Abstract
Introduction
Genetic modification, or transgenesis, is a powerful technique to investigate the molecular interactions between vector-borne pathogens and their arthropod hosts, as well as a potential novel approach for vector-borne disease control. Transgenesis requires the use of specific regulatory regions, or promoters, to drive expression of genes of interest in desired target tissues. In mosquitoes, the vast majority of described promoters are from Anopheles and Aedes mosquitoes.
Results
Culex tarsalis is one of the most important vectors of arboviruses (including West Nile virus) in North America, yet it has not been the subject of molecular genetic study. In order to facilitate molecular genetic work in this important vector species, we isolated four fat body-specific promoter sequences located upstream of the Cx. tarsalis vitellogenin genes (Vg1a, Vg1b, Vg2a and Vg2b). Sequences were analyzed in silico to identify requisite cis-acting elements. The ability for promoter sequences to drive expression of green fluorescent protein (GFP) in vivo was investigated using transgenic Drosophila melanogaster. All four promoters were able to drive GFP expression but there was dramatic variation between promoters and between individual Drosophila lines, indicating significant position effects. The highest expression was observed in line Vg2bL3, which was >300-fold higher than the lowest line Vg1aL2.
Conclusions
These new promoters will be useful for driving expression of genes of interest in transgenic Cx. tarsalis and perhaps other insects.
Introduction
Some genetic modification-mediated approaches for control of mosquito-borne diseases are based on deploying transgenic mosquitoes expressing genes that will affect mosquito vectorial capacity [1]. An essential step in this process is to identify suitable stage- and tissue-specific promoters to drive expression of anti-pathogen genes of interest, work on which has been primarily focused on midgut, salivary gland and fatbody promoters of Anopheles and Aedes mosquitoes. The promoter regions of the Anopheles gambiae carboxypeptidase (AgCP) and Aedes aegypti carboxypeptidase (AeCP) genes have been isolated and shown to drive midgut-specific bloodmeal-inducible gene expression [2]. The regulatory regions of the Ae. aegypti salivary gland specific maltase-like I (MalI) and apyrase (Apy) genes were shown to direct tissue-specific expression of a luciferase reporter gene in transgenic Ae. aegypti [3]. The promoter fragments of An. gambiae female salivary gland-specific genes AgApy and D7r4 directed expression of a LacZ reporter gene in the An. stephensi salivary gland [4]. A female salivary gland-specific promoter for a gene encoding anopheline antiplatelet protein (AAPP) isolated from An. stephensi was able to drive expression of DsRed in the salivary glands [5]. Several fatbody specific promoters have also been reported. The Ae. aegypti vitellogenin gene promoter was successfully used for fatbody expression and secretion of defensin into the hemolymph [1], [6]. A 1.7 kb promoter fragment of An. gambiae vitellogenin gene (VgT2) was able to direct expression of green fluorescent protein (GFP) in a tissue-, stage-, and sex-specific manner in transgenic An. stephensi [7].
By using transgenic Drosophila, some regulatory regions isolated from non-Drosophila insects (ranging from flies to lepidopterans) have been confirmed to be functional in Drosophila, providing evidence for transcriptional regulation similarities between Drosophila and non-Drosophila insects. The regulatory elements in promoter regions of the midgut-specific carboxypeptidase gene in Simulium (blackflies), and trypsin genes in An. gambiae were recognized and able to drive expression of reporter genes in a gut-specific manner in Drosophila [8]–[9]. The promoter regions of the apyrase and D7-related genes expressed in An. gambiae salivary glands were able to drive tissue-specific reporter gene expression in transgenic Drosophila [4], [10]. A regulatory region of the fatbody specific vitellogenin gene could direct the expression of the reporter in a correct stage- and tissue-specific manner in Drosophila similar to the endogenous Drosophila yolk protein genes [6]. A portion of the 5′-flanking region of the female-specific hexamerin gene, Hex-1.2, from the mosquito Ochlerotatus atropalpus drove expression of the luciferase reporter gene in the D. melanogaster fatbody [11]. Even in more divergent lepidopterans, some gene regulatory regions are also recognized by Drosophila [12]–[13]. Therefore, Drosophila is a useful system for characterizing the transcriptional regulation of genes from non-Drosophila insects. Moreover, Drosophila transformation is simpler than mosquito transformation and is especially useful to study regulatory regions from insect species for which transformation has not been achieved.
