attenuates palmitate- or LPS-induced inflammation through increasing FA oxidation associated with ACC and Cpt1a (47). It is noteworthy that increased macrophage lipid accumulation is related to M1 macrophage polarization in adipose tissue during obesity (54) and SIRT1 is involved in AMPK-dependent FA oxidation enhancement (55). Additionally, AMPK activation also regulates GLUT4 translocation (40). Here, we found that quercetin not only activated AMPKα1/SIRT1 but also promoted ACC phosphorylation and Cpt1a expression in the LPS-stimulated condition (supplementary Fig. I). Previous studies reported that quercetin reduced macrophage (10–13) and adipocyte (10) inflammation through suppressing phosphorylation of c-Jun N-terminal kinase and c-Jun and inhibiting activation of NF-κB. Therefore, AMPK/SIRT1 activation by quercetin might increase FA oxidation, depress inflammatory signals such as c-Jun and NF-κB, and enhance glucose uptake in adipose tissues, thereby improving adipose tissue inflammation and insulin resistance.
This study has several potential limitations because we only focused on the possible action of quercetin on HFD-induced adipose tissue and macrophage inflammation in mice. First, we did not assess the effects of dietary quercetin on lean body mass, although EAT, SAT, BAT, and liver weights were detected (Fig. 1C, supplementary Table III). However, the results cannot give us any information about the alteration in lean body mass by quercetin, so we should perform a lean body mass study in the future. Second, we found here that dietary quercetin reduced the mast cell number in EAT and increased energy expenditure marker Ucp1 expression in BAT (Fig. 3), suggesting that energy expenditure levels are enhanced in mice fed quercetin-containing HFD. However, this hypothesis requires further confirmation, including studies in oxygen consumption and CO2 production, changes in the hourly respiration exchange rate, core temperatures, physical activity, morphological analysis of BAT, expression of series energy expenditure marker genes in BAT, and so on. Third, considering the paradoxical effects of quercetin on HFD-induced body weight gain in previous reports (6, 16–21), dietary quercetin intervention started at 5 weeks old, an age that may be more sensitive to HFD-induced efficacy. Thus, the metabolic impact of dietary quercetin during development needs to be investigated in our future work. Moreover, we did not use classic freeze clamp methodology here, which may lead to hypoxia and enhance AMPK activity post mortem. However, all the tissues were similarly handled in our study, and the non-freeze clamp methodology should not affect the relative difference of AMPK activities among EATs from the tested mice. Indeed, in accordance with a previously published study (29), we showed here that HFD reduced AMPK activities in EAT in mice (Fig. 5A). Therefore, dietary quercetin-abolished HFD-induced AMPK inactivation (Fig. 5A) should not be compromised by our non-freeze clamping manipulation.
In summary, as shown in supplementary Fig. III, we demonstrate here that dietary quercetin ameliorates HFD-induced obesity and insulin resistance in mice. Meanwhile, quercetin attenuates inflammatory mast cell and macrophage infiltration into adipose tissue and reduces adipose tissue inflammation. More importantly, quercetin antagonizes M1 polarization and inflammation of ATMs, which play a critical role in obesity-associated adipose tissue inflammation and insulin resistance (3). Finally, we confirm that quercetin enhances LKB1 activity and the AMP/ATP ratio, activates AMPKα1/SIRT1 and other associated anti-inflammation (e.g., AMPK/ACC) signal pathways, and thereby inhibits macrophage polarization and inflammation. Considering that quercetin is an abundant dietary flavonoid in plants and its inherent antiadipogenesis, anti-inflammation, and anti-insulin resistance actions, it might become a benefic dietary supplement to protect against obesity-associated insulin resistance and diabetes.
