Abstract
Adipose tissue contains one of the largest reservoirs of cholesterol in the body. Adipocyte dysfunction in obesity is associated with intracellular cholesterol accumulation, and alterations in cholesterol homeostasis have been shown to alter glucose metabolism in cultured adipocytes. ABCA1 plays a major role in cholesterol efflux, suggesting a role for ABCA1 in maintaining cholesterol homeostasis in the adipocyte. However, the impact of adipocyte ABCA1 on adipose tissue function and glucose metabolism is unknown. Our aim was to determine the impact of adipocyte ABCA1 on adipocyte lipid metabolism, body weight, and glucose metabolism in vivo. To address this, we used mice lacking ABCA1 specifically in adipocytes (ABCA1−ad/−ad). When fed a high-fat, high-cholesterol diet, ABCA1−ad/−ad mice showed increased cholesterol and triglyceride stores in adipose tissue, developed enlarged fat pads, and had increased body weight. Associated with these phenotypic changes, we observed significant changes in the expression of genes involved in cholesterol and glucose homeostasis, including ldlr, abcg1, glut-4, adiponectin, and leptin. ABCA1−ad/−ad mice also demonstrated impaired glucose tolerance, lower insulin sensitivity, and decreased insulin secretion. We conclude that ABCA1 in adipocytes influences adipocyte lipid metabolism, body weight, and whole-body glucose homeostasis.
Keywords: cholesterol, glucose homeostasis, insulin resistance, lipid metabolism, obesity, type 2 diabetes, ATP binding cassette transporter A1
Adipose tissue has a crucial role in energy metabolism because it is the major storage site for TGs. Furthermore, adipose tissue is an important exocrine organ secreting various adipokines important in metabolism (1, 2). In addition to being the main site for TG storage, adipose tissue also contains one of the largest pools of cholesterol in the body (3, 4). Cholesterol and TGs are taken up by adipocytes from plasma lipoproteins via lipoprotein receptors including the LDL receptor (LDLR), scavenger receptor B1 (SR-B1), and CD36 (3–5). TGs leave adipose tissue as NEFAs after TG lipolysis (6), while cholesterol is removed from adipocytes via transporters such as ABCA1 and ABCG1 (7, 8).
ABCA1 is responsible for the efflux of cholesterol to apoA1 and small HDL particles and is the rate-limiting protein in HDL production (9, 10). ABCA1 is widely expressed (11), has been shown to be a crucial regulator of intracellular cholesterol stores, and has an important role in the function of multiple tissues (12, 13). For example, lack of ABCA1 in the brain leads to changes in motor activity and sensorimotor function (12), whereas ABCA1 deficiency in beta cells reduces insulin release (14). ABCA1 has been shown to be expressed and functional in adipocytes (7, 8). Gonadal adipose tissue (GAT) lacking ABCA1 has reduced cholesterol efflux concomitant with increased cholesterol stores (9, 15), indicating that ABCA1-mediated cholesterol efflux is important in adipocyte cholesterol metabolism. Changes in adipocyte cholesterol homeostasis are associated with adipose dysfunction and obesity (3, 16, 17). However, the role of ABCA1 and intracellular cholesterol in adipose function with regard to body weight and glucose metabolism is unclear.
Intracellular cholesterol has been suggested to play a role in adipose tissue dysfunction (3, 16, 17). Adipocytes from obese individuals contain increased stores of both TGs and cholesterol (3, 17). Furthermore, in 3T3 adipocytes, stimulation of TG lipolysis has been shown to induce cholesterol efflux (7). Therefore, it has been suggested that cholesterol may directly affect TG stores (16, 17). This also suggests that ABCA1 may be linked to TG metabolism in adipocytes and adipose tissue function. Adipose tissue dysfunction is a characteristic of obesity (18) and is an important risk factor for insulin resistance, glucose intolerance, and the eventual development of type 2 diabetes (18, 19). Adipose tissue dysfunction affects glucose tolerance through altered release of NEFAs and adipokines in plasma (1, 2, 19, 20). NEFA release from adipose tissue reduces insulin sensitivity and insulin secretion (19, 21). Expression and release of the various adipokines regulate insulin sensitivity, insulin release, and glucose tolerance (2, 19, 21).
Adipocyte cholesterol may also directly regulate glucose homeostasis in the adipocyte. Membrane cholesterol depletion from the adipocyte using methyl-β-cyclodextrin reduces glut-4 expression and impairs insulin-stimulated adipocyte glucose transport in 3T3 cells (16). This provides further evidence for a direct link between cholesterol and adipocyte glucose homeostasis.
Collectively, this evidence suggests that cholesterol regulation in adipocytes may have important implications for glucose homeostasis. ABCA1 has been shown to regulate adipocyte cholesterol levels (7, 8). However, the role of ABCA1 in adipocyte function with regard to body weight and glucose metabolism is unknown. In this study, we suggest a link between the regulation of cholesterol in adipocytes by ABCA1 with TG storage, body weight, and whole-body glucose homeostasis.
MATERIALS AND METHODS
Animals
Mice lacking ABCA1 in the adipose tissue (ABCA1−ad/−ad) were generated by crossing ABCA1 floxed mice with mice expressing the cre transgene under the adipose-specific aP2 promoter (The Jackson Laboratory, Bar Harbor, ME). These mice have been shown to be deficient in ABCA1 specifically in their adipose tissues (15). Mice were on a C57BL/6 background, were housed under 12 h light-dark cycles, and had ad libitum access to chow or high-fat, high-cholesterol (HFHC) diet and water. Male mice were used for all experiments. To study the role of adipocyte ABCA1 in diet-induced obesity, mice were fed an HFHC diet containing 21% milk fat weight/weight (w/w) and 0.21% cholesterol (Western diet D12079B; Research Diets, New Brunswick, NJ) at 10 weeks of age. All experiments were in conformity with Public Health Service policy, approved by the University of British Columbia Animal Care Committee, and conducted in accordance with their guidelines.
Tissue analysis
Mice were euthanized using CO2 after 4 h fasting, and tissues were isolated, frozen in liquid nitrogen, and stored at −80°C. Subcutaneous adipose tissue (SAT) was isolated from the hip region, and brown adipose tissue (BAT) from the intrascalpular region. For quantitative PCR, RNA was isolated using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol, cDNA was synthesized using the SuperScript First-Strand Synthesis kit (Invitrogen), and PCR was performed using SYBR Green (ABI, Carlsbad, CA) in a 7500 ABI fast machine. Lipids were isolated from tissues after homogenization in 10 μl/mg methanol, 25 μl homogenate was added to 225 μl methanol, 675 μl chloroform was added, samples were mixed and centrifuged, the supernatant containing lipids was dried, and lipids were dissolved in 2% Triton X-100. TGs were measured with TG reagent (Roche, Penzberg, Germany), and cholesterol with infinity cholesterol reagent (Fisher, Waltham, MA). For Western blotting, tissue lysates were prepared, separated by SDS-PAGE, and blotted to a polyvinylidene difluoride membrane, and proteins were detected using the following antibodies: LDLR (Abcam, Cambridge, MA); actin and GAPDH (Millipore, Billerica, MA); glucose transporter 4 (GLUT-4), protein kinase B (AKT), and phosphorylated AKT (pAKT) (Cell Signaling, Danvers, MA); anti-mouse Alexa Fluor 800 (Rockland, Gilbertsville, PA); and anti-rabbit Alexa Fluor 680 (Molecular Probes, Invitrogen). Proteins were visualized and analyzed using Licor Odyssey (Licor Biosciences, Lincoln, NE). ABCA1 was detected with our in-house antibody and HRP-labeled secondary antibodies (Jackson ImmunoResearch, West Grove, PA) and visualized using ECL (Pierce, Rockford, IL). For the lipolysis assay, adipose tissue was isolated, minced, and cultured in DMEM F12. Adipose tissue was treated with isoproterenol (Sigma, St. Louis, MO), glycerol release in the medium was measured over a 4 h period using glycerol and TG reagent (Roche), and glycerol release per hour normalized by tissue weight was calculated.
