Abstract
The first line of defense in plants against pathogens is induced by the recognition of microbe-associated molecular patterns (MAMP). Perception of bacterial flagellin (flg22) by the pattern recognition receptor flagellin-sensing 2 (FLS2) is the best characterized MAMP response, although the underlying molecular mechanisms are not fully understood. Here we studied the relationship between salicylic acid (SA) or jasmonic acid (JA) signaling and FLS2-mediated signaling by monitoring flg22-triggered responses in known SA or JA related mutants of Arabidopsis thaliana (L.) Heynh. The sid2 mutant, impaired in SA biosynthesis, had less basal FLS2 mRNA accumulation than the wild type, which correlated with suppression of early flg22 responses such as ROS production and induction of marker genes, WRKY29 and FRK1. The JA-signaling mutants, jar1 and coi1, exhibited an enhanced flg22-triggered oxidative burst and more callose accumulation than the wild type, and pretreatment with SA or coronatine (COR), a structural mimic of JA-isoleucine, altered these flg22-induced responses. Nonexpressor of pathogenesis-related genes 1 (NPR1) acted downstream of SID2 and required SA-dependent priming for the enhanced flg22-triggered oxidative burst and callose deposition. Activation of JA signaling by COR pretreatment suppressed the flg22-triggered oxidative burst and callose accumulation in a coronatine insensitive 1 (COI1) dependent manner. COR had a negative effect on flg22 responses but only the flg22-triggered oxidative burst depended on SA-JA/COR signaling antagonism. Thus the activated SA and JA signaling pathways have an influence on flg22-triggered oxidative burst and callose deposition. These results may explain how SA and JA signaling are cross talked for regulation of flg22-triggered responses.
Introduction
Current models suggest two forms of innate immunity in plants [1]. In one model, resistance is triggered by microbe-associated molecular patterns (MAMPs) and is referred to as MAMP-triggered immunity (MTI). In the second model, effector-triggered immunity (ETI), the plant response is triggered by pathogen effectors. MTI is initiated through the recognition of conserved MAMPs by specific pattern recognition receptors (PRRs) in the plant. The best-characterized MAMP is flagellin [2], [3]. Flg22 is a 22-amino acid synthetic polypeptide that corresponds to a highly conserved epitope of the Pseudomonas aeruginosa flagellin protein [2]. It is widely used as a proxy for flagellin in flagellin-mediated signaling in Arabidopsis thaliana (L.) Heynh. Flg22 is recognized by the Arabidopsis flagellin sensing 2 protein (FLS2), a leucine-rich repeat receptor kinase [4], [5]. Activity of the downstream pathways is marked by common signaling events, such as ion fluxes, protein phosphorylation cascades, accumulation of reactive oxygen species (ROS), induction of defense genes, and cell-wall reinforcement by callose deposition [6]–[8]. By contrast, effector-triggered immunity results from the highly specific, direct or indirect interaction of pathogen effectors and the products of plant R genes. This recognition event leads to a strong local defense response that stops pathogen growth [9].
To survive, plants have to respond rapidly and effectively to each intruder. Plant defense signal interactions, upon an intruder's attack, can be either mutually antagonistic or synergistic and are thought to further optimize the specificity of the defense response. One of the best-studied examples of defense-related signal crosstalk is the antagonistic interaction between the salicylic acid (SA) and the jasmonic acid-ethylene (JA/ET) response pathways [10]–[12]. Biotrophic and hemi-biotrophic pathogens are generally more sensitive to SA-dependent responses, whereas necrotrophic pathogens and herbivorous insects are commonly deterred by JA/ET-dependent defense [13], [14]. ET modulates SA related plant defense signaling both positively and negatively [12]: ET has synergistic effects on SA-induced expression of PATHOGENESIS-RELATED PROTEIN 1 (PR 1) [15], whereas the ET-responsive transcription factor EIN3 and EIN3-LIKE1 (EIL1) attenuate SA biosynthesis by direct binding and repression of SALICYLIC ACID INDUCTION DEFICIENT 2 (SID2), encoding an SA biosynthesis enzyme [16]. SA can suppress both JA biosynthesis and sensitivity [17]. However, some of the JA biosynthetic genes are positively regulated by JA, and it does not seem to be required for the SA-mediated depression of JA signaling [18]. The protein NPR1 (for NONEXPRESSOR OF PR1) plays an important role in mediating the suppressive effect of SA down streanm of JA [17], [19].
The positive and negative regulatory components of hormone pathways are potential targets for modification of hormonal crosstalk during disease and defense. Microbial pathogens have developed the ability to manipulate plant defense responses by producing phytohormones or their functional mimics [20]. For example, coronatine (COR), a structural mimic of JA-isoleucine (JA-Ile) produced by Pseudomonas syringae pv. tomato (Pst) bacterium, triggers the activation of JA-dependent defense responses leading to the suppression of SA-dependent defense responses [21].
Recent studies show that SA signaling is an integral part of both the MTI and ETI defense responses. Treatment with flg22 causes SA accumulation and induces expression of canonical SA-related genes, including SID2, enhanced disease susceptibility 5 gene (EDS5), NPR1, and PR1 [22], [23]. Previous studies show flg22-induced SA accumulation to be dependent on SID2, which encodes isochorismate synthase, a SA biosynthetic enzyme [24], [25]. MAMPs have also been reported to stimulate JA and ET production [26]–[28] by up regulating genes that encode the proteins involved in JA and ET biosynthesis [29].
Several key regulatory proteins involved in SA-JA crosstalk have been identified in Arabidopsis. The major positive regulator of the SA response, NPR1, is a possible modulator of crosstalk between the SA and JA signals [19]. The cytosolic function of the NPR1 protein is important during SA-JA crosstalk [17], [30], while the nuclear function of NPR1 is important during the activation of SA-responsive genes [19]. Coronatine insensitive 1 (COI1) encodes an F-box protein that regulates JA-signaling by inactivating negative regulators of JA-mediated responses [31]. The coi1 mutant exhibits enhanced expression of SA-dependent defenses and enhanced resistance to P. syringae [32], [33]. The SA-mediated defense pathway is sensitized in coi1 plants, so that SA-dependent defenses are hyper-activated in response to attack by P. syringae. Exogenous COR also triggered re-opening of stomata that had closed during the plants' response to MAMPs; closed stomata are part of the defense response as closure should inhibit bacterial entry into the leaf [34]. Recent reports provide evidence that COR activates three NAC genes (petunia NAM and Arabidopsis ATAF1, ATAF2, and CUC2): the transcription factors ANAC019, ANAC055, and ANAC072. These transcription factors then inhibit SA accumulation by regulating genes involved in SA synthesis and metabolism [35]. These reports suggested that COR-triggered SA suppression may be the molecular mechanism for COR-mediated virulence in stomata, as well as in tissues local to the infection and tissues involved in the systemic response. These findings are consistent with the hypothesis that activation of JA signaling pathway negatively results SA-dependent inducible defenses.
The goal of this study was to determine how the flg22 response and SA or JA signaling are linked. Here we investigated flg22 responses in known SA or JA related mutants and have identified SID2 as an important component of flg22-triggered oxidative burst and early response gene induction, partially through activating the accumulation of FLS2 mRNA. Pretreatment with SA enhanced flg22 responses through NPR1 downstream of SID2. Activated JA signaling, by COR pretreatment, acts through COI1 to suppress the flg22 induced ROS production and callose deposition downstream of JAR1. These findings indicated that both SA signaling and COR mediated JA signaling are critical components in regulating flg22 responses and significantly extend our understanding of the relationship between defense-related hormone signaling and flg22 responses.
Results
Both SA and JA signaling are involved in flg22-triggered oxidative burst
One of the early reactions triggered by perception of flg22 is an oxidative burst, a rapid and transient accumulation of ROS [2]. To investigate the involvement of SA and JA in early flg22-induced responses, we monitored the flg22-triggered oxidative burst in intact seedlings of a collection of known SA- or JA-related mutants (Figs 1 and S1). The oxidative burst was diminished in the ethylene-insensitive mutant, ein2 as described earlier [36]. In the auto-immune mutant, cim6, which exhibits high levels of SA accumulation and constitutive activation of SA signaling [37], the flg22-dependent ROS generation was evidently greater than that in the wild type (Fig. 1). By contrast, in sid2 and eds5 (also known as sid1) mutants [38], [39], which do not accumulate SA after either biotic or abiotic stresses, the oxidative burst was much smaller than in the wild-type (Figs 1 and S1). A clear increase in ROS production was detected in jar1 and fad7/fad8 mutants, which have impaired JA-signaling (Figs 1 and S1) [40], [41]. These findings indicate that the SA and JA signaling pathways are antagonistically regulated the flg22-triggered oxidative burst.
