Abstract
X-linked adrenoleukodystrophy (X-ALD) is a peroxisomal disorder caused by impaired degradation of very long-chain fatty acids (VLCFAs) due to mutations in the ABCD1 gene responsible for VLCFA transport into peroxisomes. Lorenzo's oil, a 4:1 mixture of glyceryl trioleate and glyceryl trierucate, has been used to reduce the saturated VLCFA level in the plasma of X-ALD patients; however, the mechanism by which this occurs remains elusive. We report the biochemical characterization of Lorenzo's oil activity toward elongation of very long-chain fatty acid (ELOVL) 1, the primary enzyme responsible for the synthesis of saturated and monounsaturated VLCFAs. Oleic and erucic acids inhibited ELOVL1, and, moreover, their 4:1 mixture (the FA composition of Lorenzo's oil) exhibited the most potent inhibitory activity. The kinetics analysis revealed that this was a mixed (not a competitive) inhibition. At the cellular level, treatment with the 4:1 mixture reduced the level of SM with a saturated VLCFA accompanied by an increased level of SM with a monounsaturated VLCFA, probably due to the incorporation of erucic acid into the FA elongation cycle. These results suggest that inhibition of ELOVL1 may be an underlying mechanism by which Lorenzo's oil exerts its action.
Keywords: ABCD1, erucic acid, oleic acid, peroxisome, sphingolipids, X-linked adrenoleukodystrophy, elongation of very long-chain fatty acid 1
FAs are the major constituents of cellular lipids and are highly diverse in their chain length and degree of saturation. This structural diversity underlies the various functions of FAs. The most abundant cellular FAs are long-chain fatty acids (LCFAs) having a chain length of 11 to 20 carbons (C11–C20). FAs with >C20 are called very long-chain fatty acids (VLCFAs) and have essential physiological functions that cannot be compensated for by LCFAs, such as skin barrier formation, retinal function, myelin maintenance, and spermatogenesis (1–5). VLCFAs are components of the two important lipid classes, glycerolipids and sphingolipids, which are characterized by their lipid backbone that in the former is a glycerol and in the latter a sphingoid base. Although the sphingoid base is structurally similar to the glycerol with an FA at the sn-1 position, VLCFAs linked to these backbones are strikingly different: Those of glycerolipids are mostly polyunsaturated, whereas those of sphingolipids are saturated and monounsaturated (especially C24). In mammals, a polar head group, phosphocholine or sugar, is attached to the N-acylated sphingoid base (ceramide) forming SM or glycosphingolipid, respectively. Sphingolipids containing VLCFAs have unique cellular functions such as microdomain/raft-mediated signal transduction and cell survival (6–8). Ceramides with ≥C26 VLCFAs are also present in the skin and essential for epidermal permeability barrier function (3, 9, 10).
X-linked adrenoleukodystrophy (X-ALD) is the most common peroxisomal disorder characterized by progressive demyelination and adrenal insufficiency (11, 12). The gene mutated in X-ALD is the ABCD1 gene (13), which encodes a peroxisomal ABC protein responsible for transporting VLCFAs (as VLCFA-CoAs) into peroxisomes (14, 15) where these FAs are broken down by β-oxidation (16). The defective ABCD1 protein impairs this normal VLCFA degradation process, resulting in the accumulation of VLCFAs, specifically C24:0 and C26:0, in the plasma and tissues (11, 17). The accumulation of these VLCFAs is believed to play a crucial role in the pathogenesis of X-ALD such as inflammatory demyelination and oxidative damage (18, 19). Therefore, reducing or preventing the accumulation of VLCFAs by either promoting their degradation or suppressing their synthesis may lead to the treatment of X-ALD.
VLCFAs are synthesized by the FA elongation cycle in the endoplasmic reticulum by sequential addition of C2 units from malonyl-CoA to long-chain acyl-CoA (5, 20). Each elongation cycle is composed of four reactions: condensation, reduction, dehydration, and reduction. The first condensation reaction is the rate-limiting step catalyzed by elongation of very long-chain fatty acid (ELOVL) family proteins. There are seven known ELOVL isozymes (ELOVL1–7) in mammals (5, 20), each of which exhibits different specificity for chain length and degree of saturation of the acyl-CoA substrate (7). Among the seven isozymes, ELOVL1 is highly active toward saturated and monounsaturated C20- to C24-CoAs (7) and thus is responsible for the synthesis of C22- to C26-VLCFAs such as those accumulated in X-ALD. Knockdown of ELOVL1 in X-ALD fibroblasts is reported to lower the level of C26:0 (21), suggesting ELOVL1 as a potential pharmacological target for the treatment of X-ALD (17, 21, 22).
Lorenzo's oil, a 4:1 mixture of glyceryl trioleate (C18:1 n-9) and glyceryl trierucate (C22:1 n-9), was introduced in 1989 as a dietary treatment for X-ALD patients (23). After oral administration, the triglycerides are hydrolyzed by lipases in the digestive tract to liberate oleic and erucic acids, which are then absorbed from the intestine and transported via lymph and blood to the tissues where they exert their effects. Lorenzo's oil normalizes the levels of C24:0 and C26:0 in the plasma of X-ALD patients (23, 24); however, it does not alter the clinical progression of patients with preexisting neurological or adrenal dysfunction (24, 25) but may have a preventive effect in asymptomatic patients (26).
The development of Lorenzo's oil goes back to 1986, when oleic and erucic acids were found to lower the level of C26:0 in X-ALD fibroblasts (27). Since then, despite much research, the mechanism of action of Lorenzo's oil has remained ambiguous. Some possible mechanisms include the following: 1) prior conversion of oleic and erucic acids to their CoA esters followed by competitive inhibition of ELOVL isozymes; 2) direct inhibition of VLCFA synthesis by oleic and erucic acids themselves; 3) enhanced degradation/removal of saturated VLCFAs induced by oleic and erucic acids and/or their metabolites; and 4) alteration of lipid homeostasis mediated by oleic and erucic acids, followed by induction of various cellular responses including metabolic changes and gene expression (28). In the present study, we biochemically characterized the activity of Lorenzo's oil toward ELOVL1 using in vitro and cellular FA elongation assays to understand how Lorenzo's oil reduces saturated VLCFAs.
MATERIALS AND METHODS
Cell culture and transfection
HeLa and HEK 293T cells were grown in DMEM (D6046 for HeLa cells and D6429 for HEK 293T cells; Sigma, St. Louis, MO) containing 10% fetal bovine serum and supplemented with 100 units/ml penicillin and 100 μg/ml streptomycin. HEK 293T cells were grown in dishes coated with 0.3% collagen. Transfections were performed using Lipofectamine Plus Reagent (Life Technologies, Carlsbad, CA) according to the manufacturer's instructions.