Culex tarsalis is one of the most important vectors of arboviruses in North America. It has been associated with or is capable of transmission of West Nile Virus [14]–[18], Western Equine Encephalitis virus [19]–[21], St. Louis Encephalitis virus [20]–[21], Japanese Encephalitis virus, Venezuelan Equine Encephalitis virus [22] and Rift valley fever virus [23]. To our knowledge, no tissue-specific promoters have been isolated or tested from this significant vector species. Previously we isolated four vitellogenin genes (Vg1a, Vg1b, Vg2a and Vg2b) from Cx. tarsalis [24]–[25]. In this study we cloned, bioinformatically and functionally characterized the four vitellogenin gene promoters from Cx. tarsalis. These putative promoter sequences were used to drive a reporter gene (GFP) in the fat bodies of transgenic D. melanogaster. All 4 sequences were functional in Drosophila. There was dramatic variation between promoters and between individual Drosophila lines, indicating significant position effects. These promoter sequences will be useful for future molecular genetic studies in this important mosquito species.
Results
Cloning and in silico analysis of the 5′ putative regulatory regions of four vitellogenin genes from Cx. Tarsalis
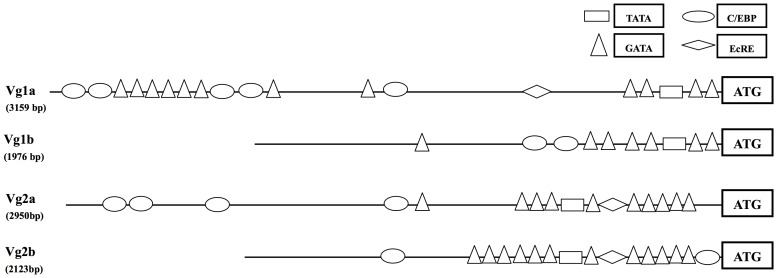
The transcription factor binding sites (GATA, C/EBP, EcRE) which are required for tissue- and stage-specific vitellogenin expression in mosquito vitellogenin genes are usually located approximately 2 kb 5′ upstream of the initial vitellogenin transcriptional start site [1]. Four vitellogenin genes from Cx. tarsalis were isolated previously [24]–[25]. In this study, we cloned the putative 5′ regulatory regions for the four distinct vitellogenin genes using a genome walking approach [24]. Isolated sequences were 3159-bp, 1976-bp, 2950-bp and 2133-bp for Vg1a, Vg1b, Vg2a and Vg2b, respectively. Sequences were deposited into GenBank under accession numbers KF271781 - KF271784. Analysis showed that the sequences of the two Vg1 promoters were highly conserved in the region from translation start codon “ATG” to 123-bp upstream, then increased in divergence. Likewise, sequences of the two Vg2 promoters were highly-conserved in the region from “ATG” to 166-bp upstream, then increased in divergence. Sequences between the Vg1 and Vg2 regulatory regions were highly divergent.
Despite this divergence, rtPCR indicated that all four vitellogenin promoters had similar expression profiles and were active both pre- and post-bloodmeal [25], suggesting that they may share similar cis-regulatory elements. We analyzed these sequences to identify putative responsive elements. Even with highly divergence sequences, most of the transcription factor binding sites (GATA, TATA, C/EBP, EcRE), which are required for tissue- and stage- specific vitellogenin expression in the Ae. aegypti Vg1 gene could be found in all four Cx. tarsalis vitellogenin promoters. Differences between each vitellogenin promoter were also identified (Figure 1).
Figure 1. Schematic illustration of putative of regulatory elements in the promoter regions of the four Culex tarsalis vitellogenin genes.
Activity analysis of four vitellogenin promoters in transgenic D. melanogaster
Germline transformation of Cx. tarsalis has not yet been achieved, so we used transgenic D. melanogaster to investigate the function of the Cx. tarsalis vitellogenin promoters in vivo. We designed 4 piggyBac constructs that each had the general organization: pBac[3XP3-DsRed-CxVg-promoter-EGFP] and generated transgenic Drosophila by embryonic microinjection with pBac helper plasmid. Three lines for each promoter were randomly selected for reporter gene expression analysis.