Supplementary Material
Footnotes
Abbreviations:
- ACC
- acetyl-CoA carboxylase
- AICAR
- 5-aminoimidazole-4-carboxamide-1-β4-ribofuranoside
- AKT
- protein kinase B
- AMPK
- adenosine monophosphate-activated protein kinase
- ATM
- adipose tissue macrophage
- BAT
- brown adipose tissue
- BMDM
- bone marrow-derived macrophage
- Cpt1a
- carnitine palmitoyltransferase 1a
- EAT
- epididymis adipose tissue
- GLUT4
- glucose transporter 4
- HFD
- high-fat diet
- IL
- interleukin
- LFD
- low-fat diet
- LKB1
- liver kinase B1
- LPS
- lipopolysaccharide
- MCP-1
- monocyte chemotactic protein-1
- NF-κB
- nuclear factor-κB
- PM
- plasma membrane
- SAT
- subcutaneous adipose tissue
- SIRT1
- silent information regulator 1
- UCP1
- uncoupling protein 1
- WAT
- white adipose tissue
This work was supported by a project from National Natural Science Foundation of China, Grant No. 31171315 (J.L.); the Specialized Research Fund for the Doctoral Program of Higher Education of China, Grant Nos. 20100111110009 (J.L.); the Anhui Provincial Natural Science Foundation of China, Grant No. 11040606M92 (J.L.); the Fundamental Research Funds for the Central Universities, China, Grant Nos. 2012HGCX0003 and 2012HGQC0019 (J.L. and B.B.); and the China Postdoctoral Science Foundation, Grant No. 2013M541817 (B.B.). The authors declare no conflict of interest.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures and three tables.
REFERENCES
- 1.Sun S., Ji Y., Kersten S., Qi L. 2012. Mechanisms of inflammatory responses in obese adipose tissue. Annu. Rev. Nutr. 32: 261–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ouchi N., Parker J. L., Lugus J. J., Walsh K. 2011. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 11: 85–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Osborn O., Olefsky J. M. 2012. The cellular and signaling networks linking the immune system and metabolism in disease. Nat. Med. 18: 363–374 [DOI] [PubMed] [Google Scholar]
- 4.Lumeng C. N., Bodzin J. L., Saltiel A. R. 2007. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117: 175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patsouris D., Li P. P., Thapar D., Chapman J., Olefsky J. M., Neels J. G. 2008. Ablation of CD11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 8: 301–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Panchal S. K., Poudyal H., Brown L. 2012. Quercetin ameliorates cardiovascular, hepatic, and metabolic changes in diet-induced metabolic syndrome in rats. J. Nutr. 142: 1026–1032 [DOI] [PubMed] [Google Scholar]
- 7.Kobori M., Masumoto S., Akimoto Y., Takahashi Y. 2009. Dietary quercetin alleviates diabetic symptoms and reduces streptozotocin-induced disturbance of hepatic gene expression in mice. Mol. Nutr. Food Res. 53: 859–868 [DOI] [PubMed] [Google Scholar]
- 8.Ahn J., Lee H., Kim S., Park J., Ha T. 2008. The anti-obesity effect of quercetin is mediated by the AMPK and MAPK signaling pathways. Biochem. Biophys. Res. Commun. 373: 545–549 [DOI] [PubMed] [Google Scholar]
- 9.Yang L., Li X. F., Gao L., Zhang Y. O., Cai G. P. 2012. Suppressive effects of quercetin-3-O-(6’’-Feruloyl)-beta-D-galactopyranoside on adipogenesis in 3T3–L1 preadipocytes through down-regulation of PPARgamma and C/EBPalpha expression. Phytother. Res. 26: 438–444 [DOI] [PubMed] [Google Scholar]
- 10.Overman A., Chuang C. C., McIntosh M. 2011. Quercetin attenuates inflammation in human macrophages and adipocytes exposed to macrophage-conditioned media. Int. J. Obes. (Lond). 35: 1165–1172 [DOI] [PubMed] [Google Scholar]
- 11.Comalada M., Ballester I., Bailon E., Sierra S., Xaus J., Galvez J., de Medina F. S., Zarzuelo A. 2006. Inhibition of pro-inflammatory markers in primary bone marrow-derived mouse macrophages by naturally occurring flavonoids: analysis of the structure-activity relationship. Biochem. Pharmacol. 72: 1010–1021 [DOI] [PubMed] [Google Scholar]