Plasmids
Both the pCE-puro 3xFLAG-1 vector and the pCE-puro 3xFLAG-ELOVL1 plasmid were described previously (7). The pCE-puro 3xFLAG-ELOVL1(AA) plasmid was created by site-directed mutagenesis using the QuikChange kit (Stratagene, Agilent Technologies, La Jolla, CA) with primers 5′-TTCCTACATGTCTTCGCTGCCTCTGTGCTTCCCTG-3′ and 5′-CAGGGAAGCACAGAGGCAGCGAAGACATGTAGGAA-3′.
Production of HeLa stable transformants expressing 3xFLAG-ELOVL1
HeLa cells transfected with the pCE-puro 3xFLAG-ELOVL1 plasmid were subjected to selection with puromycin at 10 μg/ml. Among the isolated clones, clone #40 (HeLa-ELOVL1) expressed the highest level of 3xFLAG-ELOVL1 protein and was used for the experiment.
In vitro FA elongation assay
The assay was performed using the total membrane fraction as described previously (7). Briefly, the total membrane fraction was incubated with C22:0-CoA and 0.075 μCi (27.3 μM) [2-14C]malonyl-CoA (American Radiolabeled Chemicals, St. Louis, MO) in 50 μl reaction buffer [50 mM HEPES/NaOH (pH 6.8), 150 mM NaCl, 10% glycerol, 4 mM MgCl2, 2 mM CaCl2, 1 mM DTT] at 37°C for 30 min. The effect of oleic acid, erucic acid, and their mixture, as well as DHA and EPA (all from Sigma), on ELOVL1 activity was examined by including each FA dissolved in 2 μl ethanol or the same volume of ethanol only (vehicle control) in the assay mixture. The reaction was terminated by adding 25 μl 75% (w/v) KOH-water and 50 μl ethanol followed by heating at 70°C for 1 h, and then the mixture was acidified with 100 μl 5 M HCl in 50 μl ethanol. Lipids were extracted with 700 μl hexane and dried. The lipid residue was suspended in 20 μl chloroform and separated by TLC on a Silica Gel 60 high-performance TLC plate (Merck, Whitehouse Station, NJ) with hexane-diethyl ether-acetic acid (30:70:1, v/v/v) as the solvent system. Radiolabeled lipids were detected by autoradiography and quantified by a bioimaging analyzer BAS-2500 (Fuji Photo Film, Tokyo, Japan).
Analysis of FA incorporation into HeLa cells
The [14C]FAs used include the following: [1-14C]palmitic acid (55 mCi/mmol; Moravek Biochemicals and Radiochemicals, Brea, CA); [1-14C]stearic, [1-14C]oleic, and [1-14C]erucic acids (55 mCi/mmol; American Radiolabeled Chemicals); and [2-14C]arachidic and [2-14C]behenic acids (55 mCi/mmol). The latter two [14C]FAs were prepared in house by alkaline hydrolysis of [2-14C]arachidoyl- and [2-14C]behenoyl-CoAs, respectively, which were in turn synthesized from stearoyl- and arachidoyl-CoAs (Sigma), respectively, with [2-14C]malonyl-CoA (55 mCi/mmol) using the membrane fraction of HEK 293T cells overproducing ELOVL3 (7).
HeLa cells cultured in 35 mm dishes were incubated with 4.5 nCi [14C]FA (dissolved in 4 μl ethanol) in 1 ml serum-free medium at 37°C for 4 h. After removal of medium, cells were washed with 1 ml PBS containing 1 mg/ml FA-free BSA, detached from the dish using a cell scraper and suspended in 200 μl PBS. Lipids were extracted from the cells by successive addition and mixing of 750 μl chloroform-methanol-12 M HCl (100:200:1, v/v/v), 250 μl chloroform, and 250 μl 1% KCl. After centrifugation at 9,000 g and room temperature for 1 min, the organic phase was recovered and dried. The lipid residue was suspended in chloroform-methanol (2:1, v/v) and separated by TLC on a Silica Gel 60 high-performance TLC plate with 1-butanol-acetic acid-water (3:1:1, v/v/v) as the solvent system. Radiolabeled lipids were detected and quantified by a bioimaging analyzer BAS-2500. For the determination of the chain length of the incorporated [14C]FAs, the lipids extracted from the cells were subjected to methanolysis, and the liberated FA methyl esters were analyzed using reversed-phase TLC (C18; chloroform-methanol-water, 5:15:1, v/v/v) and a bioimaging analyzer BAS-2500 as described previously (7).
Immunoblotting
Immunoblotting was performed as described previously (7) using the anti-FLAG antibody M2 (1 μg/ml; Stratagene) as the primary antibody and HRP-conjugated anti-mouse IgG F(ab’)2 fragment (1:7,500 dilution; GE Healthcare Life Sciences, Buckinghamshire, UK) as the secondary antibody. The signal was detected with Pierce Western Blotting Substrate (Thermo Fisher Scientific, Waltham, MA).
Lipid analysis by ultraperformance liquid chromatography MS
Cell pellets were spiked with an internal standard, C17:0-SM (500 pmol; Avanti Polar Lipids, Alabaster, AL), and homogenized in 1,450 μl chloroform-methanol-water-12 M HCl (200:200:180:1, v/v/v/v). After centrifugation at 9,000 g and room temperature for 1 min, the organic phase was recovered and treated with 135 μl 0.5 M NaOH in methanol at 37°C for 1 h followed by neutralization with 67.5 μl 1 M HCl. After addition of 450 μl water, the organic phase was recovered and dried, and the lipid residue was suspended in chloroform-methanol (1:2, v/v). SM was quantified by Xevo G2 QTof LC/MS system (Waters, Milford, MA): Lipids were resolved by ultraperformance liquid chromatography (UPLC) on a reversed-phase column (AQUITY UPLC BEH C18 column, length 150 mm; Waters), and the identity was confirmed by MS in the positive ion mode with a scan range of m/z 500–1,200. The data were analyzed by MassLynx software (version 4.1; Waters).