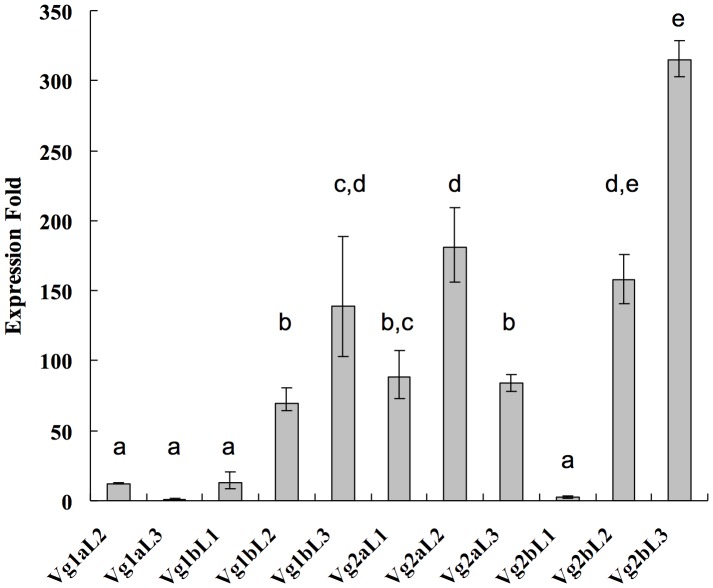
To monitor the expression level of GFP gene, RT-PCR and qRT-PCR analysis were employed. The results showed that GFP expression in D. melanogaster fat bodies was observed when driven by all Cx. tarsalis promoters, but expression levels were highly variable between different vitellogenin promoters and between different independent fly lines of the same promoter (Figures 2 and 3). In general, GFP expression driven by Vg2a and Vg2b promoters was higher than when driven by Vg1a and Vg1b promoters. The highest expression was observed in line Vg2bL3, which had 315-fold higher GFP expression compared to line Vg1aL3, the line with the lowest detectable GFP expression (no expression was detectable in line Vg1aL1). These results show that different lines transformed with the same promoter sequence had highly variable reporter gene expression, indicating position effects due to the integration site of the transposon in the Drosophila genome.
Figure 2. Expression of GFP gene in transgenic Drosophila by RT-PCR.

Three independent lines for each promoter were assayed. The constitutive ribosomal protein 49 gene (rp49) in D. melanogaster was examined as an endogenous control. N = negative control.
Figure 3. Variation in reporter gene (GFP) expression among transgenic Drosophila melanogaster lines.
Relative expression levels of GFP were normalized to the expression of ribosomal protein 49 (rp49). Expression folds are calculated by comparison to line Vg1aL3, which had the lowest detectable GFP expression. Error bars represent standard errors. Letters represent statistical differences.
Discussion
We isolated four putative promoter regions of vitellogenin genes from Cx. tarsalis and their function was analyzed using transgenic D. melanogaster. All four promoter sequences were able to drive reporter gene expression in Drosophila fat bodies, suggesting that these regulatory regions contain sufficient cis-acting elements for the correct expression of the gene. These analyses also demonstrated a conservation of regulatory element function between D. melanogaster and Cx. tarsalis, two distantly related species.
Our results suggest that position effects occur due to the site of integration of the transposon in the Drosophila genome. Position effects are very common and can result from novel enhancer/silencer-promoter interactions [26]–[30]. In Drosophila, positive position effects have been detected by inserting a transgene with a weak promoter near an enhancer element where high-level expression detected is due to the effect of the enhancer. In other instances, transcription is downregulated due to the negative action of neighboring silencing elements silencer.
The lowest activity was detected in Drosophila lines containing Vg1a promoter constructs, although this promoter contains the longest 5′ flanking fragment size (3.1 kb). It is possible that all three lines we used in the study had strong negative position effects due to (1) silencers near their insertion sites, although this regulatory region contains sufficient cis-elements for expression, (2) the cis-regulation elements controlling the expression of the vitellogenin gene may be located in 3′ regulatory region that is not included in the construct, or (3) the sequence we identified may contain repressor elements. Deletion analysis will help to clarify this in future studies.
To date, molecular genetic studies in Cx. tarsalis have been lacking compared to other mosquito species. These 4 identified promoter sequences will be useful for transgenic studies in this important vector insect.
Materials and Methods
Ethics statement
Mosquitoes were bloodfed on commercially obtained chicken blood using a membrane feeder.
Mosquitoes
Cx. tarsalis used in this study were from the KNWR colony, which was collected in the Kern National Wildlife Refuge (KNWR) in Kern County, California [25]. Larvae were reared at a standard density of 200 larvae/pan and fed a 1∶2∶2 blend of fish food, rabbit pellets and bovine liver extract. Adults were maintained on 10% sucrose solution at 27±1°C and 80±5% relative humidity with a 14 h/10 h light/dark cycle.