Plasma analysis
Blood samples were drawn from mice from the saphenous vein after 4 h fasting (8 AM to 12 PM). Blood glucose was measured with a glucose meter (OneTouch) or using QuantiChrom glucose assay reagents (BioAssay Systems, Hayward, CA) according to the manufacturer's protocol. Insulin was measured using the Mouse Insulin ELISA (Mercodia, Uppsala, Sweden). TGs were measured with TG reagent (Roche), NEFAs with NEFA half-micro test (Roche), and cholesterol with infinity cholesterol reagent (Fisher). HDL cholesterol was measured after precipitation of apoB-containing lipoproteins from plasma using heparin (500 U/ml) and MnCl2 (0.2 M) 2:1:1 (22). Adiponectin, visfatin, and leptin were measured with commercially available ELISA kits (adiponectin: Alpco, Salem, NH; visfatin: Abnova, Tapei City, Taiwan; leptin: Abcam, Cambridge, UK).
Glucose tolerance and insulin sensitivity testing
Mice were fasted for 4 h (8 AM to 12 PM) and were injected intraperitoneally with 1 g/kg (HFHC) or 2 g/kg (chow) glucose (Sigma) in PBS for glucose tolerance testing or with 1 U/kg insulin (Novo Nordisk, Bagsværd, Denmark) in PBS for insulin sensitivity testing. Blood was drawn via the saphenous vein before injection and at 15, 30, 60, and 90 min after injection and was assayed for glucose.
Insulin secretion
Islets were isolated from mice anesthetized with avertin intraperitoneally, and the pancreas was perfused with collagenase (Sigma) in calcium-free HBSS via the bile duct. The pancreas was homogenized after digestion at 37°C, and islets were handpicked in RPMI 10% FBS and penicillin/streptomycin. For glucose secretion experiments, islets were incubated in Krebs-Ringer Bicarbonate (KRB) buffer for 2 h followed by 1 h incubation in low- or high-glucose KRB buffer (1.67 vs. 16.7 mM). Insulin secreted in the supernatant was assayed using Mouse Insulin ELISA (Mercodia).
Statistical analysis
Data are expressed as means ± SEM. Data were interpreted using Student's t-test or two-way ANOVA followed by Bonferroni post hoc test using Prism 5.0a (GraphPad, La Jolla, CA). Differences were considered statistically significant when P < 0.05.
RESULTS
Adipocyte ABCA1 deficiency increases adipose tissue cholesterol content
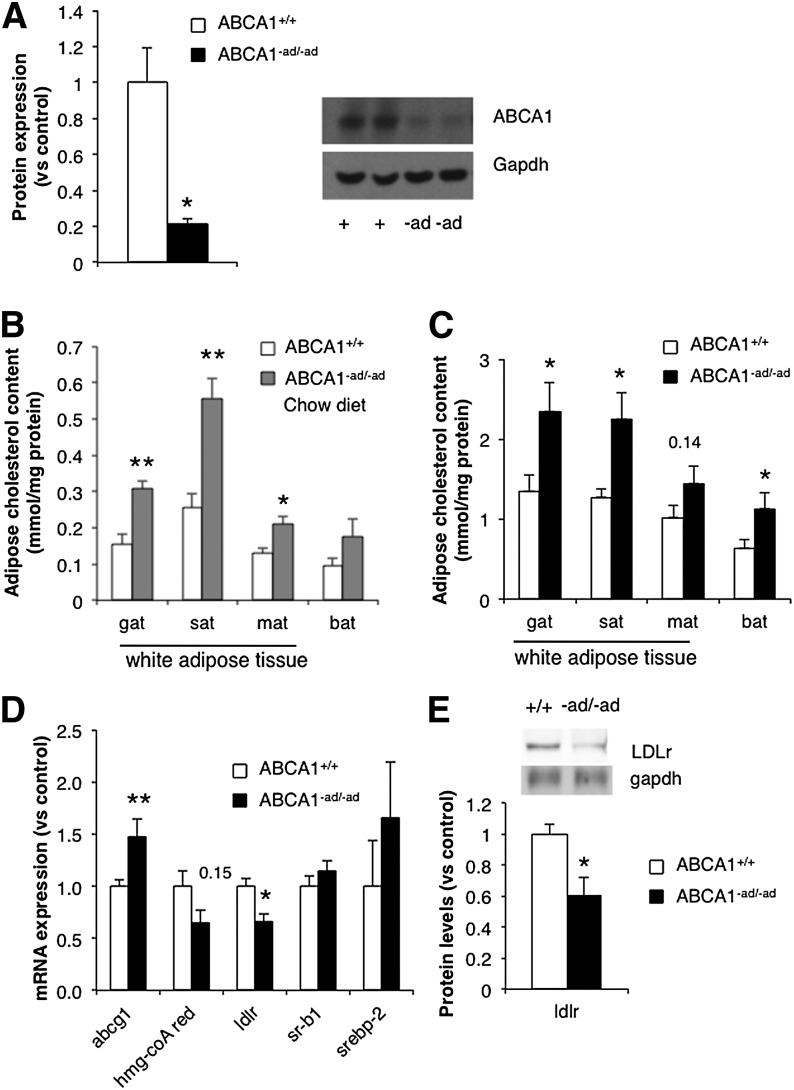
ABCA1 protein expression in adipose tissue was measured by Western blotting to confirm the specific loss of ABCA1 in adipose tissue from ABCA1−ad/−ad mice. A significant reduction in adipocyte ABCA1 protein levels in ABCA1−ad/−ad mice was observed (Fig. 1A). To ensure that ABCA1 knockdown due to expression of the ap2-cre transgene was only localized to adipocytes, we also measured ABCA1 in liver, muscle, and brain and found no change in ABCA1 protein levels (supplementary Fig. IA).
Fig. 1.
Adipocyte ABCA1 deficiency increases adipose tissue cholesterol content. GAT was isolated from ABCA1+/+ and ABCA1−ad/−ad mice, and ABCA1 levels were measured by Western blotting (A). Adipose tissue depots were isolated from 10-week-old ABCA1+/+ mice and ABCA1−ad/−ad mice fed a chow diet and were assessed for cholesterol content (B). ABCA1+/+ mice and ABCA1−ad/−ad mice were fed an HFHC diet from 10 weeks of age until 18 weeks of age, adipose tissue was extracted for quantification of cholesterol content (C), mRNA expression of genes in cholesterol homeostasis was measured by RT-PCR (D), and protein levels of the LDLR were assessed by Western blotting (E). Values are means ± SEM; N = 4 (A), N = 5–6 (B, E), N = 6–10 (D), N = 6–7 (C). * P < 0.05; ** P < 0.01. Hmg-coA red, HMG-CoA reductase; MAT, mesenteric adipose tissue; Srebp, sterol regulatory element binding protein.