RESULTS
Oleic acid inhibits ELOVL1 activity in vitro
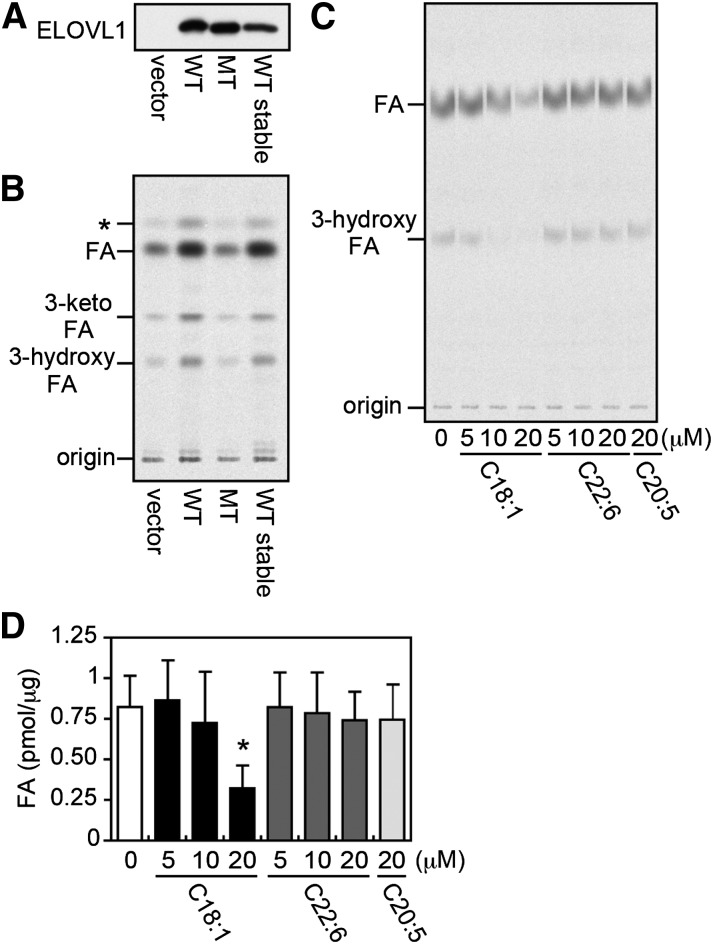
Of the seven mammalian ELOVLs, ELOVL1 is primarily responsible for the synthesis of C22- to C26-VLCFAs (7). We therefore generated HeLa cells transiently or stably expressing 3xFLAG-tagged ELOVL1 (3xFLAG-ELOVL1), thus overproducing ELOVL1. To confirm their increased ELOVL1 activities, we also generated HeLa cells expressing either vector control or 3xFLAG-tagged ELOVL1 mutant (negative control) in which the conserved HXXHH motif for ELOVL family members (where XX represents VF for ELOVL1) was replaced with HVFAA [i.e., 3xFLAG-ELOVL1(AA)]. The conserved motif is thought to be located in the active site, and its mutations in the yeast ELOVL homolog Fen1 and in ELOVL7 result in the loss of their activities (29, 30). Both the mutant 3xFLAG-ELOVL1(AA) and the wild-type 3xFLAG-ELOVL1 proteins were expressed at a comparative level (Fig. 1A). The total membrane fractions were prepared from these cells and examined for their ELOVL1 activities in the in vitro FA elongation assay with [2-14C]malonyl-CoA as the C2 donor and C22:0-CoA as the substrate. The reaction products were analyzed by TLC autoradiography after alkaline hydrolysis to the corresponding FAs (Fig. 1B). As expected, the membrane fractions from those HeLa cells overproducing ELOVL1, but not from the cells expressing ELOVL1(AA), significantly increased ELOVL1 activity as compared with the vector control cells. Therefore, the total membrane fraction from HeLa cells stably overproducing ELOVL1 (HeLa-ELOVL1) was used in the following FA elongation assays, except where indicated, in which case the total membrane fraction from HEK 293T cells transiently overexpressing ELOVL1 (7) was used.
Fig. 1.
Oleic acid inhibits ELOVL1 activity. (A, B) The total membrane fraction was prepared from HeLa cells transfected with a vector (pCE-puro 3xFLAG-1), a plasmid encoding the wild-type (WT) 3xFLAG-ELOVL1 protein (pCE-puro 3xFLAG-ELOVL1), or a plasmid encoding the mutant (MT) 3xFLAG-ELOVL1 protein [pCE-puro 3xFLAG-ELOVL1(AA)], and from HeLa cells stably expressing the WT 3xFLAG-ELOVL1 protein (HeLa-ELOVL1). For transient expression, cells were harvested 24 h after transfection. (A) The total membrane fraction (5 μg protein) was subjected to immunoblotting with anti-FLAG antibody. (B) The total membrane fraction (20 μg protein) was incubated with 50 μM C22:0-CoA and 0.075 μCi (27.3 μM) [2-14C]malonyl-CoA at 37°C for 30 min. After a sequential workup (see “Materials and Methods”), lipids were separated by normal-phase TLC and detected by a bioimaging analyzer BAS-2500; *, a possible decarboxylation product of 3-keto-FA formed during saponification. (C) The in vitro FA elongation assay was performed as in (B) with the total membrane fraction (10 μg protein) prepared from HEK 293T cells transiently expressing the WT 3xFLAG-ELOVL1 protein in the presence of the indicated concentration of oleic acid (C18:1), docosahexaenoic acid (C22:6), or eicosapentaenoic acid (C20:5) in ethanol or ethanol alone (vehicle control). (D) The radioactivity associated with the product FA in (C) was quantified. Each bar represents the amount of FA generated per microgram of the total membrane protein and the mean ± SD of three independent experiments. The statistically significant difference from vehicle-treated control cells (0 μM) is indicated (* P < 0.05, Student's t-test).
We first investigated the effect of FAs with different chain length and degree of saturation on ELOVL1 activity. Thus, the in vitro FA elongation assay was performed in the presence of oleic acid (C18:1, a major component of Lorenzo's oil), DHA (C22:6), EPA (C20:5), or ethanol (vehicle control). Oleic acid was found to inhibit ELOVL1 activity in a dose-dependent manner with ∼60% inhibition at 20 μM, whereas neither DHA nor EPA exhibited any inhibition even at 20 μM (Fig. 1C, D). The saturated analog of oleic acid, stearic acid (C18:0), had no effect on ELOVL1 activity even at 100 μM (supplementary Fig. I). The longer-chain saturated FAs, including arachidic acid (C20:0) and behenic acid (C22:0), could not be evaluated due to their poor solubility in the assay medium.