- 12.Hämäläinen M., Nieminen R., Vuorela P., Heinonen M., Moilanen E. 2007. Anti-inflammatory effects of flavonoids: genistein, kaempferol, quercetin, and daidzein inhibit STAT-1 and NF-kappaB activations, whereas flavone, isorhamnetin, naringenin, and pelargonidin inhibit only NF-kappaB activation along with their inhibitory effect on iNOS expression and NO production in activated macrophages. Mediators Inflamm. 2007: 45673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Puangpraphant S., de Mejia E. G. 2009. Saponins in yerba mate tea (Ilex paraguariensis A. St.-Hil) and quercetin synergistically inhibit iNOS and COX-2 in lipopolysaccharide-induced macrophages through NFkappaB pathways. J. Agric. Food Chem. 57: 8873–8883 [DOI] [PubMed] [Google Scholar]
- 14.Chuang C. C., Shen W., Chen H., Xie G., Jia W., Chung S., McIntosh M. K. 2012. Differential effects of grape powder and its extract on glucose tolerance and chronic inflammation in high-fat-fed obese mice. J. Agric. Food Chem. 60: 12458–12468 [DOI] [PubMed] [Google Scholar]
- 15.Chuang C. C., Martinez K., Xie G., Kennedy A., Bumrungpert A., Overman A., Jia W., McIntosh M. K. 2010. Quercetin is equally or more effective than resveratrol in attenuating tumor necrosis factor-{alpha}-mediated inflammation and insulin resistance in primary human adipocytes. Am. J. Clin. Nutr. 92: 1511–1521 [DOI] [PubMed] [Google Scholar]
- 16.Stewart L. K., Wang Z., Ribnicky D., Soileau J. L., Cefalu W. T., Gettys T. W. 2009. Failure of dietary quercetin to alter the temporal progression of insulin resistance among tissues of C57BL/6J mice during the development of diet-induced obesity. Diabetologia. 52: 514–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wein S., Behm N., Petersen R. K., Kristiansen K., Wolffram S. 2010. Quercetin enhances adiponectin secretion by a PPAR-gamma independent mechanism. Eur. J. Pharm. Sci. 41: 16–22 [DOI] [PubMed] [Google Scholar]
- 18.Stewart L. K., Soileau J. L., Ribnicky D., Wang Z. Q., Raskin I., Poulev A., Majewski M., Cefalu W. T., Gettys T. W. 2008. Quercetin transiently increases energy expenditure but persistently decreases circulating markers of inflammation in C57BL/6J mice fed a high-fat diet. Metabolism. 57: S39–S46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boesch-Saadatmandi C., Wagner A. E., Wolffram S., Rimbach G. 2012. Effect of quercetin on inflammatory gene expression in mice liver in vivo - role of redox factor 1, miRNA-122 and miRNA-125b. Pharmacol. Res. 65: 523–530 [DOI] [PubMed] [Google Scholar]
- 20.Kobori M., Masumoto S., Akimoto Y., Oike H. 2011. Chronic dietary intake of quercetin alleviates hepatic fat accumulation associated with consumption of a Western-style diet in C57/BL6J mice. Mol. Nutr. Food Res. 55: 530–540 [DOI] [PubMed] [Google Scholar]
- 21.Zhou M., Wang S., Zhao A., Wang K., Fan Z., Yang H., Liao W., Bao S., Zhao L., Zhang Y., et al. 2012. Transcriptomic and metabonomic profiling reveal synergistic effects of quercetin and resveratrol supplementation in high fat diet fed mice. J. Proteome Res. 11: 4961–4971 [DOI] [PubMed] [Google Scholar]
- 22.Kim J. H., Kang M. J., Choi H. N., Jeong S. M., Lee Y. M., Kim J. I. 2011. Quercetin attenuates fasting and postprandial hyperglycemia in animal models of diabetes mellitus. Nutr. Res. Pract. 5: 107–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rivera L., Moron R., Sanchez M., Zarzuelo A., Galisteo M. 2008. Quercetin ameliorates metabolic syndrome and improves the inflammatory status in obese Zucker rats. Obesity (Silver Spring). 16: 2081–2087 [DOI] [PubMed] [Google Scholar]