Because ABCA1 is a major mediator of cholesterol efflux (9, 10), we evaluated whether loss of ABCA1 in adipocytes raises adipose cholesterol content. We observed a significant increase in the cholesterol content in GAT, SAT, and MAT in ABCA1−ad/−ad mice compared with wild-type control mice (ABCA1+/+) on a chow diet (Fig. 1B). Following an HFHC diet, we observed a significant increase of cholesterol in GAT, SAT, and BAT in ABCA1−ad/−ad mice (Fig. 1C).
To maintain intracellular cholesterol homeostasis, cholesterol has been shown to regulate the expression of genes affecting cholesterol metabolism via SREBP2 and the liver X receptor (LXR) (16, 17, 23). Thus, we evaluated how the loss of adipocyte ABCA1 affects the expression of genes involved in maintaining cholesterol homeostasis. An increase in abcg1 mRNA expression as well as a reduction in ldlr gene expression and LDLR protein levels was observed (Fig. 1D, E). ABCG1 is a cholesterol transporter important in the maintenance of tissue cholesterol homeostasis by mediating cholesterol efflux to HDL (24). The LDLR mediates uptake of cholesterol by cells (25, 26). Our results indicate that the loss of ABCA1 significantly alters adipose tissue cholesterol homeostasis and that adipocytes compensate for disturbances in lipid homeostasis by altering the expression of genes involved in cholesterol metabolism.
Adipocyte ABCA1 deficiency increases fat pad weight on an HFHC diet
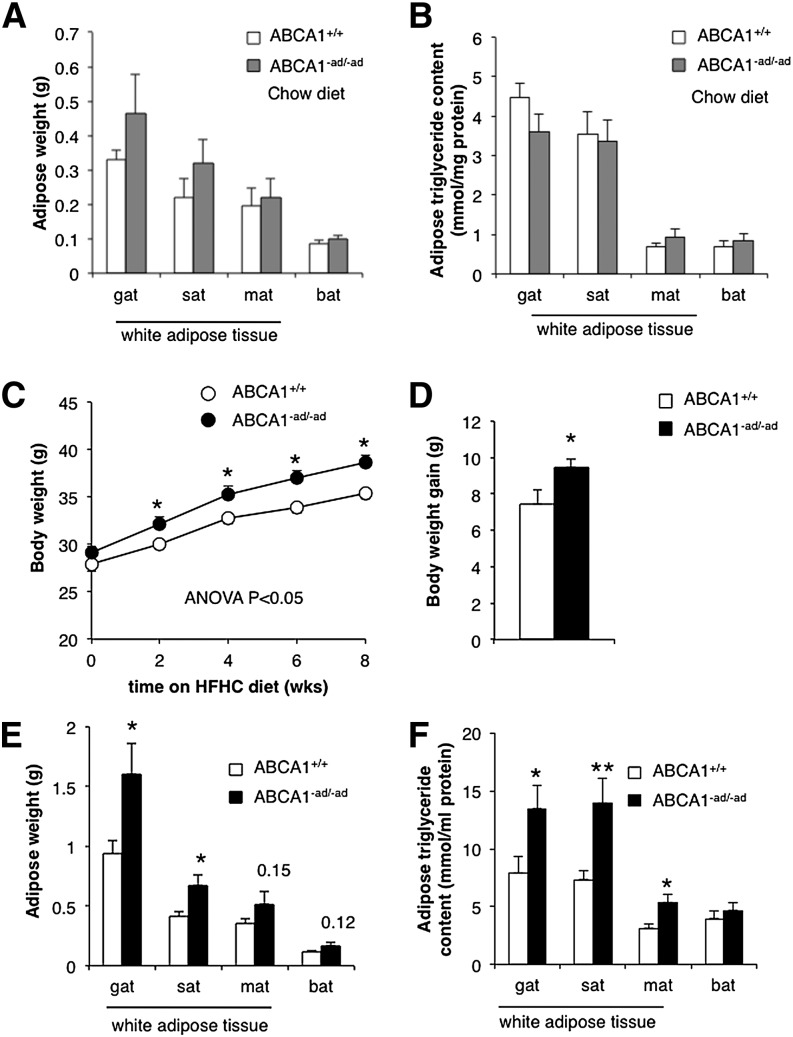
Obesity is associated with the accumulation of both cholesterol and TGs in adipose tissue (3, 17). We evaluated whether changes in adipocyte ABCA1 expression and cholesterol content influence body weight, fat pad weight, and adipocyte TG levels.
On a chow diet, ABCA1−ad/−ad mice demonstrated no difference in body weight, fat pad weight, and adipose tissue TG content compared with wild-type littermate controls (Fig. 2A, B). However, on an HFHC diet containing 21% milk fat (w/w) and 0.21% of cholesterol (w/w), ABCA1−ad/−ad mice gain more weight than their littermate controls (Fig. 2C, D). To assess whether these changes in body weight gain were reflected in changes to adipose tissue mass, we isolated adipose tissue depots. Concomitant with increased body weight gain, GAT and SAT depots showed a significant increase in weight (Fig. 2E). To evaluate changes in TG content, we extracted lipids from different types of adipose tissue depots and observed that TG content was significantly increased in GAT, SAT, and MAT following correction for protein levels (Fig. 2F). Our results indicate that lack of adipocyte ABCA1 leads to cholesterol accumulation, increased TG content in adipocytes, enlarged fat pads, and increased body weight.
Fig. 2.
Adipocyte ABCA1 deficiency increases fat pad weight and adipose TG content on an HFHC diet. Adipose tissue depots were isolated from 10-week-old ABCA1+/+ and ABCA1−ad/−ad mice on a chow diet and weighed (A), and TG content was measured (B). ABCA1+/+ mice and ABCA1−ad/−ad mice were fed an HFHC diet starting at 10 weeks of age and were weighed biweekly to assess weight gain (C, D). After 8 weeks of diet, adipose depots were isolated and weighed (E), and TG content was quantified (F). Values are means ± SEM; N = 5–6 (A, B), N = 15 (C, D), N = 6–7 (E, F). * P < 0.05; ** P < 0.01.