Figure 1. SA and JA signaling are required for flg22-triggered oxidative burst.
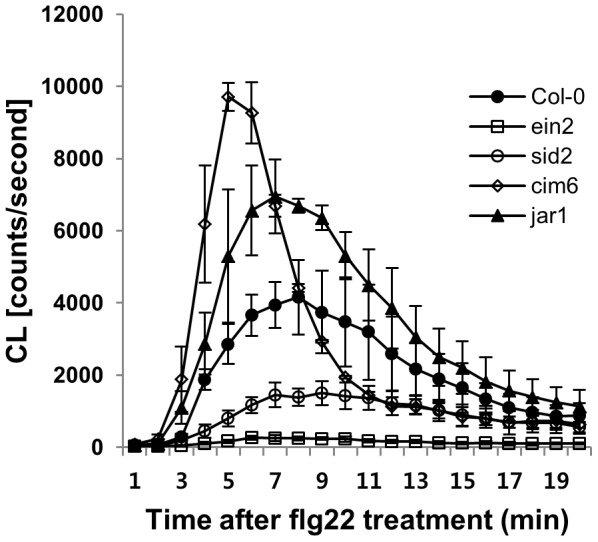
Flg22-induced ROS generation was monitored in liquid-grown intact seedlings of indicated Arabidopsis genotypes after treatment with 1 µM flg22. Error bars represent the SD of five independent samples (n = 10) and similar results were obtained in multiple independent experiments.
SA or COR pretreatment induce marked changes in flg22-triggered oxidative burst
To investigate whether exogenous SA or JA affects the flg22-triggered oxidative burst in Arabidopsis seedlings, we measured ROS levels in hormone-treated Arabidopsis seedlings. The effect of COR on the oxidative burst was also measured as many strains of the P. syringae synthesize COR, a JA-Ile mimic that suppresses flg22 responses by antagonizing SA-activated defense pathways [35], [42]. When seedlings were treated with SA, MeJA, or COR simultaneously with flg22, there was little effect on ROS accumulation compared to the control (Fig. 2). On the other hand, there was a marked enhancement in the flg22-triggered oxidative burst when seedlings were pretreated with SA for 24 h (Fig. 2A). This finding is similar to that of a previous report in parsley suspension cultures, in which pre-incubation with SA enhanced both spontaneous and elicitor-induced production of H2O2. The greatest effect in this study required pretreatment with >500µM SA for longer than 24 h [43].
Figure 2. Effect of exogenous chemical treatments (SA, MeJA, or COR) on the flg22-triggered oxidative burst.
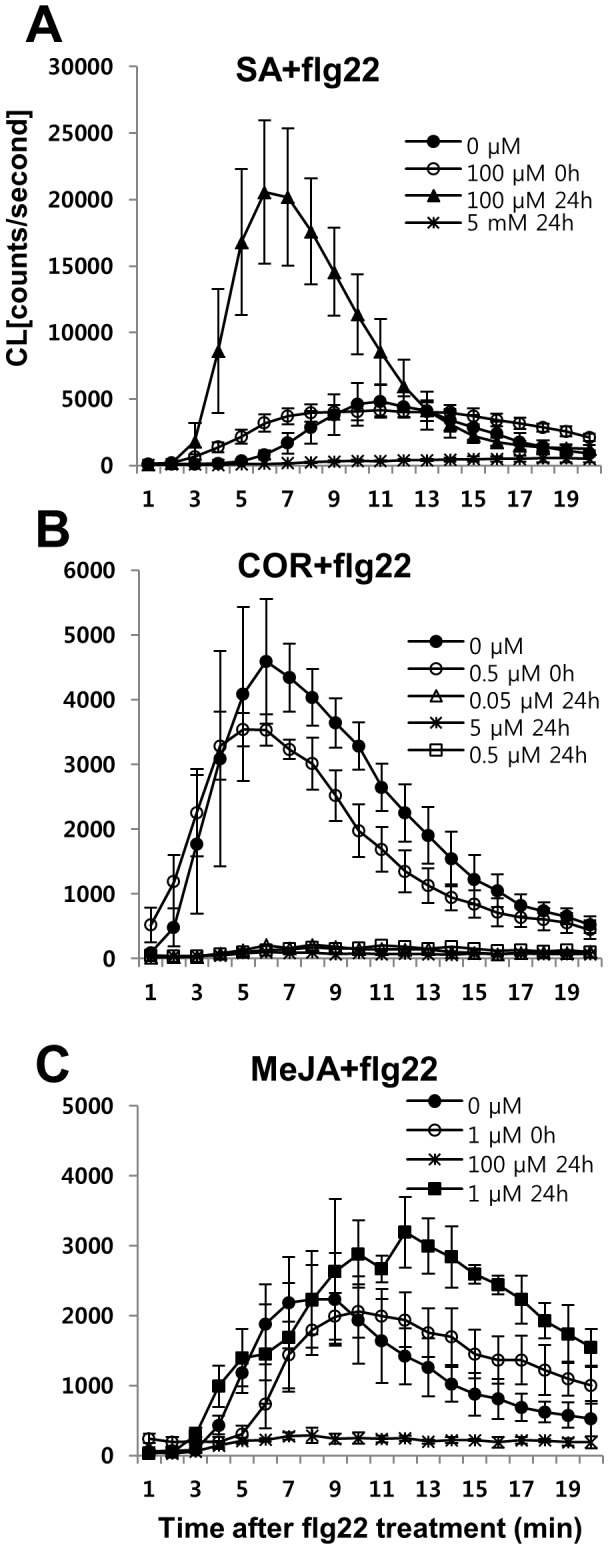
(A-C) Arabidopsis seedlings were pre-incubated with various concentrations of chemicals for the indicated time periods (0 and 24 h) before the start of ROS measurements. Flg22 (1 µM) was added at zero time. Error bars represent the SD of five independent samples (n = 10) and similar results were obtained in three independent experiments.
Since the mutants jar1, fad7/fad8, and coi1, which are impaired in either JA biosynthesis or signaling, showed enhanced flg22-triggered oxidative burst as compared to the wild type (Figs 3C, 3D, and S1), we expected that exogenous MeJA and COR would reduce the burst in these mutants. Interestingly, the effect of MeJA pre-incubation on the flg22-triggered oxidative burst was relatively weak (Fig. 2C), although it was clearly suppressed by 24-h pretreatment with 0.5 µM COR and even with 0.05 µM COR (Fig. 2B). It has been suggested that COI1 directly binds to JA-Ile and COR and serves as a receptor for jasmonates [44]. Furthermore, interaction of tomato COI1 with jasmonate ZIM domain (JAZ) family proteins is highly specific for JA-Ile and structurally related JA conjugates and COR is ∼1000-fold more active than JA-Ile in promoting this interaction in vitro [45], which could explain the different results with MeJA (1 µM) or COR (0.05 µM).
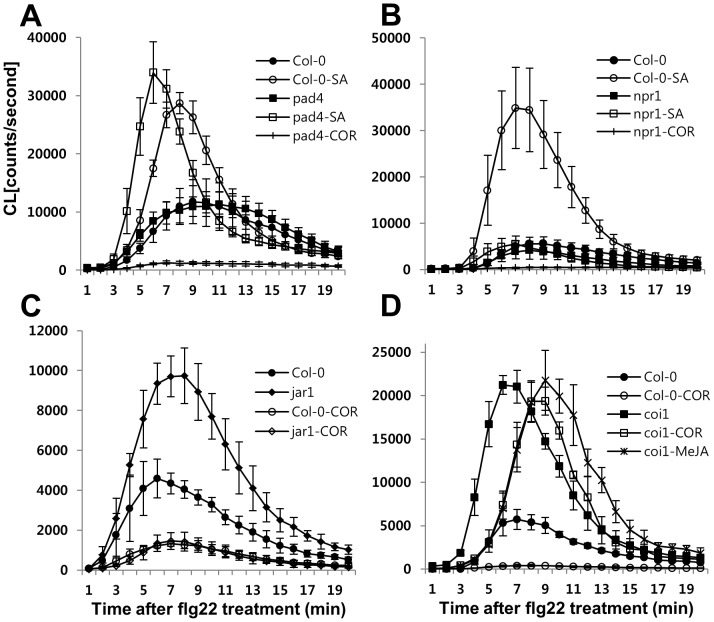
Figure 3. The effect of SA and COR in the flg22-triggered oxidative burst is dependent on NPR1 and COI1, respectively.