- 24.Jeong S. M., Kang M. J., Choi H. N., Kim J. H., Kim J. I. 2012. Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice. Nutr. Res. Pract. 6: 201–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ying H. Z., Liu Y. H., Yu B., Wang Z. Y., Zang J. N., Yu C. H. 2013. Dietary quercetin ameliorates nonalcoholic steatohepatitis induced by a high-fat diet in gerbils. Food Chem. Toxicol. 52: 53–60 [DOI] [PubMed] [Google Scholar]
- 26.Marcolin E., San-Miguel B., Vallejo D., Tieppo J., Marroni N., Gonzalez-Gallego J., Tunon M. J. 2012. Quercetin treatment ameliorates inflammation and fibrosis in mice with nonalcoholic steatohepatitis. J. Nutr. 142: 1821–1828 [DOI] [PubMed] [Google Scholar]
- 27.Weng S. Y., Schuppan D. 2013. AMPK regulates macrophage polarization in adipose tissue inflammation and NASH. J. Hepatol. 58: 619–621 [DOI] [PubMed] [Google Scholar]
- 28.Salminen A., Hyttinen J. M., Kaarniranta K. 2011. AMP-activated protein kinase inhibits NF-kappaB signaling and inflammation: impact on healthspan and lifespan. J. Mol. Med. (Berl). 89: 667–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang Z., Kahn B. B., Shi H., Xue B. Z. 2010. Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J. Biol. Chem. 285: 19051–19059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sag D., Carling D., Stout R. D., Suttles J. 2008. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J. Immunol. 181: 8633–8641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Escande C., Nin V., Price N. L., Capellini V., Gomes A. P., Barbosa M. T., O'Neil L., White T. A., Sinclair D. A., Chini E. N. 2013. Flavonoid apigenin is an inhibitor of the NAD+ase CD38: implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes. 62: 1084–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kellenberger E., Kuhn I., Schuber F., Muller-Steffner H. 2011. Flavonoids as inhibitors of human CD38. Bioorg. Med. Chem. Lett. 21: 3939–3942 [DOI] [PubMed] [Google Scholar]
- 33.Howitz K. T., Bitterman K. J., Cohen H. Y., Lamming D. W., Lavu S., Wood J. G., Zipkin R. E., Chung P., Kisielewski A., Zhang L. L., et al. 2003. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 425: 191–196 [DOI] [PubMed] [Google Scholar]
- 34.Liu J., Divoux A., Sun J., Zhang J., Clement K., Glickman J. N., Sukhova G. K., Wolters P. J., Du J., Gorgun C. Z., et al. 2009. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat. Med. 15: 940–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Altintas M. M., Azad A., Nayer B., Contreras G., Zaias J., Faul C., Reiser J., Nayer A. 2011. Mast cells, macrophages, and crown-like structures distinguish subcutaneous from visceral fat in mice. J. Lipid Res. 52: 480–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee W. H., Lin R. J., Lin S. Y., Chen Y. C., Lin H. M., Liang Y. C. 2011. Osthole enhances glucose uptake through activation of AMP-activated protein kinase in skeletal muscle cells. J. Agric. Food Chem. 59: 12874–12881 [DOI] [PubMed] [Google Scholar]
- 37.Wang L., Trebicka E., Fu Y., Waggoner L., Akira S., Fitzgerald K. A., Kagan J. C., Cherayil B. J. 2011. Regulation of lipopolysaccharide-induced translation of tumor necrosis factor-alpha by the toll-like receptor 4 adaptor protein TRAM. J. Innate Immun. 3: 437–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cheng Z., Pang T., Gu M., Gao A. H., Xie C. M., Li J. Y., Nan F. J., Li J. 2006. Berberine-stimulated glucose uptake in L6 myotubes involves both AMPK and p38 MAPK. Biochim. Biophys. Acta. 1760: 1682–1689 [DOI] [PubMed] [Google Scholar]