Adipocyte ABCA1 deficiency alters lipolysis but does not lead to major changes in genes involved in TG metabolism
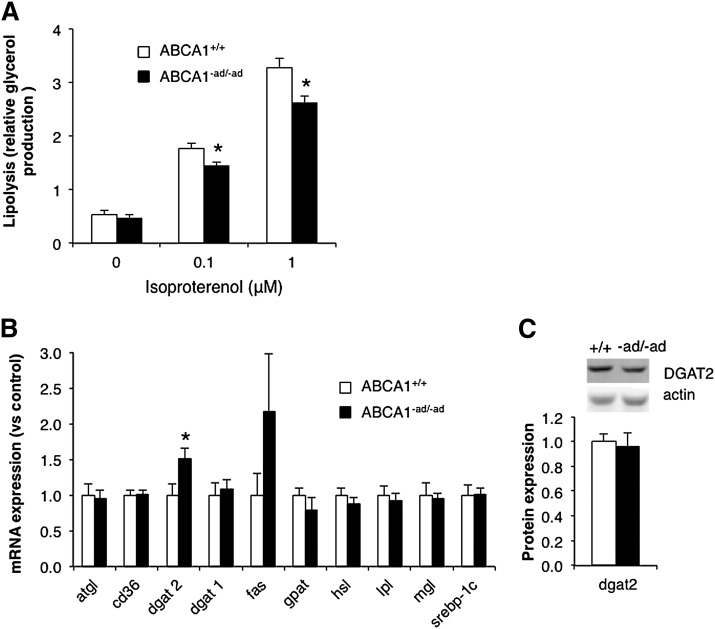
Adipose tissue stores large amounts of TGs, which can act as sources of energy and can be used to synthesize membrane and signaling lipids. However, in order to cross the adipocyte plasma membrane and be released into the plasma, adipocyte TGs must be hydrolyzed into fatty acids by a process known as lipolysis (6).
Sustained lipolysis has been shown to stimulate cholesterol efflux through ABCA1 (7). This suggests that there may be an association between ABCA1 function and TG lipolysis. In order to address this relationship, we investigated whether ABCA1 expression can affect TG lipolysis. We stimulated lipolysis using isoproterenol and observed reduced glycerol release in the medium from adipose tissue lacking ABCA1 (Fig. 3A). This suggests that ABCA1-mediated cholesterol efflux activity may influence TG lipolysis.
Fig. 3.
Adipocyte ABCA1 deficiency reduces lipolysis. GAT was isolated from ABCA1+/+ and ABCA1−ad/−ad mice, and isoproterenol-stimulated lipolysis was assessed ex vivo (A). In addition, mRNA expression of genes involved in TG homeostasis was measured in GAT by RT-PCR (B), and diglyceride acyltransferase (DGAT) 2 protein levels were measured by Western blotting (C). Values are means ± SEM; N = 4–6 (A), N = 10 (B), N = 5–6 (C). * P < 0.05. Atgl: adipose triglyceride lipase; Gpat, glycerol-3-phosphate acyltransferase; Hsl, hormone sensitive lipase; Mgl, monoglyceride lipase.
TG stores in adipose tissue are regulated by the combined effect of TG uptake, TG synthesis, and lipolysis. To further explore possible mechanisms linking adipocyte ABCA1 and TG homeostasis, we assessed the expression of 10 major genes in TG metabolism. A significant increase was observed only in dgat2 mRNA expression (Fig. 3B). DGAT2 is a crucial enzyme involved in TG biosynthesis (27). However, the consequence of increased dgat2 mRNA on TG metabolism is unclear because protein levels of DGAT2 were unaltered (Fig. 3C).
Adipocyte ABCA1 deficiency alters plasma and liver lipid levels
In humans, adipose dysfunction is associated with dyslipidemia including elevated plasma TG levels and low HDL (19, 28). In order to assess the contribution of adipocyte ABCA1 to plasma lipid metabolism, we measured total cholesterol, HDL, TGs, and NEFAs in the plasma of wild-type and ABCA1−ad/−ad mice. We would expect decreased plasma cholesterol levels because of decreased efflux of cholesterol from adipose tissue in ABCA1−ad/−ad mice. We indeed observed a decrease in plasma cholesterol levels; however, plasma levels of HDL cholesterol showed no significant difference (Table 1). There was a significant increase in plasma TG levels in ABCA1−ad/−ad mice (Table 1). Plasma NEFA levels were not significantly affected (Table 1).
TABLE 1.
Plasma parameters in adipose ABCA1 knockout mice
| ABCA1+/+ | ABCA1−ad/−ad | P | |
| Chow | |||
| Cholesterol | 2.51 ± 0.09 | 2.21 ± 0.09 | 0.034 |
| HDL | 2.10 ± 0.07 | 1.96 ± 0.08 | 0.234 |
| TG | 1.06 ± 0.06 | 1.22 ± 0.04 | 0.030 |
| NEFA | 0.31 ± 0.017 | 0.31 ± 0.014 | 0.780 |
| Glucose | 9.01 ± 0.28 | 9.91 ± 0.22 | 0.017 |
| Insulin | 0.84 ± 0.09 | 0.96 ± 1.00 | 0.410 |
| HFHC | |||
| Cholesterol | 3.94 ± 0.22 | 3.59 ± 0.10 | 0.260 |
| HDL | 3.25 ± 0.23 | 3.07 ± 0.31 | 0.643 |
| TG | 0.71 ± 0.01 | 0.79 ± 0.08 | 0.047 |
| NEFA | 0.36 ± 0.043 | 0.43 ± 0.041 | 0.340 |
| Glucose | 8.65 ± 0.49 | 9.45 ± 0.21 | 0.240 |
| Insulin | 1.11 ± 0.09 | 1.92 ± 0.32 | 0.028 |
Blood was drawn from the saphenous vein after 4 h fasting and assessed for the parameters above. Values are means ± SEM. P values reaching statistical significance are bold. Chow: n = 15 (cholesterol, TG, glucose, HDL), n = 5–10 (insulin, NEFA); HFHC: n = 8–6.
Obesity and adipose dysfunction not only have consequences on plasma lipids but also have been associated with hepatosteatosis (21). Although the precise mechanism by which adipose tissue affects lipid accumulation in the liver remains unclear, increased NEFA flux, decreased adiponectin and visfatin expression, and insulin resistance may all play a role (1, 20, 21, 28). To assess the effect of adipose ABCA1 on the liver, we measured liver weight and hepatic lipid content in ABCA1−ad/−ad mice. After an HFHC diet, ABCA1−ad/−ad mice had an increased liver weight and demonstrated elevated levels of TG and cholesterol content in hepatic tissue (supplementary Fig. IIIA, B).