(A–D) Effect of pretreatment with SA (100 µM) or COR (0.5 µM) for 24 h on the flg22-triggered oxidative burst in mutant [pad4 (A), npr1 (B), jar1 (C), coi1 (D)] and wild-type Columbia seedlings. Flg22 (1 µM) was added at zero time. Error bars represent the SD of five independent samples (n = 10) and similar results were obtained in three independent experiments.
To determine whether high dosages of MeJA suppress the flg22-triggered oxidative burst, we also measured ROS levels in Arabidopsis seedlings after 24 h of pre-incubation with 100 µM MeJA or 5 µM COR. As expected, the flg22-triggered oxidative burst was clearly suppressed by both chemical treatments (Figs 2B and 2C). High doses of SA (5 mM) pretreatment also obviously suppressed the flg22-triggered ROS production, which may cause indirect effects from modification of endogenous phytohormone balance (Fig. 2A). We did not detect altered ROS production by SA (5 mM), MeJA (100 µM), or COR (5 µM) pretreatment alone (data not shown). In summary, we conclude that pretreatment with low concentrations of SA enhances the flg22-triggered oxidative burst while COR or MeJA pretreatment reduces it.
NPR1 is required for SA-mediated priming for enhancing the flg22-triggered oxidative burst; COR acts through COI1 to suppress the burst
To study the relevance of the signal component of SA in the flg22 response, we analyzed the flg22-triggered oxidative burst in the SA-signaling mutants, pad4 [46] and npr1 [47]. Both pad4 and npr1 mutants exhibited wild type like flg22-induced ROS production, while there was no SA-mediated priming effect in the npr1 mutant compared to the wild type (Fig. 3B). This finding suggests that NPR1, but not PAD4, is required for SA-mediated priming for the enhanced flg22-triggered oxidative burst.
To investigate whether COR treatment can function as a JA-Ile mimic downstream of JAR, we measured the flg22-induced ROS level in jar1 and coi1. As shown in Fig. 3C, COR still suppressed the flg22-triggered oxidative burst in jar1, whereas COR and MeJA were not able to suppress the burst in the coi1 mutant (Fig. 3D). This finding indicated that COR signals act through COI1 downstream of JAR1 to suppress the flg22-induced ROS burst.
COR compromises SA signaling-mediated priming effect on flg22-triggered oxidative burst in cim6
To identify an association of JA-SA antagonism with the flg22-triggered ROS response, we determined if JA signaling activated by COR suppressed auto-activated SA signaling in cim6 [37] compared to the wild type. Fig. 4A shows that the flg22-triggered oxidative burst was suppressed in cim6 plants by pre-incubation with COR. This finding indicates that COR antagonizes activated SA signaling to suppress the flg22-triggered oxidative burst in cim6. When mutants seedlings were pretreated with SA and COR simultaneously, however, the SA-mediated ROS amplification was not affected by COR (Fig. 4B), suggesting that the effect of SA dominated.
Figure 4. COR is required to overcome the SA effect during the flg22-triggered oxidative burst.
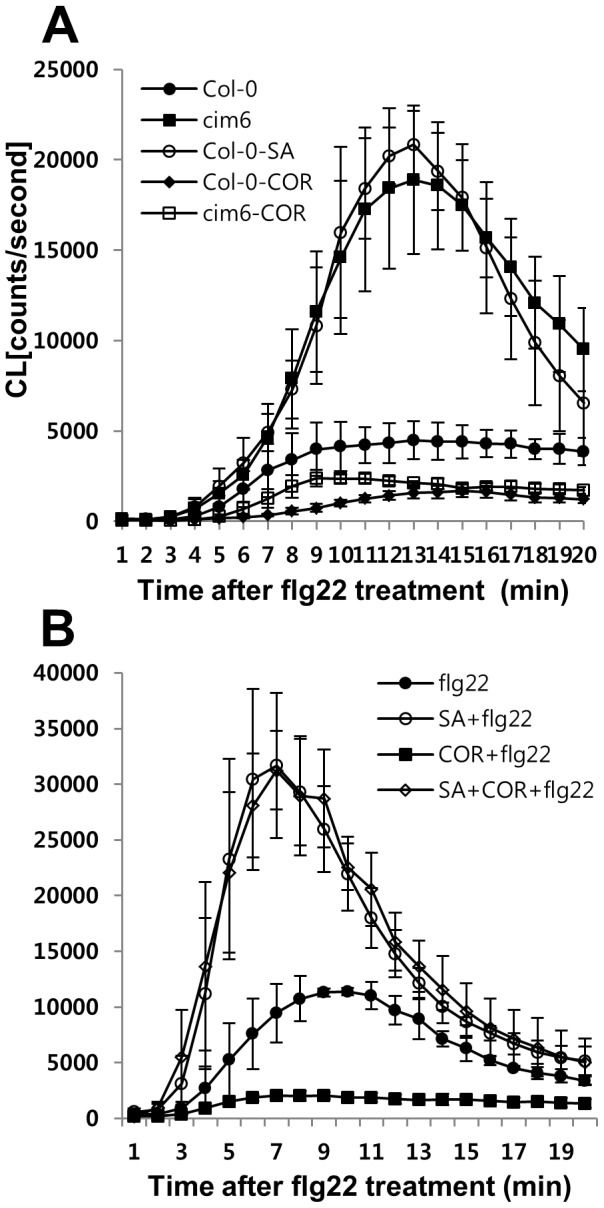
(A) Effect of pretreatment with SA (100 µM) or COR (0.5 µM) for 24 h on the flg22- triggered oxidative burst in cim6 and wild-type Columbia seedlings. Flg22 (1 µM) was added at zero time. (B) COR did not suppress flg22-induced ROS generation when applied simultaneously with SA. Eight-day-old seedlings were pre incubated with SA (100 µM), COR (0.5 µM), or SA plus COR for 24 h. Flg22 (1 µM) was added at zero time. Error bars represent the SD of five independent samples (n = 10) and similar results were obtained in at least two independent experiments.
SA signaling contributes to FLS2 transcript accumulation and early flg22 responses
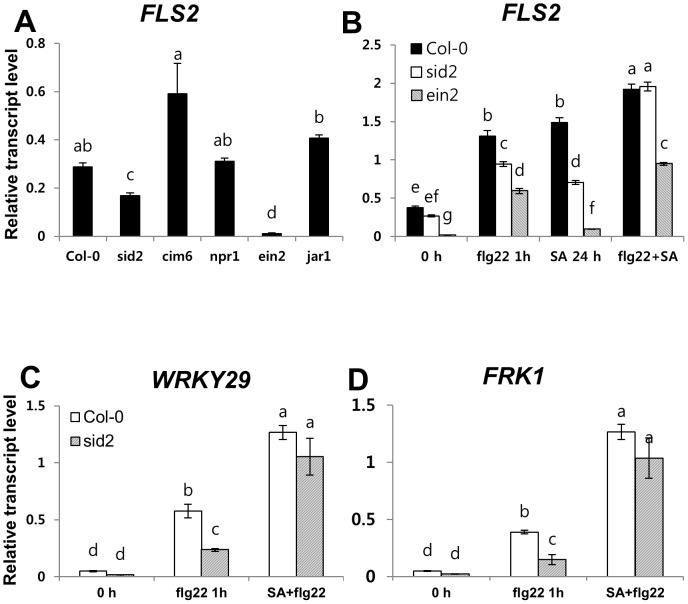
FLS2 transcript accumulation and FLS2 protein abundance affect flg22-triggered ROS generation [36]. This led us to compare transcript levels of the wild type and the mutants that were impaired in SA and JA signaling. We used the ein2 mutant as a negative control for basal FLS2 transcript accumulation because it is impaired in FLS2-mediated responses and these correlated with reduced FLS2 transcription and protein accumulation [36]. The cim6 mutant also had a high level of basal FLS2 transcription (Fig. 5A). The sid2 mutant is impaired in SA biosynthesis [38], and had reduced basal and flg22-induced FLS2 transcript levels (Figs 5A and 5B). This observation was predicted, as exogenous SA alone induced FLS2 transcript accumulation in Arabidopsis seedlings (Fig. 5B). However, the effect of SID2 mutation on FLS2 transcript accumulation was relatively weak when compared with that in the ein2 (Figs 5A and 5B) indicating that SA signaling is required for full induction of FLS2, together with other components. These results indicate that SA signaling components play a role for FLS2 transcript accumulation, which may affect the magnitude of the flg22-triggered oxidative burst in cim6 and sid2 plants.