- 39.Yang M., Zhang Y., Pan J., Sun J., Liu J., Libby P., Sukhova G. K., Doria A., Katunuma N., Peroni O. D., et al. 2007. Cathepsin L activity controls adipogenesis and glucose tolerance. Nat. Cell Biol. 9: 970–977 [DOI] [PubMed] [Google Scholar]
- 40.Huang S., Czech M. P. 2007. The GLUT4 glucose transporter. Cell Metab. 5: 237–252 [DOI] [PubMed] [Google Scholar]
- 41.Leto D., Saltiel A. R. 2012. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat. Rev. Mol. Cell Biol. 13: 383–396 [DOI] [PubMed] [Google Scholar]
- 42.Park H. H., Lee S., Son H. Y., Park S. B., Kim M. S., Choi E. J., Singh T. S., Ha J. H., Lee M. G., Kim J. E., et al. 2008. Flavonoids inhibit histamine release and expression of proinflammatory cytokines in mast cells. Arch. Pharm. Res. 31: 1303–1311 [DOI] [PubMed] [Google Scholar]
- 43.Kempuraj D., Castellani M. L., Petrarca C., Frydas S., Conti P., Theoharides T. C., Vecchiet J. 2006. Inhibitory effect of quercetin on tryptase and interleukin-6 release, and histidine decarboxylase mRNA transcription by human mast cell-1 cell line. Clin. Exp. Med. 6: 150–156 [DOI] [PubMed] [Google Scholar]
- 44.Chalkiadaki A., Guarente L. 2012. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab. 16: 180–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carling D., Sanders M. J., Woods A. 2008. The regulation of AMP-activated protein kinase by upstream kinases. Int. J. Obes. (Lond). 32(Suppl. 4): S55–S59 [DOI] [PubMed] [Google Scholar]
- 46.Galic S., Fullerton M. D., Schertzer J. D., Sikkema S., Marcinko K., Walkley C. R., Izon D., Honeyman J., Chen Z. P., van Denderen B. J., et al. 2011. Hematopoietic AMPK beta1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. J. Clin. Invest. 121: 4903–4915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anhê G. F., Okamoto M. M., Kinote A., Sollon C., Lellis-Santos C., Anhe F. F., Lima G. A., Hirabara S. M., Velloso L. A., Bordin S., et al. 2012. Quercetin decreases inflammatory response and increases insulin action in skeletal muscle of ob/ob mice and in L6 myotubes. Eur. J. Pharmacol. 689: 285–293 [DOI] [PubMed] [Google Scholar]
- 48.Rickard A. J., Young M. J. 2009. Corticosteroid receptors, macrophages and cardiovascular disease. J. Mol. Endocrinol. 42: 449–459 [DOI] [PubMed] [Google Scholar]
- 49.Neves A. L., Coelho J., Couto L., Leite-Moreira A., Roncon-Albuquerque R., Jr 2013. Metabolic endotoxemia: a molecular link between obesity and cardiovascular risk. J. Mol. Endocrinol. 51: R51–R64 [DOI] [PubMed] [Google Scholar]
- 50.Olefsky J. M., Glass C. K. 2010. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 72: 219–246 [DOI] [PubMed] [Google Scholar]
- 51.Lawrence T., Natoli G. 2011. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat. Rev. Immunol. 11: 750–761 [DOI] [PubMed] [Google Scholar]
- 52.Leiherer A., Mundlein A., Drexel H. 2013. Phytochemicals and their impact on adipose tissue inflammation and diabetes. Vascul. Pharmacol. 58: 3–20 [DOI] [PubMed] [Google Scholar]
- 53.Jiang S., Wang W., Miner J., Fromm M. 2012. Cross regulation of sirtuin 1, AMPK, and PPARgamma in conjugated linoleic acid treated adipocytes. PLoS ONE. 7: e48874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prieur X., Mok C. Y., Velagapudi V. R., Nunez V., Fuentes L., Montaner D., Ishikawa K., Camacho A., Barbarroja N., O'Rahilly S., et al. 2011. Differential lipid partitioning between adipocytes and tissue macrophages modulates macrophage lipotoxicity and M2/M1 polarization in obese mice. Diabetes. 60: 797–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cantó C., Gerhart-Hines Z., Feige J. N., Lagouge M., Noriega L., Milne J. C., Elliott P. J., Puigserver P., Auwerx J. 2009. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 458: 1056–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.