Adipocyte ABCA1 deficiency alters the expression of proteins involved in glucose homeostasis
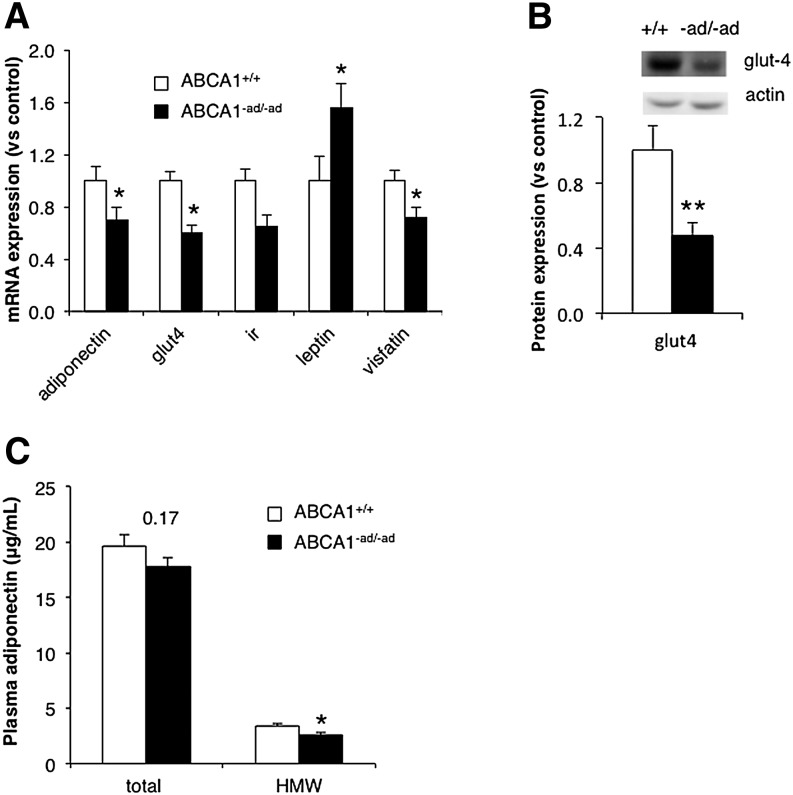
Adipose tissue is critical in regulating whole-body glucose homeostasis through the release of adipokines such as leptin, adiponectin, and visfatin (1, 2). These act on various tissues including the pancreas to stimulate insulin secretion and the muscle to enhance insulin sensitivity. Low adiponectin levels are associated with reduced insulin secretion, insulin resistance, and hepatosteatosis. Visfatin is an insulin-mimicking protein, while leptin levels increase with weight gain and serve to regulate food intake and energy expenditure (1, 2, 20). Obesity and adipose dysfunction can alter adipokine expression and secretion.
We evaluated whether adipose tissue dysfunction due to the loss of ABCA1 could affect the expression of adipokine genes critical to whole-body glucose homeostasis. In ABCA1-deficient adipose tissue, we found that mRNA expression of leptin was significantly increased, whereas adiponectin and visfatin expression were significantly reduced (Fig. 4A). Plasma leptin and visfatin levels were unaltered (supplementary Fig. IID, E). HMW adiponectin levels showed a 30% decrease (P < 0.05) (Fig. 4C). These changes in adipokine expression may affect glucose tolerance.
Fig. 4.
Adipocyte ABCA1 deficiency influences expression of genes in glucose metabolism. Levels of mRNA expression of genes involved in glucose homeostasis were assessed in GAT from ABCA1+/+ mice and ABCA1−ad/−ad mice fed an HFHC for 8 weeks by RT-PCR (A). GLUT-4 levels were measured using Western blotting (B), and plasma adiponectin levels were measured using ELISA (C). Values are means ± SEM; N = 6–7 (A), N = 9–10 (B), N = 14–15 (C). * P < 0.05; ** P < 0.01. HMW, high molecular weight.
GLUT-4 is a protein crucial for insulin-stimulated glucose uptake. Depletion of membrane adipocyte cholesterol by methyl-β-cyclodextrin in vivo reduces glut-4 mRNA expression and decreases insulin-stimulated glucose uptake (16). Moreover, GLUT-4 deficiency in adipose tissue has been shown to affect whole-body glucose tolerance (29). We evaluated whether ABCA1 affects glut-4 mRNA expression and GLUT-4 protein levels in adipose tissue. Both mRNA and protein expression of GLUT-4 were reduced in GAT from ABCA1−ad/−ad mice (Fig. 4A, B). The changes in adipokine expression and reduced glut-4 expression suggest that adipose tissue dysfunction as a result of ABCA1 deficiency may impact whole-body glucose tolerance and insulin sensitivity.
Adipocyte ABCA1 deficiency impairs glucose tolerance, reduces insulin sensitivity, and impairs beta-cell function
We demonstrate that adipose ABCA1 deficiency increases cholesterol and TG storage in adipocytes and reduces adiponectin, visfatin, and glut-4 expression. These alterations in lipid homeostasis and gene expression are linked to impaired glucose tolerance and reduced insulin sensitivity (1, 2, 19, 20). Based on these findings, we hypothesize that adipocyte ABCA1 deficiency leads to glucose intolerance and insulin resistance in vivo.
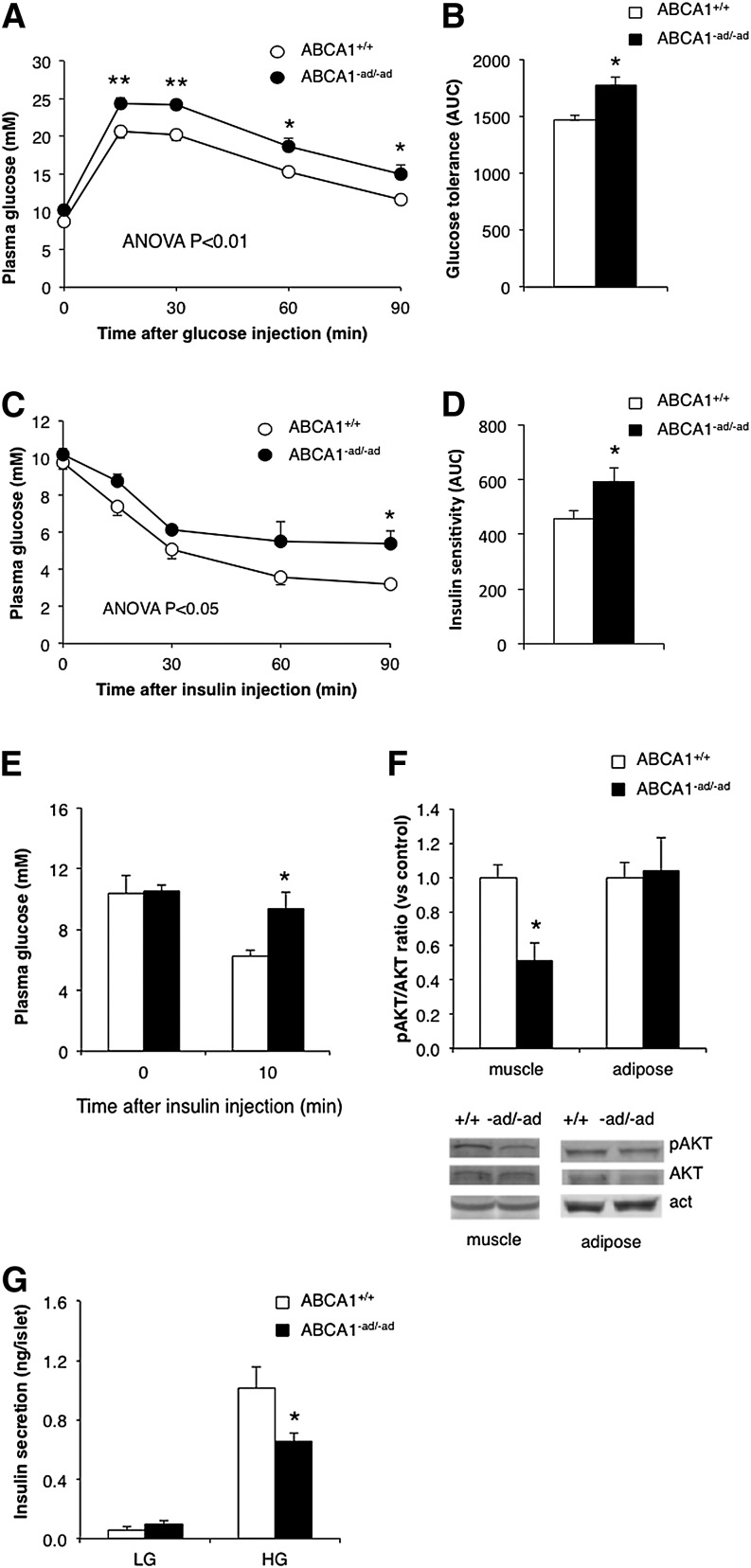
To address this, we assessed glucose tolerance in ABCA1−ad/−ad versus control mice. Although we observed that glucose tolerance was unaltered in mice lacking adipocyte ABCA1 on a chow diet (data not shown), ABCA1−ad/−ad mice had significantly impaired glucose tolerance following an HFHC diet (Fig. 5A, B). To determine whether impaired glucose tolerance could be explained by a reduction in insulin sensitivity, we measured the response in plasma glucose levels after intraperitoneal insulin injection. A significant impairment of insulin sensitivity was observed in ABCA1−ad/−ad mice (Fig. 5C, D). We then evaluated in which tissues insulin sensitivity was affected through measurement of AKT phosphorylation, a marker for intracellular insulin signaling. Because basal pAKT levels were too low to quantify (data not shown), we stimulated AKT phosphorylation by giving mice an intraperitoneal insulin injection followed by tissue extraction and immunoblotting analysis. Western blots revealed that the pAKT/AKT ratio was reduced in muscle from ABCA1−ad/−ad following insulin injection. The pAKT/AKT ratio was unaltered in adipose tissue (Fig. 5E, F). In the liver, AKT levels were very low, even following the injection of insulin, and we found no obvious changes in pAKT/AKT ratio (data not shown). Thus, adipocyte ABCA1 deficiency specifically impairs muscle tissue insulin sensitivity.