Figure 5. Down regulation of the flg22 response genes in sid2 plants.
For Quantitative RT-PCR analysis, 8-day-old seedlings were pre-treated with 100 µM of salicylic acid for 24 h and then incubated in 1 µM flg22 solution for 1 h. ACT2 [74] was used as a control. Data represent SD. All quantitative gene expression measurements were performed using technical triplicate and biological duplicates. Differential letter types indicated significant differences (α = 0.05) by one-way ANOVA and Tukey HSD test of comparisons between plant genotypes with individual treatment.
In our study, NPR1 is required for SA-mediated priming for the enhanced flg22-triggered oxidative burst (Fig. 2A). Thus, we also analyzed SA priming effects on flg22-induced FLS2 transcript accumulation and ROS production in sid2 plants. SA pretreatment restored and enhanced flg22-induced FLS2 transcription (Fig. 5B) and ROS production in sid2 (Fig. S3). This finding indicated that NPR1 acts at SID2 downstream to regulate SA-mediated priming for enhancing the flg22-triggered oxidative burst.
Previous report showed that the mRNA levels of WRKY29, flg22-induced receptor-like kinase 1 (FRK1), and glutathion S-transferase 1 (GST1) were increased in Arabidopsis protoplasts within 30 min after flg22 treatment [48]. In our system, transcript levels of WRKY29 and FRK1 were increased in seedlings 1 h after flg22 treatment (Figs 5C and 5D) and the induction levels of WRKY29 and FRK1 transcripts were reduced by approximately 50% in the sid2 mutant compared to wild-type plants (Figs 5C and 5D). SA pretreatment recovered the flg22-induced expression of WRKY29 and FRK1in the sid2 mutant to the wild-type levels (Figs 5C and 5D). These findings indicate that SA signaling involves in the regulation of the early flg22 response genes WRKY29 and FRK1. In our system, SA signaling was not only required for FLS2 mRNA accumulation but also for downstream events, including ROS production and early flg22 response gene accumulation. We suggest that SA signaling contributes to early flg22 responses through activating FLS2 mRNA accumulation. Consistent with our results, Assai and colleagues demonstrate that flg22 signaling leading to the expression of WRKY29 and FRK1 requires FLS2 [48].
SA or COR pretreatment trigger marked changes in flg22-induced callose deposition
Another well-studied flg22-elicited response in Arabidopsis is the deposition of callose, a β (1-3)-glucan polymer, which is regulated by indole glucosinolates (IGs) [7]. MYB51 is a transcription factor essential for the regulation of IGs biosynthesis [49]. The SID2 mutation had little effect on flg22-induced expression of MYB51 (Fig. S4) and sid2 plant exhibited wild-type-like flg22-induced callose response. This finding indicated that MYP51 functions downstream of SID2 or a SID2-independent pathway to regulate the flg22-induced callose accumulation. However, SA or COR pretreatment markedly affected the flg22-induced MYB51 mRNA level (Fig. 6A). COR pretreatment significantly reduced flg22-induced expression of MYB51, while SA pretreatment greatly enhanced its transcript abundance in cotyledons 1 h after flg22 treatment (Fig. 6A). To determine whether altered MYB51 transcript abundance is correlated with flg22-triggered callose deposition, we measured callose deposition in COR or SA pretreated Arabidopsis cotyledons. Pretreatment with COR suppressed flg22-induced callose deposition in the wild-type and jar1 cotyledons, but not in coi1 (Figs 6C and S5B). This finding indicated that COR signals act through COI1 to suppress the flg22-induced callose deposition the downstream of JAR1. Pretreatment with SA enhanced flg22-induced callose deposition in all of the mutants tested except npr1, indicating that SA primes callose deposition through NPR1 downstream of SID2 (Figs 6B and S5A). In summary, NPR1 is required for SA-mediated priming for enhancing both flg22-induced ROS production and callose deposition, while COR suppresses flg22-induced ROS production as well as callose response through COI1 (Fig. 2B). Based on these results, we suggest that the altered flg22-triggered oxidative burst resulting from COR or SA pre-incubation might affect flg22-induced callose deposition. Actually, a model system has been used to demonstrate that ROS act as positive signals in flg22- and oligogalacturonides (OGs)-induced callose deposition [50], [51].
Figure 6. Effect of SA or COR pretreatment on flg22-induced MYB51 transcript accumulation and callose deposition of Arabidopsis seedlings.
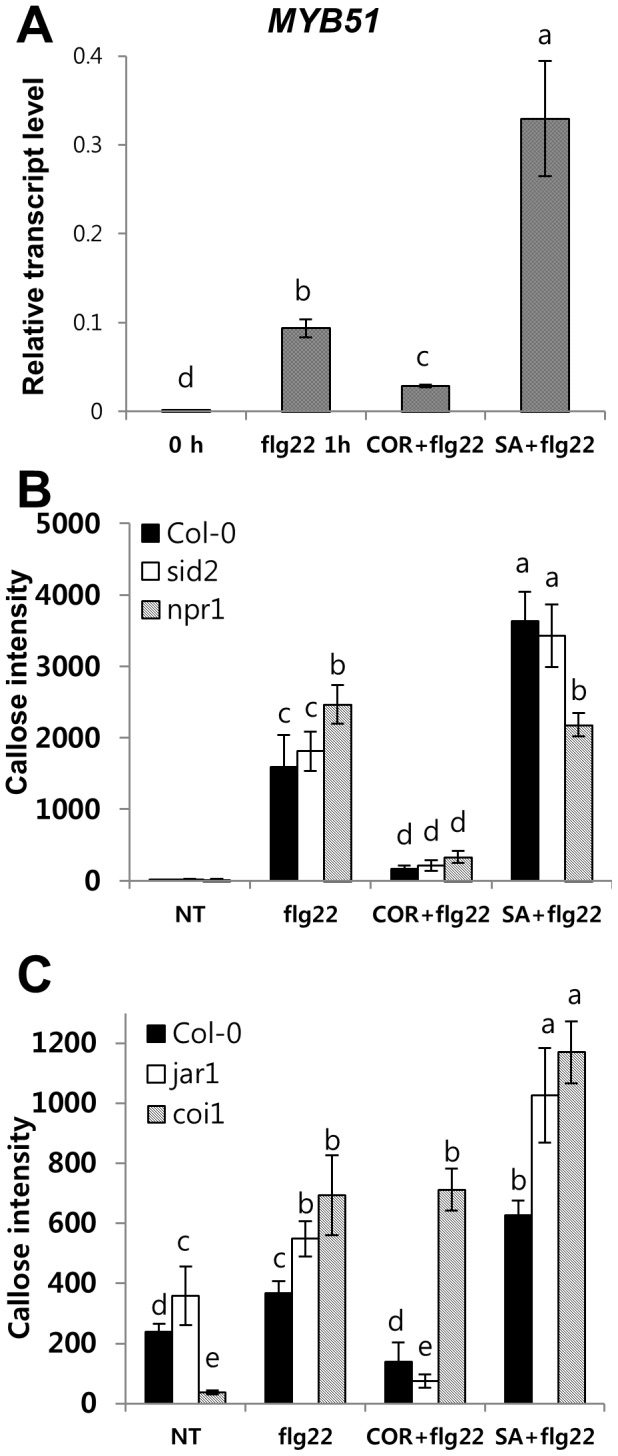
(A) MYB51 transcripts were measured in 8-day-old seedlings 1 h after treatment with 1 µM flg22. Data represent SD. All quantitative gene expression measurements were performed using technical triplicates and biological duplicates. (B–C) Eight-day-old seedlings were pre-incubated with SA (100 µM) or COR (0.5 µM) for 24 h, after which the seedlings treated with flg22 for 1 h were stained with aniline blue. Relative callose intensities were quantified as the number of fluorescent callose-corresponding pixels relative to the total number of pixels covering plant material. Values represent SE, n>6. Differential letter types indicated significant differences (α = 0.05) by one-way ANOVA and Tukey HSD test of comparisons between plant genotypes with individual treatment.