A 4:1 mixture of oleic and erucic acids (Lorenzo's oil) strongly inhibits ELOVL1 activity
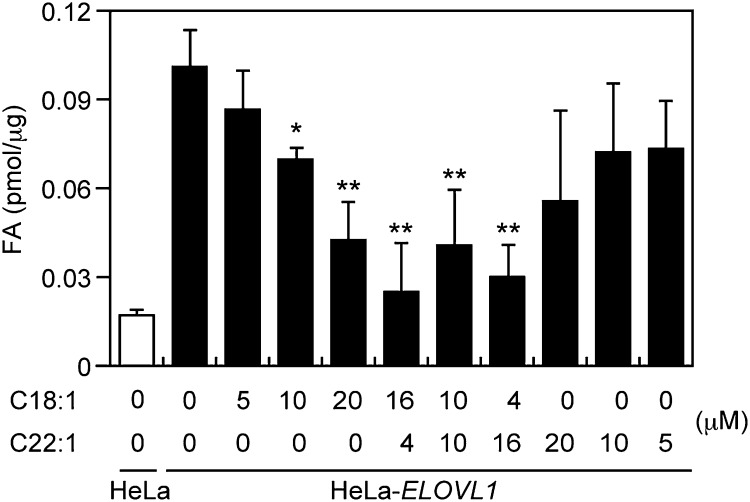
Because Lorenzo's oil is a 4:1 mixture of oleic (C18:1) and erucic (C22:1) acids, the in vitro FA elongation assay was conducted next in the presence of a mixture in varying proportions of oleic and erucic acids (4:1, 1:1, and 1:4) (Fig. 2). The concentration of the mixture was kept constant at 20 μM, which is the concentration at which oleic acid showed maximum inhibitory activity. Erucic acid alone appeared to inhibit some ELOVL1 activity at 20 μM, but the effect was not statistically significant. Interestingly, all mixtures of oleic and erucic acids tested showed inhibitory activity comparable to or even more profound than that of oleic acid, with the 4:1 mixture (the exact FA ratio of Lorenzo's oil) being the most potent.
Fig. 2.
A mixture of oleic and erucic acids inhibits ELOVL1 activity. The total membrane fraction (10 μg protein) prepared from either HeLa cells or HeLa-ELOVL1 cells was incubated with 50 μM C22:0-CoA and 0.075 μCi (27.3 μM) [2-14C]malonyl-CoA at 37°C for 30 min in the presence of the indicated concentration of oleic acid (C18:1), erucic acid (C22:1), or their mixture in ethanol or ethanol alone (vehicle control). After a sequential workup (see “Materials and Methods”), lipids were separated by normal-phase TLC and quantified by a bioimaging analyzer BAS-2500. Each bar represents the amount of FA generated per microgram of the total membrane protein and the mean ± SD of three independent experiments. Statistically significant differences from vehicle-treated control HeLa-ELOVL1 cells are indicated (* P < 0.05 and ** P < 0.01, Student's t-test).
Lorenzo's oil inhibits ELOVL1 activity by a mechanism other than competitive inhibition
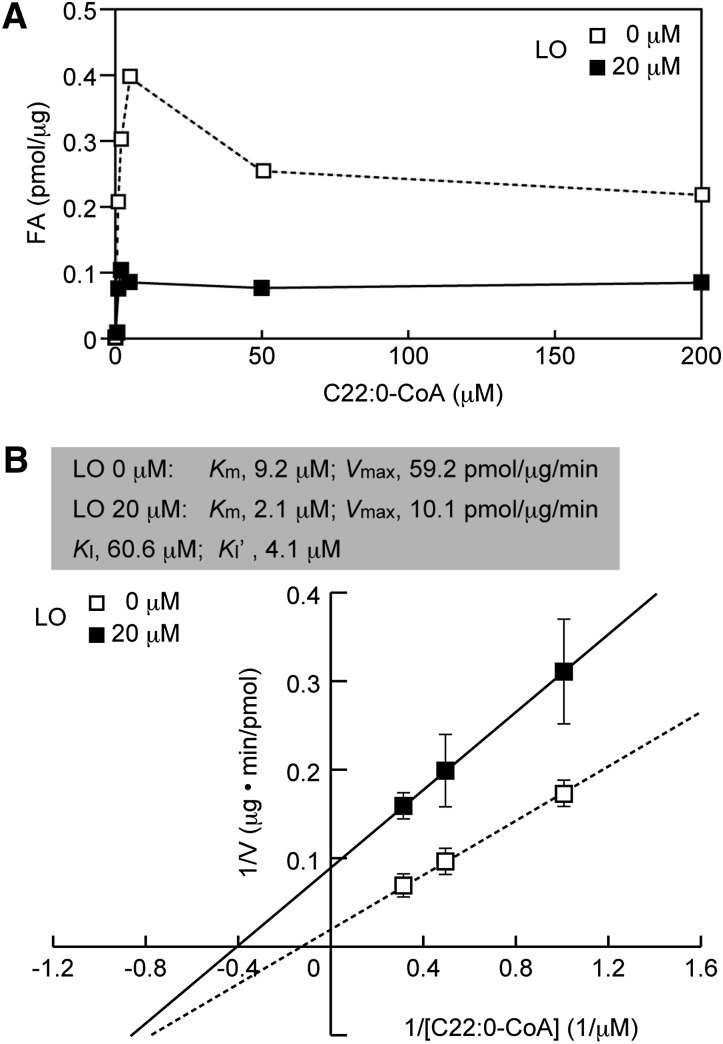
Following the identification of a 4:1 mixture of oleic and erucic acids (Lorenzo's oil) as a potent inhibitor of ELOVL1, we undertook a kinetic characterization of this inhibition process. One possible mechanism of inhibition was that oleic and erucic acids might compete with the actual substrate, C22:0-CoA, for the active site of ELOVL1 (i.e., competitive inhibition). If this is the case, the inhibition can be overcome by raising the substrate concentration. The in vitro FA elongation assay was then carried out at various concentrations of C22:0-CoA in the absence or presence of a 4:1 mixture of oleic and erucic acids at a concentration of 20 μM (Fig. 3A). In the absence of the 4:1 mixture, ELOVL1 activity reached a peak at a C22:0-CoA concentration of 5 μM and declined slightly thereafter. The high concentration of C22:0-CoA may have a detergent-like effect on ELOVL1, which is known to be sensitive to solubilization by Triton X-100 (30). In the presence of the 4:1 mixture, inhibition of ELOVL1 remained essentially constant even at 200 μM C22:0-CoA, indicating that the mechanism of inhibition is not competitive.
Fig. 3.