Genomic DNA extraction and partial coding sequence isolation
Genomic DNA was isolated from female adults using the procedure previously described [24]. Based on the available An. gambiae, Ae. aegypti and Cx. pipiens vitellogenin sequences from GenBank, primers were designed to PCR-amplify the partial coding sequence at the 5′ end of the genes (Table 1). PCR products were cloned using the TOPO TA cloning kit for sequencing (Invitrogen).
Table 1. PCR primers list.
| Gene | Forward Primer(5′->3′) | Reverse Primer(5′->3′) | Amplicon Size (bp) |
| Vg1a promoter fragment | AGCTGGCCGGCCAGACAACTAAGACGTCCATG | GTTGATCGTTGAGGCAGGCAG | 3159 |
| Vg1b promoter fragment | CTGGCCGGCCATCCTCGTAACTCCTACCAC | GTTGATCGTTGAGGCAGGCAG | 1976 |
| Vg2a promoter fragment | AGCTGGCCGGCCGATGTGCAGCCATCCCCTTAG | CACGATCACTCGAGCTTTTTTCTTCAC | 2950 |
| Vg2b promoter fragment | AGCTGGCCGGCCGATGTTTTGCCTAAGACCTTTTG | CACGATCACTCGAGCTTTTTTCTTCAC | 2133 |
| Vg1ap+GFP+BGHR | AGCTGGCCGGCCAGACAACTAAGACGTCCATG | AGCTGGCCGGCC CCTAGAGCCCCAGCTGGTTC | 4197 |
| Vg1bp+GFP+BGHR | CTGGCCGGCCATCCTCGTAACTCCTACCAC | AGCTGGCCGGCC CCTAGAGCCCCAGCTGGTTC | 3047 |
| Vg2ap+GFP+BGHR | AGCTGGCCGGCCGATGTGCAGCCATCCCCTTAG | AGCTGGCCGGCC CCTAGAGCCCCAGCTGGTTC | 4021 |
| Vg2bp+GFP+BGHR | AGCTGGCCGGCCGATGTTTTGCCTAAGACCTTTTG | AGCTGGCCGGCC CCTAGAGCCCCAGCTGGTTC | 3204 |
| GFP (for RT-PCR) | AGGTGATGCTACATACGGAAAG | CATGCCATGTGTAATCCCAG | 598 |
| GFP (for qPCR) | TACAAGACGCGTGCTGAAGT | CAATGTTGTGGCGAATTTTG | 199 |
| rp49 | ATCGGTTACGGATCGAACAA | GACAATCTCCTTGCGCTTCT | 165 |
Genomic DNA extraction, 5′ promoter region isolation and bioinformatics analysis of putative transcriptional factors binding site
Isolation of the 5′-flanking regions of vitellogenin promoter was performed by genome walking as previously described [24]. Sequence analyses were performed using BLAST (http://www.ncbi.nlm.nih.gov/blast/) and protein sequence alignments conducted using Vector NTI Advance 10 (Invitrogen). Putative transcription factor binding sites were identified using the web-based Conreal server (http://conreal.niob.knaw.nl/).
Plasmid construction and transgenic Drosophila melanogaster
For each vitellogenin gene, a pair of primers was designed to amplify the region 2–3 kb upstream from the start codon (Table 1). PCR reactions proceeded with incubation at 94°C for 2 min, followed by 35 cycles consisting of 94°C for 30 s, 58°C for 30 s and 72°C for 2 min.
Each amplified putative promoter fragment was cloned upstream of the GFP coding sequence in pGlow (Invitrogen). Each promoter, together with GFP and the BGH polyadenylation sequence from pGlow was PCR-amplified and digested using Asc I and Fse I (for Vg1 promoters) or Fse I (for Vg2 promoters) and inserted into the Fse I site in pBac[3XP3-DsRed] to generate four constructs which each had the general organization: pBac[3XP3-DsRed-CxVg-promoter-GFP-BGH]. Each construct was injected into D. melanogaster embryos (w 1118) with the helper plasmid phsp-pBac [31]. Transgenic D. melanogaster were identified by red eye fluorescence using a Leica MZ95 microscope with DsRed filter (exciter HQ545/30x; emitter HQ620/60m) and lines established.