Fig. 5.
Adipocyte ABCA1 deficiency impairs glucose tolerance. ABCA1+/+ mice and ABCA1−ad/−ad mice fed an HFHC diet received an intraperitoneal injection of 1 g/kg glucose in PBS, blood samples were drawn at the indicated time points, and glucose was measured to assess glucose tolerance (A, B). Insulin sensitivity was assessed by injecting mice intraperitoneally with 1 U/kg insulin in PBS, and glucose was measured at the indicated time points (C, D). To assess tissue-specific insulin sensitivity, mice were injected with 10 U/kg insulin, tissues were isolated, and pAKT and total AKT levels were measured by Western blotting (E, F). Pancreatic islets were isolated from ABCA1+/+ mice and ABCA1−ad/−ad mice fed an HFHC diet and incubated in low- (1.67 mM) and high-glucose (16.7 mM) medium, and insulin secretion was measured (G). Values are means ± SEM; N = 6–7 (A–D), N = 3–4 (E, F), N = 10–13 (G). * P < 0.05; ** P < 0.01.
Decreased insulin sensitivity in peripheral tissues results in increased insulin secretion by the pancreas to compensate for this insulin resistance (30, 31). Increased production of insulin may eventually lead to beta-cell failure and reduced insulin secretion (30, 31). This is consistent with our findings because ABCA1−ad/−ad mice demonstrated both decreased insulin sensitivity in muscle tissue and a significant increase in circulating plasma insulin levels on an HFHC diet (Table 1). To explore whether beta-cell function was affected, pancreatic islets were isolated from ABCA1−ad/−ad and wild-type mice and incubated in KRB buffer with low (1.67 mM) or high (16.67 mM) glucose, and insulin secretion in the media was measured. We observed that insulin secretion from beta cells was reduced in islets from ABCA1−ad/−ad mice after HFHC feeding (Fig. 5G). Our findings support the paradigm that reduced beta-cell function can result from an increase in insulin demand as a consequence of insulin resistance in tissues. Collectively, our data suggest that intracellular cholesterol homeostasis in adipocytes is maintained by ABCA1 and is crucial for glucose tolerance, insulin sensitivity, and beta-cell function.
DISCUSSION
Adipose tissue contains one of the largest pools of cholesterol in the body, is the primary storage depot for TGs, and is crucial in energy metabolism (20). Alterations in adipocyte cholesterol are associated with adipose dysfunction and obesity, and ABCA1 has been shown to mediate cholesterol efflux from adipose tissue (7, 9, 15). Adipose tissue dysfunction is an important risk factor for the development of glucose intolerance, insulin resistance, and type 2 diabetes (3, 19, 28); this indicates a role for adipocyte cholesterol and ABCA1 in these conditions.
Previous studies showing that cholesterol may be crucial in adipocyte function were mainly carried out in the 3T3 adipocyte cell line or observations in obese mice (3, 4, 16). In our study, we directly investigated how adipocyte ABCA1 affects adipose lipid content and adipose function using a mouse model specifically lacking ABCA1 in adipocytes. In this study, we demonstrated significantly increased intracellular cholesterol levels in the adipose tissue of adipocyte ABCA1-deficient mice. Concomitant with increased cholesterol levels, we observed an enlargement of fat pads and an accumulation of TGs in the adipose tissue of ABCA1−ad/−ad mice on an HFHC diet. These changes in adipose tissue have significant metabolic consequences because we demonstrated that ABCA1−ad/−ad mice manifest with impaired glucose tolerance, reduced muscle insulin sensitivity, and lower glucose-stimulated insulin secretion from beta cells ex vivo.
Using ABCA1−ad/−ad mice, we showed that cholesterol accumulates in ABCA1-deficient adipose tissue in mice on both a chow and an HFHC diet. This increase in cholesterol content is likely a direct effect of reduced ABCA1-mediated cholesterol efflux from adipose tissue. Increased intracellular cholesterol has a major impact on lipid metabolism because cholesterol regulates the expression of multiple genes involved in intracellular lipid homeostasis. For example, increased intracellular cholesterol reduces the expression of hmgcoA reductase and the ldlr via SREBP2 to reduce cholesterol synthesis and uptake (16, 17). In addition, the generation of oxysterols from intracellular cholesterol activates the LXR transcription factor, which increases the expression of cholesterol efflux genes including abca1 and abcg1 (16, 23). In this study, we observed decreased ldlr expression and an increase in abcg1 expression in ABCA1-deficient adipose tissue. We believe that the absence of ABCA1 induces cholesterol accumulation and influences various compensatory mechanisms to regulate cholesterol balance in adipocytes. Increased ABCG1 to enhance cholesterol efflux and decreased LDLR to reduce cholesterol uptake suggest that compensatory mechanisms are conserved in the adipocyte to regulate cholesterol homeostasis.