A 4:1 mixture of oleic and erucic acids inhibits ELOVL1 by a mixed-type inhibition mechanism. The total membrane fraction (10 μg protein) from HeLa-ELOVL1 cells was incubated with the indicated concentration of C22:0-CoA and 0.075 μCi (27.3 μM) [2-14C]malonyl-CoA at 37°C for 30 min in the presence of 20 μM 4:1 mixture of oleic (C18:1) and erucic (C22:1) acids in ethanol or ethanol vehicle alone. After a sequential workup (see “Materials and Methods”), lipids were separated by normal-phase TLC and quantified by a bioimaging analyzer BAS-2500. (A) Values presented are the amount of FA generated per microgram of the total membrane protein. (B) The mean values from three independent experiments are expressed in a Lineweaver-Burk plot with a linear regression line. LO, 4:1 mixture of oleic and erucic acids.
We analyzed these data by using a Lineweaver-Burk plot to determine the kinetic parameters (Km and Vmax) of ELOVL1 toward C22:0-CoA. To exclude any nonspecific effects seen at the higher concentration of C22:0-CoA, we used the data obtained at 1, 2, and 3 μM C22:0-CoA, yielding a linear increase of the ELOVL1 activity (Fig. 3B). The Km and Vmax values of ELOVL1 for C22:0-CoA were found to be 9.2 μM and 59.2 pmol/min/μg protein, respectively, and those in the presence of the 4:1 mixture were 2.1 μM and 10.1 pmol/min/μg protein, respectively. The KI and KI’ values were also calculated to be 60.6 and 4.1 μM, respectively. These findings suggest that ELOVL1 inhibition by a 4:1 mixture of oleic and erucic acids (Lorenzo's oil) is a mixed inhibition in which these acids preferentially bind to the ELOVL1-substrate (C22:0-CoA) complex.
Erucic acid is preferentially metabolized to sphingolipids
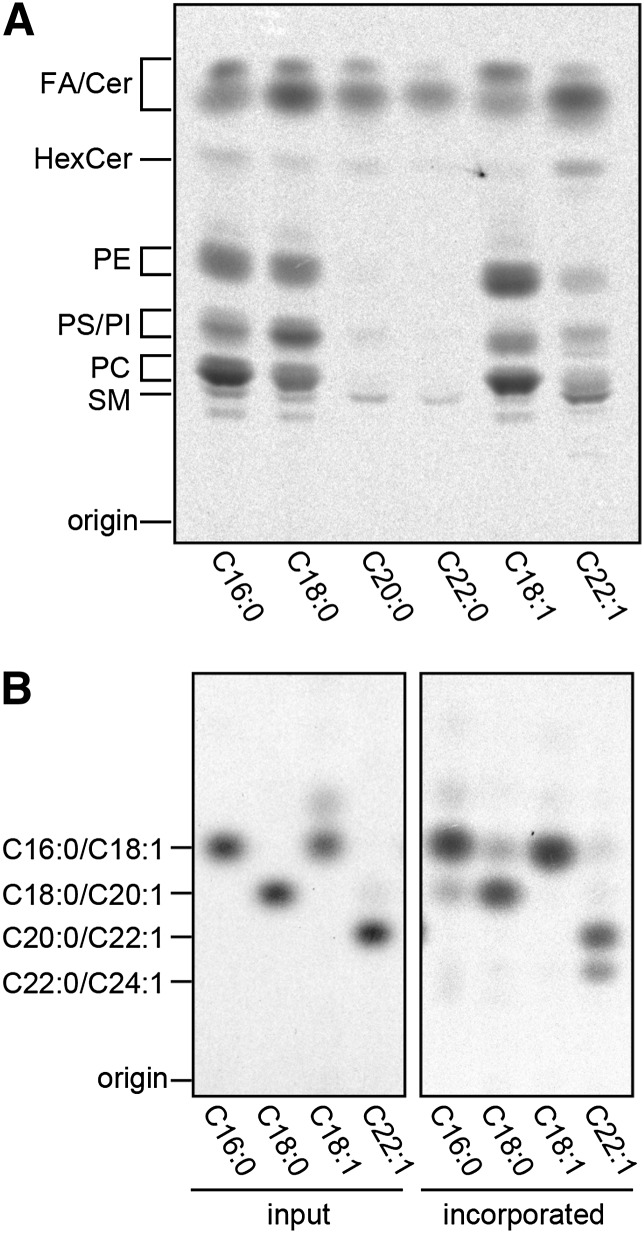
We next investigated the metabolism of oleic and erucic acids using HeLa cells. The cells were incubated with 14C-labeled oleic and erucic acids, as well as with 14C-labeled palmitic, stearic, arachidic, and behenic acids for comparison. Similar to [14C]palmitic and [14C]stearic acids, ∼70% of [14C]oleic acid was taken up by cells and metabolized mainly to glycerophospholipids [phosphatidylethanolamine (PE), phosphatidylserine (PS), phosphatidylinositol (PI), and phosphatidylcholine (PC)] and to a much lesser extent to sphingolipids [hexosylceramide (HexCer) and SM] (Fig. 4A). In contrast, [14C]erucic acid, of which ∼60% was taken up by cells, was metabolized mainly to sphingolipids. Both [14C]arachidic and [14C]behenic acids were rather poorly taken up by cells (∼20%) and seemed to be metabolized to sphingolipids.
Fig. 4.
Erucic acid is elongated and incorporated into sphingolipids. HeLa cells were incubated with the indicated [14C]FAs (4.5 nCi) at 37°C for 4 h. (A) Following extraction from the cells, lipids were separated by normal-phase TLC and detected by a bioimaging analyzer BAS2500. Cer, ceramide. (B) Following extraction from the cells, lipids were subjected to methanolysis, and the liberated FA methyl esters were analyzed by reversed-phase TLC with a bioimaging analyzer BAS-2500.
The chain length of the [14C]FA metabolites was determined by methanolysis of these lipids followed by reverse-phase TLC analysis of [14C]FA methyl esters (Fig. 4B). Almost one-third of [14C]erucic acid was elongated to [14C]nervonic acid (C24:1), whereas the rest of [14C]FAs were found mostly unchanged. The elongation of erucic to nervonic acids is catalyzed by ELOVL1 (7).