RT-PCR gel analysis of GFP transcripts in transgenic D. melanogaster
Drosophila fatbodies were dissected from adult female transgenic flies and total RNA extracted using the SV Total RNA Isolation system (Promega). 10–15 transgenic female adults (3 to 5 days old) for each sample were processed for RNA extraction. To remove genomic DNA contamination, the DNA-free™ kit (Ambion) was employed following the manufacturer's protocol. Two micrograms of total RNA was used as template for first strand cDNA synthesis using M-MLV Reverse Transcriptase (Promega). A pair of GFP specific primers (Table 1) was used for PCR. The PCR reaction was 2 min at 94°C, followed by 35 cycles consisting of 94°C for 30 s, 50°C for 30 s, and 72°C for 4 min 30 sec, and a final 72°C extension for 10 min. No-RT controls were included with every reaction.
Quantitative RT-PCR assays
To quantify expression levels of GFP driven by Cx. tarsalis vitellogenin promoters in transgenic D. melanogaster, we conducted quantitative real-time PCR using GFP-specific and the rp49-specific (single-copy nuclear gene) primer sets (Table 1). Quantitative PCR (qPCR) was performed using the Rotor-Gene Q Real-Time PCR System (QIAGEN) using the Rotor-Gene SYBR Green PCR Kit (QIAGEN). qPCR for both GFP and rp49 proceeded with incubation at 95°C for 5 min, followed by 40 PCR cycles consisting of 95°C denaturation for 5 s, 60°C annealing for 5 s and 72°C extension for 5 s and a final melting curve analysis of the PCR product to verify the specificity and identity, with ramp from 60°C to 95°C, rising by 1°C each step and 90 s for the pre-melt conditioning on first step, 5 s for each step afterwards. We tested 5 samples per treatment and each sample was replicated 3 times. Threshold cycle (Ct) values of the target gene GFP and rp49 were calculated for each replicate. Expression folds of GFP between different constructs of vitellogenin promoters or lines were calculated using the comparative CT method of relative quantization [32]. No-RT controls were included with every reaction. Expression values between lines were statistically compared by Kruskal-Wallis test with the Conover-Inman method for multiple comparisons using StatsDirect software (statsdirect.com).
Acknowledgments
We thank Dr. Vladimir Kokoza for helpful comments on an earlier draft of this manuscript, and BestGene Inc. for assistance with generating transgenic Drosophila lines.
Funding Statement
This research was supported by NIH grant R01AI067371 to JLR. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kokoza V, Ahmed A, Cho WL, Jasinskiene N, James AA, et al. (2000) Engineering blood meal-activated systemic immunity in the yellow fever mosquito, Aedes aegypti . Proc Natl Acad Sci USA 97: 9144–9149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moreira LA, Edwards MJ, Adhami F, Jasinskiene N, James AA, et al. (2000) Robust gut-specific gene expression in transgenic Aedes aegypti mosquitoes. Proc Natl Acad Sci USA 97: 10895–10898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coates CJ, Jasinskiene N, Pott GB, James AA (1999) Promoter-directed expression of recombinant fire-fly luciferase in the salivary glands of Hermes-transformed Aedes aegypti. Gene. 226: 317–325. [DOI] [PubMed] [Google Scholar]
- 4. Lombardo F, Nolan T, Lycett G, Lanfrancotti A, Stich N, et al. (2005) An Anopheles gambiae salivary gland promoter analysis in Drosophila melanogaster and Anopheles stephensi . Insect Mol Biol 14: 207–216. [DOI] [PubMed] [Google Scholar]
- 5. Yoshida S, Watanabe H (2006) Robust salivary gland-specific transgene expression in Anopheles stephensi mosquito. Insect Mol Biol 15: 403–410. [DOI] [PubMed] [Google Scholar]
- 6. Kokoza VA, Martin D, Mienaltowski MJ, Ahmed A, Morton CM, et al. (2001) Transcriptional regulation of the mosquito vitellogenin gene via a blood meal-triggered cascade. Gene 274: 47–65. [DOI] [PubMed] [Google Scholar]
- 7. Chen XG, Marinotti O, Whitman L, Jasinskiene N, James AA, et al. (2007) The Anopheles gambiae vitellogenin gene (VGT2) promoter directs persistent accumulation of a reporter gene product in transgenic Anopheles stephensi following multiple bloodmeals. Am J Trop Med Hyg 76: 1118–1124. [PubMed] [Google Scholar]