Discussion
Recent studies have shown that SA signaling is an integral part of the flg22 responses. Flg22 treatments caused SA accumulation in a SID2-dependent manner that caused gene expression changes and pathogen growth [23]. Resistance to Pst DC3000 induced by pre-treatment with flg22 was compromised in sid2 plants, demonstrating that flg22-induced SA is important for MAMP-triggered resistance [23], [52]. However, flg22-elicited bacterial resistance corresponds to a late flg22 response. Our study points to the impact of SA signaling at an early stage of the flg22 response, oxidative burst that may be involved in late flg22 response, callose deposition. Here, we found that cim6 and sid2 had altered FLS2 mRNA levels, which correlated with the level of the flg22-triggerd oxidative burst. SA signaling is also involved in the regulation of the early flg22 response genes, WRKY29 and FRK1 (Figs 5C and 5D). These findings demonstrated that SA signaling is required for not only a late flg22 response but also for early flg22 responses. Furthermore, we provide new evidence that NPR1 is involved in SA-dependent priming for enhancing the flg22-triggered oxidative burst and callose deposition (Figs 3B and 6B). SA signaling and COI1-dependent signaling are antagonistic to one another [53]. Similarly, in our system, COR had a negative effect on flg22 responses but only the flg22-triggered oxidative burst depended on SA-JA/COR signaling antagonism. COR suppressed flg22-induced ROS production in cim6 (Fig. 4A) while still reducing callose deposition in sid2 (Fig. 6B). This finding suggests that, in addition to antagonizing one another, they regulate flg22-induced responses independently.
SA signaling contributes to basal FLS2 mRNA accumulation
SID2, an essential gene for SA synthesis [25] was required for the flg22-triggered oxidative burst (Fig. 1). Because the flg22-triggered oxidative burst occurred within a few minutes of elicitation, we hypothesized that SA signaling components may modulate early flg22-responses, possibly by controlling FLS2 accumulation. To test this hypothesis, we measured the basal levels of the FLS2 transcript in sid2 and cim6 by qRT-PCR analysis. Interestingly, basal FLS2 mRNA was strongly enhanced in cim6 and suppressed in sid2, compared to the wild type (Fig. 5A). However, the effect of the SID2 mutation on FLS2 transcript accumulation was relatively weak when compared to that in the ein2 mutant (Figs 5A and 5B), suggesting that SA signaling components accompany other factors to regulate FLS2 mRNA accumulation. According to a recent report, ET signaling also contributes to FLS2 expression. EIN3 and EIN3-like transcription factors, which require EIN2 activity to accumulate, directly control FLS2 expression [54]. Our results confirm this previous report: suppressed expression of EIN2 in sid2 plants before flg22 treatment (Fig. S2). In the absence of flg22, the intact SID2 might be required for EIN2 transcript accumulation. Importantly, SID2 is not a classical transcription regulator and therefore, it is unlikely to regulate EIN2 or FLS2 gene expression directly. Further study of the mechanism of EIN2 transcript regulation in sid2, including the relationship between ethylene-signaling and EIN2 mRNA level, may reveal any SID2 function in FLS2 transcript regulation.
NPR1 plays a role in SA-mediated priming for enhancing flg22 responses
Establishment of systemic acquired resistance (SAR) requires a functional SA signaling pathway and is closely associated with systemic SA accumulation and systemic expression of a set of pathogenesis-related (PR) and other defense genes [55]. Priming is a phenomenon that enables cells to respond to much lower stimulus in a more rapid and robust manner than do nonprime cells [56], [57]. An example of priming comes from studies of parsley by Kauss and Jeblick, 1995 and Thulke and Conrath, 1998. Our data also showed that pretreatment with low doses of SA strongly enhanced the flg22-triggered oxidative burst, marker gene accumulation, and callose deposition in Arabidopsis seedlings.
The sid2 mutant plants exhibit diminished early flg22 responses while the npr1 mutant is not defective in flg22 responses (Figs 1, 3B and 6B). The npr1 mutant accumulates wild-type-like SA levels in response to avirulent pathogen inoculation. However, npr1 mutants are unable to express induced SAR [47], [58]. In this study, exogenous SA served as an flg22-signaling enhancer. The npr1 plants, however, did not show SA-dependent enhancement of the flg22-triggered oxidative burst or callose response (Figs 3B and 6B), indicating that NPR1 is involved in SA-mediated priming that enhanced flg22-induced responses (Fig. 3B). Consistent with our results, there are other reports that NPR1 plays a role in SA-mediated priming for enhanced defense responses [56], [59]. These potentiated responses suggest that the priming of defense responses is not solely confined to the SAR response. NPR1-mediated priming of defense responses also demonstrated in flg22 responses (Figs 3B and 6B).
Although the molecular basis of SA-mediated priming for enhancing flg22 responses is unclear, we hypothesize that SA pretreatment act at the post-translational level by protein modification. SA has been shown to control the nuclear translocation of NPR1 through cellular redox changes [60], [61]. NPR1 homeostasis is controlled by SA binding to NPR3/NPR4 in a concentration-dependent manner. In wild-type plants, low basal SA levels may bind to NPR4, thereby allowing some NPR1 to accumulate to confer basal resistance [62], [63]. Free stable NPR1 monomer might not be sufficient for the activation of the FLS2 downstream event that is required for the recognition of flg22 by FLS2. In our system, pre-incubation with 100 µM SA alone promoted FLS2 transcript regulation (Fig. 5B) while it did not trigger ROS production (data not shown). The enhanced level of FLS2 mRNA and free stable NPR1, possibly due to SA pretreatment, might contribute to accelerated FLS2-dependent flg22 responses.
How does COR signaling link the flg22-triggered responses?
Antagonism between SA and JA has been reported, mostly as SA inhibiting JA [64], although a few cases show an antagonistic relationship of JA on SA signaling. The higher SA content of the coi1 mutant, compared to the wild type, is one example of this relationship [65]. A recent report provides evidence that COR pretreatment suppresses SA accumulation through three NAC genes: ANAC019, ANAC055, and ANAC072. These NAC transcription factors exert this inhibitory effect by repressing SID2 (ICS1) and SA methyl transferase 1 (BSMT1) genes involved in SA biosynthesis and metabolism, respectively [35]. In this study, COR pretreatment suppressed the enhanced flg22-triggered oxidative burst (Fig. 4A) in Arabidopsis seedlings. Furthermore, three JA-signaling mutants, jar1, coi1, and fad7/fad8 were hypersensitive to flg22. The JA signaling mutants exhibit an almost three-fold increase in flg22-dependent ROS generation over the wild type. (Figs 1, S1, 3D). Based on this result, we suggest that SA signaling is required for canonical flg22-triggered ROS production and, therefore, COR-mediated suppression of the burst, representing one mechanism that underlies JA-SA antagonism.
Flg22-induced callose deposition is regulated by ROS [50], [51], miRNA signals generated by RNA interference regulatory protein Argonautel [66] and glucosinolate-derived metabolites [7]. Furthermore, SA is also involved in microbe-triggered callose deposition [7], [67], suggesting that there are multiple signaling pathways in flg22-induced callose formation. COR pretreatment inhibited flg22-induced callose deposition in both wild type and sid2 (Fig. 6B). These results suggest that COR may function downstream of SID2 or in an SA-independent pathway to suppress flg22 induced callose response. An observation similar to ours was made in Arabisopsis roots. PAMP-induced callose deposition, which does not require SA signaling, was suppressed by COR [68]. There are also recent reports that COR suppresses an SA-independent pathway and contributes to callose deposition by reducing the accumulation of an indole glucosinolate upstream of the activity of the penetration 2 (PEN2) myrosinase [69].
What is the role of flg22-triggered oxidative burst in late flg22 responses?