Lorenzo's oil decreases the cellular level of SM with a saturated VLCFA
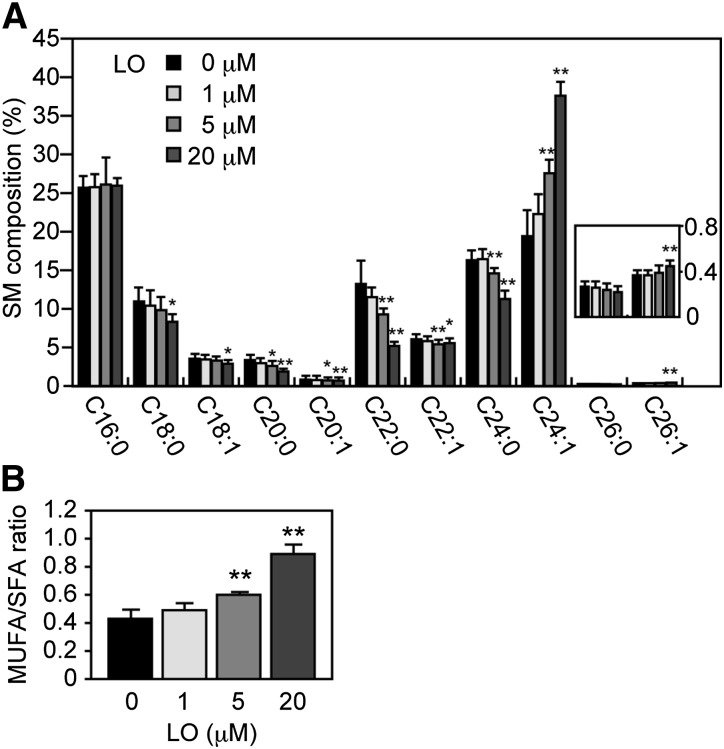
Because in eukaryotic cells saturated and monounsaturated VLCFAs are predominantly incorporated into sphingolipids, we suspected that the inhibition of ELOVL1 responsible for VLCFA synthesis would generate a characteristic sphingolipid profile. Accordingly, we investigated the profile of SM, the most abundant sphingolipid, of HeLa-ELOVL1 cells treated with a 4:1 mixture of oleic and erucic acids for 6 days with the help of UPLC-MS (Fig. 5A). We used these FAs in the free form instead of as their corresponding triglycerides (the chemical composition of Lorenzo's oil) because most triglycerides are first digested to free acids and then absorbed by cells. HeLa-ELOVL1 cells were found to contain two types of SM: one containing LCFAs (mainly C16:0 and C18:0) and the other containing VLCFAs (mainly C22:0, C24:0, and C24:1). C26:0-SM was almost undetectable in HeLa cells. Treatment with the 4:1 mixture significantly reduced the level of saturated VLCFA-SM (C22:0- and C24:0-SMs) even at 5 μM and saturated LCFA-SM (C18:0-SM) at 20 μM. This is consistent with the results in X-ALD fibroblasts reported by Rizzo et al. (27). Concurrent with the reduction of C22:0- and C24:0-SMs, monounsaturated VLCFA-SM (C24:1-SM) was increased significantly. At 20 μM, a significant increase was also detected for C26:1-SM. As a consequence, the ratio of monounsaturated to saturated SMs more than doubled at 20 μM of the 4:1 mixture compared with the untreated control (Fig. 5B). In contrast, treatment with either palmitic (C16:0) or stearic (C18:0) acid at 20 μM had almost no effect on the SM profile except for a slightly increased level of saturated SM (C18:0- and C20:0-SMs or C18:0-, C20:0-, and C22:0-SMs, respectively) (supplementary Fig. II). This is consistent with the aforementioned findings that stearic acid does not inhibit ELOVL1 activity (supplementary Fig. I) and that palmitic and stearic acids are metabolized mainly to glycerophospholipids rather than to sphingolipids (Fig. 4).
Fig. 5.
A 4:1 mixture of oleic and erucic acids reduces the cellular level of SM with a saturated VLCFA. HeLa-ELOVL1 cells were treated with a 4:1 mixture of oleic (C18:1) and erucic (C22:1) acids in a total concentration of 1, 5, or 20 μM. A proper amount of a 4:1 mixture of oleic and erucic acids was dissolved in ethanol and added to the culture media. Control cells received an equal volume of ethanol only. Cells were cultured for 6 days, during which the media were replaced every 2 days with fresh media containing either the 4:1 mixture in ethanol or ethanol only. Lipids were extracted from the cells, and SM composition was determined by UPLC-MS on a Xevo G2 QTof LC/MS system. The data were analyzed by MassLynx software. (A) Each bar represents the percent of the amount of each SM species relative to the total amount of SM and the mean ± SD from four independent assays. The inset is a blown-up portion of C26:0 and C26:1. Statistically significant differences from vehicle (ethanol)-treated control cells are indicated (* P < 0.05 and ** P < 0.01, Student's t-test). (B) Each bar represents the ratio of the amount of SM with a MUFA to the amount of SM with a saturated FA (SFA) and the mean ± SD from four independent assays. Statistically significant differences from vehicle (ethanol)-treated control cells are indicated (** P < 0.01, Student's t-test). LO, 4:1 mixture of oleic and erucic acids.
Lorenzo's oil does not affect the expression level of ELOVL1 mRNA
We also analyzed the endogenous ELOVL1 mRNA level by RT-PCR in HeLa cells treated with a 4:1 mixture of oleic and erucic acids for 6 days and found no appreciable changes caused by the treatment (supplementary Fig. III). The results suggest that Lorenzo's oil reduces the synthesis of saturated VLCFAs by inhibiting ELOVL1 at the protein level but not at the mRNA level.
DISCUSSION
It has been more than two decades since Lorenzo's oil was reported to lower the C26:0 level in X-ALD fibroblast and plasma (23, 24, 27); however, how Lorenzo's oil exerts such effects has remained largely speculative. The results of our present study elucidate for the first time the link between Lorenzo's oil and the reduction of the saturated VLCFA level.
We demonstrated that a 4:1 mixture of oleic and erucic acids potently inhibits the activity of ELOVL1 (Fig. 2) by a mixed inhibition mechanism, rather than by competitive inhibition (Fig. 3); however, at present it remains unclear how these MUFAs interact with ELOVL1. We can only speculate that because the ELOVL family members are multipass transmembrane proteins (5, 20), oleic and erucic acids may interact with ELOVL1 by intercalating between the adjacent membrane-spanning regions of ELOVL1, thereby preventing the conformational change required for its catalytic activity. This may also explain our finding that monounsaturated oleic and erucic acids, but not polyunsaturated DHA and EPA, inhibited ELOVL1 activity (Fig. 1C, D) because more cis-double bonds in the FA chain increase its rigidity and space requirement due to the bent geometry of the cis-double bond and thus limit such lateral interactions. Lorenzo's oil appears to have the optimum ratio of oleic and erucic acids (i.e., 4:1) for ELOVL1 inhibition.