It has been suggested that intracellular cholesterol influences TG stores in adipose tissue (3, 17). This hypothesis is also supported by our data because we observed that ABCA1-deficient adipose tissue accumulates TGs and cholesterol in response to an HFHC diet. It has been shown in vitro that increased ABCA1-mediated cholesterol efflux from adipocytes occurs following the stimulation of TG lipolysis (7, 8). This may indicate a possible link between ABCA1 and TG lipolysis in adipocytes. We demonstrated that TG lipolysis is reduced ex vivo in adipose tissue from ABCA1−ad/−ad mice. This suggests that ABCA1 may directly influence TG levels and lipolysis. Reduced TG lipolysis may contribute to the TG accumulation we observed in adipose tissue from ABCA1−ad/−ad mice on an HFHC diet. We ruled out feeding behavior causing weight gain because we did not observe a difference in food intake between ABCA1−ad/−ad mice and control littermates (supplementary Fig. IIC).
ABCA1−ad/−ad mice demonstrated increased body weight, impairment in glucose tolerance, and increased insulin resistance on an HFHC diet. This suggests that adipocyte ABCA1 is crucial for proper adipose tissue function in response to dietary fat and cholesterol. Our observations also indicate that loss of adipocyte ABCA1 and subsequent increase in intracellular cholesterol levels are sufficient to influence whole-body glucose tolerance. Increased body weight and adipocyte TG accumulation are associated with adipocyte dysfunction, which may lead to a number of metabolic disturbances including glucose intolerance, insulin resistance, and hepatosteatosis (18, 19, 21, 32).
Metabolic disturbances are affected by adipose tissue through the release of NEFAs and adipokines in plasma (1, 2, 19, 20). Increased NEFA flux from adipose tissue to liver, muscle, and pancreas has been shown to reduce insulin sensitivity and secretion (19, 21). However, we did not observe significantly altered NEFA levels in the plasma of our ABCA1−ad/−ad mice. Expression and release of adiponectin shows an inverse correlation with adipose mass (2, 19, 21). Decreased levels of adiponectin reduce insulin sensitivity and stimulate hepatic lipid accumulation (2, 19, 21). In our ABCA1−ad/−ad model, we observed reduced adiponectin, gene expression, and a reduction in visfatin and glut-4 gene expression along with increased leptin expression. In plasma, we observed a decrease in HMW adiponectin, which is the metabolically most active form of adiponectin. This decrease in plasma HMW adiponectin may therefore contribute to the insulin resistance and glucose intolerance, as well as the hepatic lipid accumulation, we observed in our mouse model. GLUT-4 deficiency in adipose tissue has been shown to impair whole-body glucose tolerance and muscle insulin resistance, but this effect is independent of circulating NEFAs, TGs, or leptin (29). This suggests that other unknown mechanisms associated with GLUT-4 may play a role in modulating insulin resistance.
In an effort to identify other mechanisms by which adipocyte ABCA1 deficiency affects whole-body glucose homoeostasis, we investigated the expression of genes and proteins involved in glucose metabolism in the liver. We did not observe altered levels of pAKT in the liver. Expression of the gluconeogenesis genes phosphoenolpyruvate carboxykinase and glucose 6-phosphatase in the liver were also unaltered (data not shown). We cannot exclude, however, that the hepatosteatosis we observed in ABCA1−ad/−ad mice could contribute to glucose intolerance by altering hepatic glucose output or uptake (21).
When blood glucose levels rise due to insulin resistance, the pancreas attempts to compensate by increasing beta-cell insulin secretion. However, elevated insulin secretion may eventually result in beta-cell dysfunction and failure (31, 33, 34). We observed that adipocyte-specific ABCA1 knockout mice have increased fasting insulin levels, while insulin secretion from beta cells in response to glucose was reduced. We conclude that changes in adipose tissue indirectly lead to beta-cell dysfunction as a result of insulin resistance in the muscle of ABCA1−ad/−ad mice.
The generation of mice specifically lacking ABCA1 in adipocytes has given us a unique opportunity to directly assess the role of adipocyte ABCA1 in glucose and lipid metabolism in vivo. Our data suggest a critical role for adipocyte intracellular cholesterol maintained by ABCA1 in the adipocyte in whole-body glucose homeostasis. Our findings indicate that adipocyte ABCA1 regulates adipocyte cholesterol and TG stores and thereby influences muscle insulin sensitivity and glucose tolerance. We conclude that ABCA1 in the adipocyte is an important regulator of intracellular lipid stores and has significant effects on glucose metabolism.
Supplementary Material
Acknowledgments
The authors thank Mark Wang (Centre for Molecular Medicine and Therapeutics, University of British Columbia, Vancouver, BC, Canada) for excellent animal care and technical assistance, Joanna Karasinska (Centre for Molecular Medicine and Therapeutics, University of British Columbia, Vancouver, BC, Canada) for critical reading of the manuscript and scientific discussion, and John S. Parks (Wake Forest University Health Sciences, Winston-Salem, NC) for scientific discussion.
Footnotes
Abbreviations:
- AKT
- protein kinase B
- BAT
- brown adipose tissue
- DGAT
- diglyceride acyltransferase
- GAT
- gonadal adipose tissue
- GLUT-4
- glucose transporter 4
- HFHC
- high-fat, high-cholesterol
- HMW
- high molecular weight
- LDLR
- LDL receptor
- MAT
- mesenteric adipose tissue
- pAKT
- phosphorylated AKT
- SAT
- subcutaneous adipose tissue
- SREBP
- sterol regulatory element binding protein
This work was supported by Canadian Institute for Health Research Operational Grant MOP-106684 (M.R.H.), the Canadian Diabetes Association, the Michael Smith Foundation for Health Research, and the Netherlands Organization for Scientific Research Fellowships (W.d.H.). M.R.H. is a University Killam Professor and holds a Canada Research Chair in Human Genetics.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures.