Although both Respiratory Burst Homolog proteins D and F (RbohD and RbohF) may regulate plant defense responses [70], RbohD alone was sufficient for the PAMP-triggered oxidative burst [51]. However the precise role of the flg22-triggered oxidative burst in FLS2 downstream events is unclear. Recently, Luna and associates (2011) proposed that flg22-induced callose deposition is controlled by RbohD-dependent H2O2 and that glucosinolate metabolites act downstream of RbohD-generated H2O2 in the regulation of flg22-induced callose deposition. The rbohD mutant is blocked in the flg22-induced callose response and flg22-induced H2O2 was also dramatically reduced in this mutant [71]. Because both the flg22-triggered oxidative burst and callose deposition are controlled by RbohD-dependent ROS [51], [71], it is probable that there is a relationship between the burst and callose response. To test whether alteration in the flg22-triggered oxidative burst is correlated with the abundance of flg22-induced callose, we measured flg22-induced callose deposition in SA-or COR-pretreated Arabidopsis cotyledons and assessed the correlation between ROS level and callose abundance. NPR1 regulates SA-induced priming for enhancing flg22-induced ROS, which correlated with enhancement of the flg22-induced callose response. Activated JA signaling by COR suppressed the flg22-triggered oxidative burst through COI1, which correlated with suppression of flg22-induced callose deposition. Based on these findings, we suggest that there is a relationship between the flg22-mediated oxidative burst and flg22-induced callose deposition. Interestingly, sid2 plants had a lower flg22-triggered oxidative burst than the wild type, although callose accumulation was unaltered, suggesting that NPR1 acts downstream of SID2 in the regulation of SA-mediated priming for enhanced flg22 responses. Although the molecular basis is currently unknown, an interaction between SA or JA signaling and the flg22-triggered oxidative burst seems to be required in regulation of callose deposition, a late flg22 effect. Further studies will be required to elucidate how the SA- or COR-mediated signaling acts in regulation of the flg22-triggered oxidative burst.
Materials and Methods
Plant growth conditions and chemical treatment
Arabidopsis thaliana (L.) Heynh lines used in this study were derived from the Columbia (Col) ecotype. These lines were cim6; CS6571, coi1, ein2; CS3071, eds5; CS3735, fad7fad8; CS8036, jar1; CS8072, npr1; CS3726, pad4; CS3806. The line sid2 was provided by Ken Shirasu [72]. Seeds of Arabidopsis were surface-sterilized using a gas sterilization method and planted in the wells of a 48-well microtiter plate. Each well contained MGRL nutrients [73] supplemented with 0.1% sucrose. After sealing the plates with surgical tape, they were placed at 4°C for two days to break dormancy and incubated in a 16-h light/8-h dark cycle at 22°C. Exogenous chemicals were applied at the following concentrations: 1 µM flg22 (Peptron, http://www.peptron.com), 0.05–5 µM COR (Sigma-Aldrich), 0.1–1 mM SA (Sigma-Aldrich) and 0.1–100 µM MeJA (Sigma-Aldrich).
Oxidative burst measurements
ROS were measured in eight-day-old seedlings. Seedlings were incubated in a 48-well microtiter plate containing 700 µL MGRL solution supplemented with 0.1% sucrose and 100 µM L-012 (a chemiluminescence probe; Wako, Japan). After 2 h incubation in 100 µM L-012 containing MGRL solution, 1 µM flg22 was added. A multi-label reader, VICTOR X3 (Perkin Elmer, USA), was used to verify the results we obtained from the L-012-derived chemiluminescence (CL; counts per second; cps) at 590-nm emission.
Quantitative real-time polymerase chain reaction analysis
Total RNA was isolated from the collected seedlings using RNaesy mini kit (Qiagen) according to the manufacturer's instruction. Approximately 1 µg DNA-free RNA was used for first-strand cDNA synthesis using the Moloney Murine Leukemia Virus (M-MuLV) reverse transcriptase for quantitative real-time polymerase chain reaction (qRT-PCR; Fermentas) according to the manufacturer's instruction. The qRT-PCR reactions were performed using a Thermal Cycler Dice Real Time System TP850 (TaKaRa, http://www.takara-bio.com) and SYBR Premix Ex Taq (TaKaRa). Primer sets (final concentration of 0.1 µM for each primer) were used for a final volume of 25 µL. The thermal profile of the qRT-PCR reactions was 10 min at 95°C, 40 cycles of 5 s at 95°C/20 s at 60°C. Subsequently, a dissociation curve was generated. All reactions were carried out in triplicate. Primers used for qRT-PCR are listed in the Supporting Information.
Aniline blue staining, microscopy analysis and callose quantification
Seedlings were collected, stored in 95% ethanol, and stained with aniline blue as described previously, with some modification [5]. Briefly, seedlings were incubated for at least 24 h in 95–100% ethanol until all tissues were transparent, washed in 0.07 M phosphate buffer (pH = 9), and incubated for 1–2 h in 0.07 M phosphate buffer containing 0.01% aniline blue (Sigma) prior to microscopic analysis. A minimum of eight cotyledons per condition per experiment were visualized under ultraviolet light with an epifluorescence microscope (Nikon AZ 100 M). Callose was selected manually, using the “magic wand” tool in Photoshop CS5. Callose-corresponding pixels and the number of depositions were recorded as the area covered by the total number of selected pixels and number of measurements, respectively, using the “record measurements” tool in Photoshop CS5. Average callose measurements were based on at least six photographs from different seedlings [71].
Supporting Information
Primers for qRT-PCR analysis.
(DOCX)
SA- and JA-signaling are required for the flg22-triggered oxidative burst. Flg22-induced ROS generation was monitored in liquid-grown intact seedlings of the indicated genotypes after treatment with 1 µM flg22. Error bars represent the SD from five independent samples (n = 10) and similar results were obtained in multiple independent experiments.
(TIF)
Down regulation of the ein2 gene in sid2 plants. For Quantitative RT-PCR analysis, 8-day-old seedlings were pre-treated with 100 µM of salicylic acid for 24 h and then incubated in 1 µM flg22 solution for 1 h. ACT2 [74] was used as a control. Data represent SD. All quantitative gene expression measurements were performed using technical triplicate and biological duplicates. Differential letter types indicated significant differences (α = 0.05) by one-way ANOVA and Tukey HSD test of comparisons between plant genotypes with individual treatment.
(TIF)
SA pretreatment reversed the suppressed flg22 response in sid2 mutants. For ROS measurement, 8-days-old seedlings were pretreated with 100 µM SA for 24 h and 1 µM flg22 was added at zero time. ACT2 was used as control. Error bars represent the SD of five independent samples (n = 10) and similar results were obtained in three independent experiments.
(TIF)
Effect of SA or COR pretreatment in flg22-induced MYB51 mRNA accumulation. Quantitative RT-PCR analysis of MYB51 gene expressions were measured in 8-day-old seedlings 1 h after treatment of 1 µM flg22. ACT2 was used as control. Data represent SD. All quantitative gene expression measurements were performed using technical triplicates and biological duplicates. Differential letter types indicated significant differences (α = 0.05) by one-way ANOVA and Tukey HSD test of comparisons between plant genotypes with individual treatment.
(TIF)
Effect of either SA or COR pretreatment on flg22-induced callose deposition. At 24 h post-treatment, cotyledons were stained with aniline blue. Fluorescence was observed with a NIKON AZ 100 M microscope. Representative images shown here came from eight leaves of eight independent plants, and similar results were obtained from two independent experiments.