We also demonstrated that cells treated with a 4:1 mixture of oleic and erucic acids had a reduced level of SM with either saturated C22- or C24-VLCFA (Fig. 5A) as a result of the inhibition of ELOVL1 activity, not transcriptional regulation of the ELOVL1 gene (supplementary Fig. III).
In the cell-based assay, the reduction of C22:0- and C24:0-SM was accompanied by an increase in the level of C24:1-SM (Fig. 5A). This increase probably results from the conversion of some of the oleic and erucic acids to the corresponding acyl-CoAs (C18:1- and C22:1-CoAs, respectively), followed by their incorporation into the FA elongation cycle by either the residual ELOVL1 activity or other elongases. Indeed, we observed that exogenously added [14C]erucic acid was elongated to [14C]nervonic acid and incorporated into sphingolipids, although the elongation of [14C]oleic acid was not apparent (Fig. 4A, B). The preferential incorporation of [14C]erucic/nervonic acid, but not [14C]oleic acid, to sphingolipids seems to be consistent with the fact that saturated and monounsaturated VLCFAs are predominantly found in sphingolipids (10, 31). This finding may help explain the increased levels of monounsaturated VLCFAs (C22:1 and C24:1) seen in the plasma of patients treated with Lorenzo's oil (23). Moreover, the finding raises a possibility that the increased C22:1-CoA could compete with the saturated acyl-CoAs for the ELOVL1 active site, thereby contributing to the attenuation of saturated VLCFA synthesis; however, such a possibility is not likely for oleic acid because C18:1-CoA is not a substrate for ELOVL1 (7). Consequently, Lorenzo's oil may lower the pathogenic C24:0 and C26:0 levels by a combination of two mechanisms: direct ELOVL1 inhibition by oleic and erucic acids (mixed inhibition) and indirect ELOVL1 inhibition by C22:1-CoA (competitive inhibition).
Altogether, our findings seem to indicate that the beneficial effect of Lorenzo's oil is at least in part a result of its ELOVL1 inhibition. Although Lorenzo's oil normalizes plasma C24:0 and C26:0 levels in X-ALD patients, it does not arrest or ameliorate their neurological symptoms (23, 24). Moreover, the treatment increases their plasma C22:1 and C24:1 levels (23), the effect of which on the pathology of X-ALD is currently unknown. More work is necessary to better understand the etiology of X-ALD and to develop novel treatments.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. T. Toyokuni for scientific editing of the manuscript.
Footnotes
Abbreviations:
- ELOVL
- elongation of very long-chain fatty acid
- LCFA
- long-chain fatty acid
- UPLC
- ultraperformance liquid chromatography
- VLCFA
- very long-chain fatty acid
- X-ALD
- X-linked adrenoleukodystrophy
This work was supported by Grants-in-Aid for Scientific Research (B) 23370057 (A.K.) and (C) 24590073 (T.S.) from the Japan Society for the Promotion of Science (JSPS), by a grant (Promotion for Young Research Talent and Network) from the Northern Advancement Center for Science & Technology (NOASTEC) foundation, and in part by Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program from the Ministry of Education, Culture, Sports, Science and Technology, Japan.
The online version of this article (available at http://www.jlr.org) contains supplementary data in the form of three figures and Materials and Methods.
REFERENCES
- 1.Agbaga M. P., Mandal M. N., Anderson R. E. 2010. Retinal very long-chain PUFAs: new insights from studies on ELOVL4 protein. J. Lipid Res. 51: 1624–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Imgrund S., Hartmann D., Farwanah H., Eckhardt M., Sandhoff R., Degen J., Gieselmann V., Sandhoff K., Willecke K. 2009. Adult ceramide synthase 2 (CERS2)-deficient mice exhibit myelin sheath defects, cerebellar degeneration, and hepatocarcinomas. J. Biol. Chem. 284: 33549–33560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vasireddy V., Uchida Y., Salem N., Kim S. Y., Mandal M. N., Reddy G. B., Bodepudi R., Alderson N. L., Brown J. C., Hama H., et al. 2007. Loss of functional ELOVL4 depletes very long-chain fatty acids (≥C28) and the unique ω-O-acylceramides in skin leading to neonatal death. Hum. Mol. Genet. 16: 471–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zadravec D., Tvrdik P., Guillou H., Haslam R., Kobayashi T., Napier J. A., Capecchi M. R., Jacobsson A. 2011. ELOVL2 controls the level of n-6 28:5 and 30:5 fatty acids in testis, a prerequisite for male fertility and sperm maturation in mice. J. Lipid Res. 52: 245–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kihara A. 2012. Very long-chain fatty acids: elongation, physiology and related disorders. J. Biochem. 152: 387–395 [DOI] [PubMed] [Google Scholar]
- 6.Iwabuchi K., Prinetti A., Sonnino S., Mauri L., Kobayashi T., Ishii K., Kaga N., Murayama K., Kurihara H., Nakayama H., et al. 2008. Involvement of very long fatty acid-containing lactosylceramide in lactosylceramide-mediated superoxide generation and migration in neutrophils. Glycoconj. J. 25: 357–374 [DOI] [PubMed] [Google Scholar]
- 7.Ohno Y., Suto S., Yamanaka M., Mizutani Y., Mitsutake S., Igarashi Y., Sassa T., Kihara A. 2010. ELOVL1 production of C24 acyl-CoAs is linked to C24 sphingolipid synthesis. Proc. Natl. Acad. Sci. USA. 107: 18439–18444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sassa T., Suto S., Okayasu Y., Kihara A. 2012. A shift in sphingolipid composition from C24 to C16 increases susceptibility to apoptosis in HeLa cells. Biochim. Biophys. Acta. 1821: 1031–1037 [DOI] [PubMed] [Google Scholar]