REFERENCES
- 1.Rasouli N., Kern P. A. 2008. Adipocytokines and the metabolic complications of obesity. J. Clin. Endocrinol. Metab. 93: S64–S73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Galic S., Oakhill J. S., Steinberg G. R. 2010. Adipose tissue as an endocrine organ. Mol. Cell. Endocrinol. 316: 129–139 [DOI] [PubMed] [Google Scholar]
- 3.Yu B-L., Zhao S-P., Hu J-R. 2010. Cholesterol imbalance in adipocytes: a possible mechanism of adipocytes dysfunction in obesity. Obes. Rev. 11: 560–567 [DOI] [PubMed] [Google Scholar]
- 4.Kovanen P. T., Nikkilä E. A., Miettinen T. A. 1975. Regulation of cholesterol synthesis and storage in fat cells. J. Lipid Res. 16: 211–223 [PubMed] [Google Scholar]
- 5.Acton S. L., Scherer P. E., Lodish H. F., Krieger M. 1994. Expression cloning of SR-BI, a CD36-related class B scavenger receptor. J. Biol. Chem. 269: 21003–21009 [PubMed] [Google Scholar]
- 6.Young S. G., Zechner R. 2013. Biochemistry and pathophysiology of intravascular and intracellular lipolysis. Genes Dev. 27: 459–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Lay S., Robichon C., Le Liepvre X., Dagher G., Ferre P., Dugail I. 2003. Regulation of ABCA1 expression and cholesterol efflux during adipose differentiation of 3T3–L1 cells. J. Lipid Res. 44: 1499–1507 [DOI] [PubMed] [Google Scholar]
- 8.Howard A. D., Verghese P. B., Arrese E. L., Soulages J. L. 2010. Characterization of apoA-I-dependent lipid efflux from adipocytes and role of ABCA1. Mol. Cell. Biochem. 343: 115–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McNeish J., Aiello R. J., Guyot D., Turi T., Gabel C., Aldinger C., Hoppe K. L., Roach M. L., Royer L. J., de Wet J., et al. 2000. High density lipoprotein deficiency and foam cell accumulation in mice with targeted disruption of ATP-binding cassette transporter-1. Proc. Natl. Acad. Sci. USA. 97: 4245–4250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks-Wilson A., Marcil M., Clee S. M., Zhang L. H., Roomp K., van Dam M., Yu L., Brewer C., Collins J. A., Molhuizen H. O., et al. 1999. Mutations in ABC1 in Tangier disease and familial high-density lipoprotein deficiency. Nat. Genet. 22: 336–345 [DOI] [PubMed] [Google Scholar]
- 11.Wellington C. L., Walker E. K. Y., Suarez A., Kwok A., Bissada N., Singaraja R., Yang Y-Z., Zhang L-H., James E., Wilson J. E., et al. 2002. ABCA1 mRNA and protein distribution patterns predict multiple different roles and levels of regulation. Lab. Invest. 82: 273–283 [DOI] [PubMed] [Google Scholar]
- 12.Karasinska J. M., Rinninger F., Lütjohann D., Ruddle P., Franciosi S., Kruit J. K., Singaraja R. R., Hirsch-Reinshagen V., Fan J., Brunham L. R., et al. 2009. Specific loss of brain ABCA1 increases brain cholesterol uptake and influences neuronal structure and function. J. Neurosci. 29: 3579–3589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brunham L. R., Kruit J. K., Pape T. D., Timmins J. M., Reuwer A. Q., Vasanji Z., Marsh B. J., Rodrigues B., Johnson J. D., Parks J. S., et al. 2007. Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat. Med. 13: 340–347 [DOI] [PubMed] [Google Scholar]
- 14.Brunham L. R., Singaraja R. R., Pape T. D., Kejariwal A., Thomas P. D., Hayden M. R. 2005. Accurate prediction of the functional significance of single nucleotide polymorphisms and mutations in the ABCA1 gene. PLoS Genet. 1: e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung S., Sawyer J. K., Gebre A. K., Maeda N., Parks J. S. 2011. Adipose tissue ATP binding cassette transporter A1 contributes to high-density lipoprotein biogenesis in vivo. Circulation. 124: 1663–1672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le Lay S., Krief S., Farnier C., Lefrère I., Le Liepvre X., Bazin R., Ferré P., Dugail I. 2001. Cholesterol, a cell size-dependent signal that regulates glucose metabolism and gene expression in adipocytes. J. Biol. Chem. 276: 16904–16910 [DOI] [PubMed] [Google Scholar]
- 17.Dugail I., Le Lay S., Varret M., Le Liepvre X., Dagher G., Ferré P. 2003. New insights into how adipocytes sense their triglyceride stores. Is cholesterol a signal? Horm. Metab. Res. 35: 204–210 [DOI] [PubMed] [Google Scholar]
- 18.Blüher M. 2009. Adipose tissue dysfunction in obesity. Exp. Clin. Endocrinol. Diabetes. 117: 241–250 [DOI] [PubMed] [Google Scholar]
- 19.Guilherme A., Virbasius J. V., Puri V., Czech M. P. 2008. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9: 367–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Henry S. L., Bensley J. G., Wood-Bradley R. J., Cullen-McEwen L. A., Bertram J. F., Armitage J. A. 2012. White adipocytes: more than just fat depots. Int. J. Biochem. Cell Biol. 44: 435–440 [DOI] [PubMed] [Google Scholar]
- 21.Angulo P. 2002. Nonalcoholic fatty liver disease. N. Engl. J. Med. 346: 1221–1231 [DOI] [PubMed] [Google Scholar]
- 22.van der Hoorn J. W. A., de Haan W., Berbée J. F. P., Havekes L. M., Jukema J. W., Rensen P. C. N., Princen H. M. G. 2008. Niacin increases HDL by reducing hepatic expression and plasma levels of cholesteryl ester transfer protein in APOE*3Leiden.CETP mice. Arterioscler. Thromb. Vasc. Biol. 28: 2016–2022 [DOI] [PubMed] [Google Scholar]
- 23.Zelcer N., Tontonoz P. 2006. Liver X receptors as integrators of metabolic and inflammatory signaling. J. Clin. Invest. 116: 607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennedy M. A., Barrera G. C., Nakamura K., Baldán A., Tarr P., Fishbein M. C., Frank J., Francone O. L., Edwards P. A. 2005. ABCG1 has a critical role in mediating cholesterol efflux to HDL and preventing cellular lipid accumulation. Cell Metab. 1: 121–131 [DOI] [PubMed] [Google Scholar]
- 25.Brown M. S., Dana S. E., Goldstein J. L. 1975. Receptor-dependent hydrolysis of cholesteryl esters contained in plasma low density lipoprotein. Proc. Natl. Acad. Sci. USA. 72: 2925–2929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown M. S., Goldstein J. L. 1974. Expression of the familial hypercholesterolemia gene in heterozygotes: mechanism for a dominant disorder in man. Science. 185: 61–63 [DOI] [PubMed] [Google Scholar]
- 27.McFie P. J., Banman S. L., Kary S., Stone S. J. 2011. Murine diacylglycerol acyltransferase-2 (DGAT2) can catalyze triacylglycerol synthesis and promote lipid droplet formation independent of its localization to the endoplasmic reticulum. J. Biol. Chem. 286: 28235–28246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Franssen R., Monajemi H., Stroes E. S. G., Kastelein J. J. P. 2011. Obesity and dyslipidemia. Med. Clin. North Am. 95: 893–902 [DOI] [PubMed] [Google Scholar]
- 29.Abel E. D., Peroni O., Kim J. K., Kim Y. B., Boss O., Hadro E., Minnemann T., Shulman G. I., Kahn B. B. 2001. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 409: 729–733 [DOI] [PubMed] [Google Scholar]
- 30.Tripathy D., Eriksson K. F., Orho-Melander M., Fredriksson J., Ahlqvist G., Groop L. 2004. Parallel manifestation of insulin resistance and beta cell decompensation is compatible with a common defect in type 2 diabetes. Diabetologia. 47: 782–793 [DOI] [PubMed] [Google Scholar]
- 31.Kahn S. E. 2003. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 46: 3–19 [DOI] [PubMed] [Google Scholar]
- 32.Savage D. B. 2009. Mouse models of inherited lipodystrophy. Dis. Model. Mech. 2: 554–562 [DOI] [PubMed] [Google Scholar]
- 33.Tripathy D., Chavez A. O. 2010. Defects in insulin secretion and action in the pathogenesis of type 2 diabetes mellitus. Curr. Diab. Rep. 10: 184–191 [DOI] [PubMed] [Google Scholar]
- 34.Butler A. E., Janson J., Soeller W. C., Butler P. C. 2003. Increased beta-cell apoptosis prevents adaptive increase in beta-cell mass in mouse model of type 2 diabetes: evidence for role of islet amyloid formation rather than direct action of amyloid. Diabetes. 52: 2304–2314 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.