(TIF)
Funding Statement
This work was supported by grants from Technology Development Program for Agriculture and Forestry, Ministry for Food, Agriculture, Forestry and Fisheries, Republic of Korea, the Next-Generation BioGreen 21 Program (PJ0082002011) of the Rural Development Administration of Korean, and the Korea Research Institute of Bioscience and Biotechnology (KRIBB) Research Initiative Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Jones JD, Dangl JL (2006) The plant immune system. Nature 444: 323–329. [DOI] [PubMed] [Google Scholar]
- 2. Felix G, Duran JD, Volko S, Boller T (1999) Plants have a sensitive perception system for the most conserved domain of bacterial flagellin. Plant J 18: 265–276. [DOI] [PubMed] [Google Scholar]
- 3. Gomez-Gomez L, Boller T (2002) Flagellin perception: a paradigm for innate immunity. Trends Plant Sci 7: 251–256. [DOI] [PubMed] [Google Scholar]
- 4. Gomez-Gomez L, Boller T (2000) FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol Cell 5: 1003–1011. [DOI] [PubMed] [Google Scholar]
- 5. Gomez-Gomez L, Felix G, Boller T (1999) A single locus determines sensitivity to bacterial flagellin in Arabidopsis thaliana. Plant J 18: 277–284. [DOI] [PubMed] [Google Scholar]
- 6. Boller T, Felix G (2009) A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu Rev Plant Biol 60: 379–406. [DOI] [PubMed] [Google Scholar]
- 7. Clay NK, Adio AM, Denoux C, Jander G, Ausubel FM (2009) Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323: 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim MG, da Cunha L, McFall AJ, Belkhadir Y, DebRoy S, et al. (2005) Two Pseudomonas syringae type III effectors inhibit RIN4-regulated basal defense in Arabidopsis. Cell 121: 749–759. [DOI] [PubMed] [Google Scholar]
- 9. Chisholm ST, Coaker G, Day B, Staskawicz BJ (2006) Host-microbe interactions: shaping the evolution of the plant immune response. Cell 124: 803–814. [DOI] [PubMed] [Google Scholar]
- 10. Kazan K, Manners JM (2008) Jasmonate signaling: toward an integrated view. Plant Physiol 146: 1459–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koornneef A, Pieterse CM (2008) Cross talk in defense signaling. Plant Physiol 146: 839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pieterse CM, Van der Does D, Zamioudis C, Leon-Reyes A, Van Wees SC (2012) Hormonal modulation of plant immunity. Annual review of cell and developmental biology 28: 489–521. [DOI] [PubMed] [Google Scholar]
- 13. Kessler A, Baldwin IT (2002) Plant responses to insect herbivory: the emerging molecular analysis. Annu Rev Plant Biol 53: 299–328. [DOI] [PubMed] [Google Scholar]
- 14. Thomma BP, Penninckx IA, Broekaert WF, Cammue BP (2001) The complexity of disease signaling in Arabidopsis. Curr Opin Immunol 13: 63–68. [DOI] [PubMed] [Google Scholar]
- 15. Lawton KA, Potter SL, Uknes S, Ryals J (1994) Acquired Resistance Signal Transduction in Arabidopsis Is Ethylene Independent. Plant Cell 6: 581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen H, Xue L, Chintamanani S, Germain H, Lin H, et al. (2009) ETHYLENE INSENSITIVE3 and ETHYLENE INSENSITIVE3-LIKE1 repress SALICYLIC ACID INDUCTION DEFICIENT2 expression to negatively regulate plant innate immunity in Arabidopsis. Plant Cell 21: 2527–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Spoel SH, Koornneef A, Claessens SM, Korzelius JP, Van Pelt JA, et al. (2003) NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 15: 760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leon-Reyes A, Van der Does D, De Lange ES, Delker C, Wasternack C, et al. (2010) Salicylate-mediated suppression of jasmonate-responsive gene expression in Arabidopsis is targeted downstream of the jasmonate biosynthesis pathway. Planta 232: 1423–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dong X (2004) NPR1, all things considered. Curr Opin Plant Biol 7: 547–552. [DOI] [PubMed] [Google Scholar]
- 20. Robert-Seilaniantz A, Navarro L, Bari R, Jones JD (2007) Pathological hormone imbalances. Curr Opin Plant Biol 10: 372–379. [DOI] [PubMed] [Google Scholar]
- 21. Laurie-Berry N, Joardar V, Street IH, Kunkel BN (2006) The Arabidopsis thaliana JASMONATE INSENSITIVE 1 gene is required for suppression of salicylic acid-dependent defenses during infection by Pseudomonas syringae. Mol Plant Microbe Interact 19: 789–800. [DOI] [PubMed] [Google Scholar]
- 22. Mishina TE, Zeier J (2007) Pathogen-associated molecular pattern recognition rather than development of tissue necrosis contributes to bacterial induction of systemic acquired resistance in Arabidopsis. Plant J 50: 500–513. [DOI] [PubMed] [Google Scholar]
- 23. Tsuda K, Sato M, Glazebrook J, Cohen JD, Katagiri F (2008) Interplay between MAMP-triggered and SA-mediated defense responses. Plant J 53: 763–775. [DOI] [PubMed] [Google Scholar]
- 24. Strawn MA, Marr SK, Inoue K, Inada N, Zubieta C, et al. (2007) Arabidopsis isochorismate synthase functional in pathogen-induced salicylate biosynthesis exhibits properties consistent with a role in diverse stress responses. J Biol Chem 282: 5919–5933. [DOI] [PubMed] [Google Scholar]
- 25. Wildermuth MC, Dewdney J, Wu G, Ausubel FM (2001) Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414: 562–565. [DOI] [PubMed] [Google Scholar]
- 26. Doares SH, Syrovets T, Weiler EW, Ryan CA (1995) Oligogalacturonides and chitosan activate plant defensive genes through the octadecanoid pathway. Proc Natl Acad Sci U S A 92: 4095–4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kunze G, Zipfel C, Robatzek S, Niehaus K, Boller T, et al. (2004) The N terminus of bacterial elongation factor Tu elicits innate immunity in Arabidopsis plants. Plant Cell 16: 3496–3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Simpson SD, Ashford DA, Harvey DJ, Bowles DJ (1998) Short chain oligogalacturonides induce ethylene production and expression of the gene encoding aminocyclopropane 1-carboxylic acid oxidase in tomato plants. Glycobiology 8: 579–583. [DOI] [PubMed] [Google Scholar]
- 29. Moscatiello R, Mariani P, Sanders D, Maathuis FJ (2006) Transcriptional analysis of calcium-dependent and calcium-independent signalling pathways induced by oligogalacturonides. J Exp Bot 57: 2847–2865. [DOI] [PubMed] [Google Scholar]
- 30. Pieterse CM, Van Loon LC (2004) NPR1: the spider in the web of induced resistance signaling pathways. Curr Opin Plant Biol 7: 456–464. [DOI] [PubMed] [Google Scholar]
- 31. Thines B, Katsir L, Melotto M, Niu Y, Mandaokar A, et al. (2007) JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448: 661–665. [DOI] [PubMed] [Google Scholar]
- 32. Felton GW, Korth KL, Bi JL, Wesley SV, Huhman DV, et al. (1999) Inverse relationship between systemic resistance of plants to microorganisms and to insect herbivory. Curr Biol 9: 317–320. [DOI] [PubMed] [Google Scholar]
- 33. Kloek AP, Verbsky ML, Sharma SB, Schoelz JE, Vogel J, et al. (2001) Resistance to Pseudomonas syringae conferred by an Arabidopsis thaliana coronatine-insensitive (coi1) mutation occurs through two distinct mechanisms. Plant J 26: 509–522. [DOI] [PubMed] [Google Scholar]
- 34. Melotto M, Underwood W, Koczan J, Nomura K, He SY (2006) Plant stomata function in innate immunity against bacterial invasion. Cell 126: 969–980. [DOI] [PubMed] [Google Scholar]
- 35. Zheng XY, Spivey NW, Zeng W, Liu PP, Fu ZQ, et al. (2012) Coronatine Promotes Pseudomonas syringae Virulence in Plants by Activating a Signaling Cascade that Inhibits Salicylic Acid Accumulation. Cell host & microbe 11: 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mersmann S, Bourdais G, Rietz S, Robatzek S (2010) Ethylene signaling regulates accumulation of the FLS2 receptor and is required for the oxidative burst contributing to plant immunity. Plant Physiol 154: 391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Maleck K, Neuenschwander U, Cade RM, Dietrich RA, Dangl JL, et al. (2002) Isolation and characterization of broad-spectrum disease-resistant Arabidopsis mutants. Genetics 160: 1661–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nawrath C, Metraux JP (1999) Salicylic acid induction-deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 11: 1393–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rogers EE, Ausubel FM (1997) Arabidopsis enhanced disease susceptibility mutants exhibit enhanced susceptibility to several bacterial pathogens and alterations in PR-1 gene expression. Plant Cell 9: 305–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McConn M, Browse J (1996) The Critical Requirement for Linolenic Acid Is Pollen Development, Not Photosynthesis, in an Arabidopsis Mutant. Plant Cell 8: 403–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Staswick PE, Tiryaki I (2004) The oxylipin signal jasmonic acid is activated by an enzyme that conjugates it to isoleucine in Arabidopsis. Plant Cell 16: 2117–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brooks DM, Bender CL, Kunkel BN (2005) The Pseudomonas syringae phytotoxin coronatine promotes virulence by overcoming salicylic acid-dependent defences in Arabidopsis thaliana. Mol Plant Pathol 6: 629–639. [DOI] [PubMed] [Google Scholar]