- 9.Jennemann R., Rabionet M., Gorgas K., Epstein S., Dalpke A., Rothermel U., Bayerle A., van der Hoeven F., Imgrund S., Kirsch J., et al. 2012. Loss of ceramide synthase 3 causes lethal skin barrier disruption. Hum. Mol. Genet. 21: 586–608 [DOI] [PubMed] [Google Scholar]
- 10.Sassa T., Ohno Y., Suzuki S., Nomura T., Nishioka C., Kashiwagi T., Hirayama T., Akiyama M., Taguchi R., Shimizu H., et al. 2013. Impaired epidermal permeability barrier in mice lacking Elovl1, the gene responsible for very-long-chain fatty acid production. Mol. Cell. Biol. 33: 2787–2796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berger J., Pujol A., Aubourg P., Forss-Petter S. 2010. Current and future pharmacological treatment strategies in X-linked adrenoleukodystrophy. Brain Pathol. 20: 845–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Engelen M., Tran L., Ofman R., Brennecke J., Moser A. B., Dijkstra I. M., Wanders R. J., Poll-The B. T., Kemp S. 2012. Bezafibrate for X-linked adrenoleukodystrophy. PLoS ONE. 7: e41013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosser J., Douar A. M., Sarde C. O., Kioschis P., Feil R., Moser H., Poustka A. M., Mandel J. L., Aubourg P. 1993. Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature. 361: 726–730 [DOI] [PubMed] [Google Scholar]
- 14.Morita M., Imanaka T. 2012. Peroxisomal ABC transporters: structure, function and role in disease. Biochim. Biophys. Acta. 1822: 1387–1396 [DOI] [PubMed] [Google Scholar]
- 15.van Roermund C. W., Visser W. F., IJlst L., van Cruchten A., Boek M., Kulik W., Waterham H. R., Wanders R. J. 2008. The human peroxisomal ABC half transporter ALDP functions as a homodimer and accepts acyl-CoA esters. FASEB J. 22: 4201–4208 [DOI] [PubMed] [Google Scholar]
- 16.Van Veldhoven P. P. 2010. Biochemistry and genetics of inherited disorders of peroxisomal fatty acid metabolism. J. Lipid Res. 51: 2863–2895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kemp S., Berger J., Aubourg P. 2012. X-linked adrenoleukodystrophy: clinical, metabolic, genetic and pathophysiological aspects. Biochim. Biophys. Acta. 1822: 1465–1474 [DOI] [PubMed] [Google Scholar]
- 18.Paintlia A. S., Gilg A. G., Khan M., Singh A. K., Barbosa E., Singh I. 2003. Correlation of very long chain fatty acid accumulation and inflammatory disease progression in childhood X-ALD: implications for potential therapies. Neurobiol. Dis. 14: 425–439 [DOI] [PubMed] [Google Scholar]
- 19.Vargas C. R., Wajner M., Sirtori L. R., Goulart L., Chiochetta M., Coelho D., Latini A., Llesuy S., Bello-Klein A., Giugliani R., et al. 2004. Evidence that oxidative stress is increased in patients with X-linked adrenoleukodystrophy. Biochim. Biophys. Acta. 1688: 26–32 [DOI] [PubMed] [Google Scholar]
- 20.Jakobsson A., Westerberg R., Jacobsson A. 2006. Fatty acid elongases in mammals: their regulation and roles in metabolism. Prog. Lipid Res. 45: 237–249 [DOI] [PubMed] [Google Scholar]
- 21.Ofman R., Dijkstra I. M., van Roermund C. W., Burger N., Turkenburg M., van Cruchten A., van Engen C. E., Wanders R. J., Kemp S. 2010. The role of ELOVL1 in very long-chain fatty acid homeostasis and X-linked adrenoleukodystrophy. EMBO Mol. Med. 2: 90–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engelen M., Schackmann M. J., Ofman R., Sanders R. J., Dijkstra I. M., Houten S. M., Fourcade S., Pujol A., Poll-The B. T., Wanders R. J., et al. 2012. Bezafibrate lowers very long-chain fatty acids in X-linked adrenoleukodystrophy fibroblasts by inhibiting fatty acid elongation. J. Inherit. Metab. Dis. 35: 1137–1145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rizzo W. B., Leshner R. T., Odone A., Dammann A. L., Craft D. A., Jensen M. E., Jennings S. S., Davis S., Jaitly R., Sgro J. A. 1989. Dietary erucic acid therapy for X-linked adrenoleukodystrophy. Neurology. 39: 1415–1422 [DOI] [PubMed] [Google Scholar]
- 24.Aubourg P., Adamsbaum C., Lavallard-Rousseau M. C., Rocchiccioli F., Cartier N., Jambaqué I., Jakobezak C., Lemaitre A., Boureau F., Wolf C., et al. 1993. A two-year trial of oleic and erucic acids (“Lorenzo's oil”) as treatment for adrenomyeloneuropathy. N. Engl. J. Med. 329: 745–752 [DOI] [PubMed] [Google Scholar]
- 25.Moser H. W., Moser A. B., Hollandsworth K., Brereton N. H., Raymond G. V. 2007. “Lorenzo's oil” therapy for X-linked adrenoleukodystrophy: rationale and current assessment of efficacy. J. Mol. Neurosci. 33: 105–113 [DOI] [PubMed] [Google Scholar]
- 26.Moser H. W., Raymond G. V., Lu S. E., Muenz L. R., Moser A. B., Xu J., Jones R. O., Loes D. J., Melhem E. R., Dubey P., et al. 2005. Follow-up of 89 asymptomatic patients with adrenoleukodystrophy treated with Lorenzo's oil. Arch. Neurol. 62: 1073–1080 [DOI] [PubMed] [Google Scholar]
- 27.Rizzo W. B., Watkins P. A., Phillips M. W., Cranin D., Campbell B., Avigan J. 1986. Adrenoleukodystrophy: oleic acid lowers fibroblast saturated C22–26 fatty acids. Neurology. 36: 357–361 [DOI] [PubMed] [Google Scholar]
- 28.Afman L. A., Müller M. 2012. Human nutrigenomics of gene regulation by dietary fatty acids. Prog. Lipid Res. 51: 63–70 [DOI] [PubMed] [Google Scholar]
- 29.Denic V., Weissman J. S. 2007. A molecular caliper mechanism for determining very long-chain fatty acid length. Cell. 130: 663–677 [DOI] [PubMed] [Google Scholar]
- 30.Naganuma T., Sato Y., Sassa T., Ohno Y., Kihara A. 2011. Biochemical characterization of the very long-chain fatty acid elongase ELOVL7. FEBS Lett. 585: 3337–3341 [DOI] [PubMed] [Google Scholar]
- 31.Pewzner-Jung Y., Park H., Laviad E. L., Silva L. C., Lahiri S., Stiban J., Erez-Roman R., Brügger B., Sachsenheimer T., Wieland F., et al. 2010. A critical role for ceramide synthase 2 in liver homeostasis: I. Alterations in lipid metabolic pathways. J. Biol. Chem. 285: 10902–10910 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.