- 43. Kauss H, Jeblick W (1995) Pretreatment of Parsley Suspension Cultures with Salicylic Acid Enhances Spontaneous and Elicited Production of H2O2. Plant Physiol 108: 1171–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yan J, Zhang C, Gu M, Bai Z, Zhang W, et al. (2009) The Arabidopsis CORONATINE INSENSITIVE1 protein is a jasmonate receptor. Plant Cell 21: 2220–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Katsir L, Schilmiller AL, Staswick PE, He SY, Howe GA (2008) COI1 is a critical component of a receptor for jasmonate and the bacterial virulence factor coronatine. Proc Natl Acad Sci U S A 105: 7100–7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhou N, Tootle TL, Tsui F, Klessig DF, Glazebrook J (1998) PAD4 functions upstream from salicylic acid to control defense responses in Arabidopsis. Plant Cell 10: 1021–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cao H, Bowling SA, Gordon AS, Dong X (1994) Characterization of an Arabidopsis Mutant That Is Nonresponsive to Inducers of Systemic Acquired Resistance. Plant Cell 6: 1583–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Asai T, Tena G, Plotnikova J, Willmann MR, Chiu WL, et al. (2002) MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415: 977–983. [DOI] [PubMed] [Google Scholar]
- 49. Bednarek P, Pislewska-Bednarek M, Svatos A, Schneider B, Doubsky J, et al. (2009) A glucosinolate metabolism pathway in living plant cells mediates broad-spectrum antifungal defense. Science 323: 101–106. [DOI] [PubMed] [Google Scholar]
- 50. Galletti R, Denoux C, Gambetta S, Dewdney J, Ausubel FM, et al. (2008) The AtrbohD-mediated oxidative burst elicited by oligogalacturonides in Arabidopsis is dispensable for the activation of defense responses effective against Botrytis cinerea. Plant Physiol 148: 1695–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhang J, Shao F, Li Y, Cui H, Chen L, et al. (2007) A Pseudomonas syringae effector inactivates MAPKs to suppress PAMP-induced immunity in plants. Cell host & microbe 1: 175–185. [DOI] [PubMed] [Google Scholar]
- 52. Tsuda K, Sato M, Stoddard T, Glazebrook J, Katagiri F (2009) Network properties of robust immunity in plants. PLoS Genet 5: e1000772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Spoel SH, Dong X (2008) Making sense of hormone crosstalk during plant immune responses. Cell Host Microbe 3: 348–351. [DOI] [PubMed] [Google Scholar]
- 54. Boutrot F, Segonzac C, Chang KN, Qiao H, Ecker JR, et al. (2010) Direct transcriptional control of the Arabidopsis immune receptor FLS2 by the ethylene-dependent transcription factors EIN3 and EIL1. Proc Natl Acad Sci U S A 107: 14502–14507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Durrant WE, Dong X (2004) Systemic acquired resistance. Annu Rev Phytopathol 42: 185–209. [DOI] [PubMed] [Google Scholar]
- 56. Conrath U, Pieterse CM, Mauch-Mani B (2002) Priming in plant-pathogen interactions. Trends Plant Sci 7: 210–216. [DOI] [PubMed] [Google Scholar]
- 57. Conrath U (2011) Molecular aspects of defence priming. Trends Plant Sci 16: 524–531. [DOI] [PubMed] [Google Scholar]
- 58. Delaney TP, Friedrich L, Ryals JA (1995) Arabidopsis signal transduction mutant defective in chemically and biologically induced disease resistance. Proc Natl Acad Sci U S A 92: 6602–6606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kohler A, Schwindling S, Conrath U (2002) Benzothiadiazole-induced priming for potentiated responses to pathogen infection, wounding, and infiltration of water into leaves requires the NPR1/NIM1 gene in Arabidopsis. Plant Physiol 128: 1046–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Spoel SH, Dong X (2012) How do plants achieve immunity? Defence without specialized immune cells. Nature reviews Immunology 12: 89–100. [DOI] [PubMed] [Google Scholar]
- 61. Wu Y, Zhang D, Chu JY, Boyle P, Wang Y, et al. (2012) The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell reports 1: 639–647. [DOI] [PubMed] [Google Scholar]
- 62. Moreau M, Tian M, Klessig DF (2012) Salicylic acid binds NPR3 and NPR4 to regulate NPR1-dependent defense responses. Cell research 22: 1631–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fu ZQ, Yan S, Saleh A, Wang W, Ruble J, et al. (2012) NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 486: 228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bostock RM (2005) Signal crosstalk and induced resistance: straddling the line between cost and benefit. Annu Rev Phytopathol 43: 545–580. [DOI] [PubMed] [Google Scholar]
- 65. Li J, Brader G, Palva ET (2004) The WRKY70 transcription factor: a node of convergence for jasmonate-mediated and salicylate-mediated signals in plant defense. Plant Cell 16: 319–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Li Y, Zhang Q, Zhang J, Wu L, Qi Y, et al. (2010) Identification of microRNAs involved in pathogen-associated molecular pattern-triggered plant innate immunity. Plant Physiol 152: 2222–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. DebRoy S, Thilmony R, Kwack YB, Nomura K, He SY (2004) A family of conserved bacterial effectors inhibits salicylic acid-mediated basal immunity and promotes disease necrosis in plants. Proc Natl Acad Sci U S A 101: 9927–9932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Millet YA, Danna CH, Clay NK, Songnuan W, Simon MD, et al. (2010) Innate immune responses activated in Arabidopsis roots by microbe-associated molecular patterns. Plant Cell 22: 973–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Geng X, Cheng J, Gangadharan A, Mackey D (2012) The Coronatine Toxin of Pseudomonas syringae Is a Multifunctional Suppressor of Arabidopsis Defense. The Plant cell 24: 4763–4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Torres MA, Dangl JL, Jones JD (2002) Arabidopsis gp91phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci U S A 99: 517–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Luna E, Pastor V, Robert J, Flors V, Mauch-Mani B, et al. (2011) Callose deposition: a multifaceted plant defense response. Mol Plant Microbe Interact 24: 183–193. [DOI] [PubMed] [Google Scholar]
- 72. Yoshimoto K, Jikumaru Y, Kamiya Y, Kusano M, Consonni C, et al. (2009) Autophagy negatively regulates cell death by controlling NPR1-dependent salicylic acid signaling during senescence and the innate immune response in Arabidopsis. Plant Cell 21: 2914–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Fujiwara T, Hirai MY, Chino M, Komeda Y, Naito S (1992) Effects of sulfur nutrition on expression of the soybean seed storage protein genes in transgenic petunia. Plant Physiol 99: 263–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Qiu JL, Fiil BK, Petersen K, Nielsen HB, Botanga CJ, et al. (2008) Arabidopsis MAP kinase 4 regulates gene expression through transcription factor release in the nucleus. EMBO J 27: 2214–2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers for qRT-PCR analysis.
(DOCX)
SA- and JA-signaling are required for the flg22-triggered oxidative burst. Flg22-induced ROS generation was monitored in liquid-grown intact seedlings of the indicated genotypes after treatment with 1 µM flg22. Error bars represent the SD from five independent samples (n = 10) and similar results were obtained in multiple independent experiments.
(TIF)
Down regulation of the ein2 gene in sid2 plants. For Quantitative RT-PCR analysis, 8-day-old seedlings were pre-treated with 100 µM of salicylic acid for 24 h and then incubated in 1 µM flg22 solution for 1 h. ACT2 [74] was used as a control. Data represent SD. All quantitative gene expression measurements were performed using technical triplicate and biological duplicates. Differential letter types indicated significant differences (α = 0.05) by one-way ANOVA and Tukey HSD test of comparisons between plant genotypes with individual treatment.
(TIF)
SA pretreatment reversed the suppressed flg22 response in sid2 mutants. For ROS measurement, 8-days-old seedlings were pretreated with 100 µM SA for 24 h and 1 µM flg22 was added at zero time. ACT2 was used as control. Error bars represent the SD of five independent samples (n = 10) and similar results were obtained in three independent experiments.
(TIF)
Effect of SA or COR pretreatment in flg22-induced MYB51 mRNA accumulation. Quantitative RT-PCR analysis of MYB51 gene expressions were measured in 8-day-old seedlings 1 h after treatment of 1 µM flg22. ACT2 was used as control. Data represent SD. All quantitative gene expression measurements were performed using technical triplicates and biological duplicates. Differential letter types indicated significant differences (α = 0.05) by one-way ANOVA and Tukey HSD test of comparisons between plant genotypes with individual treatment.
(TIF)
Effect of either SA or COR pretreatment on flg22-induced callose deposition. At 24 h post-treatment, cotyledons were stained with aniline blue. Fluorescence was observed with a NIKON AZ 100 M microscope. Representative images shown here came from eight leaves of eight independent plants, and similar results were obtained from two independent experiments.
(TIF)