- 8. Xiong B, Jacobs-Lorena M (1995) Gut-specific transcriptional regulatory elements of the carboxypeptidase gene are conserved between black flies and Drosophila . Proc Natl Acad Sci USA 92: 9313–9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Skavdis G, Sidén-Kiamos I, Müller HM, Crisanti A, Louis C (1996) Conserved function of Anopheles gambiae midgut-specific promoters in the fruitfly. EMBO J 15: 344–350. [PMC free article] [PubMed] [Google Scholar]
- 10. Lombardo F, Di Cristina M, Spanos L, Louis C, Coluzzi M, et al. (2000) Promoter sequences of the putative Anopheles gambiae apyrase confer salivary gland expression in Drosophila melanogaster . J Biol Chem 275: 23861–23868. [DOI] [PubMed] [Google Scholar]
- 11. Jinwal UK, Zakharkin SO, Litvinova OV, Jain S, Benes H (2006) Sex-, stage- and tissue-specific regulation by a mosquito hexamerin promoter. Insect Mol Biol 15: 301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mitsialis SA, Kafatos FC (1985) Regulatory elements controlling chorion gene expression are conserved between flies and moths. Nature. 317: 453–456. [DOI] [PubMed] [Google Scholar]
- 13. Ishida Y, Niimi T, Yamashito O (1999) The stage-and cell-specific expression of the Bombyx mori diapause hormone-pheromone biosynthesis activating neuropeptide (BomDH-PBAN) gene in the transformed Drosophila . J Seric Sci Jpn 68: 417–427. [Google Scholar]
- 14. Goddard LB, Roth AE, Reisen WK, Scott TW (2002) Vector competence of California mosquitoes for West Nile virus. Emerg Infect Dis 8: 1385–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reisen W, Lothrop H, Chiles R, Madon M, Cossen C, et al. (2004) West Nile virus in California. Emerg Infect Dis 10: 1369–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Turell MJ, Dohm DJ, Sardelis MR, O′Guinn ML, Andreadis TG, et al. (2005) An update on the potential of North American mosquitoes (Diptera: Culcidae) to transmit West Nile virus. J Med Entomol 42: 57–62. [DOI] [PubMed] [Google Scholar]
- 17. Venkatesan M, Rasgon JL (2010) Population genetic data suggest a role for mosquito-mediated dispersal of West Nile virus across the western United States. Mol Ecol 19: 1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Venkatesan M, Westbrook CJ, Hauer MC, Rasgon JL (2007) Evidence for a population expansion in the West Nile virus vector Culex tarsalis . Mol Biol Evol 24: 1208–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Barnett HC (1956) The transmission of Western Equine Encephalitis virus by the mosquito Culex tarsalis Coq. Amer J Trop Med Hyg 5: 86–98. [DOI] [PubMed] [Google Scholar]
- 20. Reisen WK, Lothrop HD, Hardy JL (1995.) Bionomics of Culex tarsalis (Diptera: Culicidae) in relation to arbovirus transmission in southeastern California. J Med Entomol 32: 316–327. [DOI] [PubMed] [Google Scholar]
- 21. Reisen WK, Meyer RP, Presser SB, Hardy JL (1993) Effect of temperature on the transmission of western equine encephalomyelitis and St. Louis encephalitis viruses by Culex tarsalis (Diptera: Culicidae). J Med Entomol 30: 151–160. [DOI] [PubMed] [Google Scholar]
- 22. Noden B, Pumpuni C, Vaughan J, Beier J (1995) Noninfectious sporozoites in the salivary glands of a minimally susceptible anopheline mosquito. J Parasitol 81: 912–915. [PubMed] [Google Scholar]
- 23. Turell MJ, Wilson WC, Bennett KE (2010) Potential for North American mosquitoes (Diptera: Culicidae) to transmit rift valley fever virus. J Med Entomol 47: 884–889. [DOI] [PubMed] [Google Scholar]
- 24. Chen S, Armistead JS, Provost-Javier KN, Sakamoto JM, Rasgon JL (2010) Duplication, concerted evolution and purifying selection drive the evolution of mosquito vitellogenin genes. BMC Evol Biol 2010 10: 142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Provost-Javier KN, Chen S, Rasgon JL (2010) Vitellogenin gene expression in autogenous Culex tarsalis . Insect Mol Biol 19: 423–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clark SH, Chovnick A (1986) Studies of normal and position-affected expression of rosy region genes in Drosophila melanogaster . Genetics 114: 819–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Spradling AC, Rubin GM (1983) The effect of chromosomal position on the expression of the Drosophila xanthine dehydrogenase gene. Cell 34: 47–57. [DOI] [PubMed] [Google Scholar]
- 28. Levis R, Hazelrigg T, Rubin GM (1985) Effects of genomic position on the expression of transduced copies of the white gene of Drosophila . Science 229: 558–561. [DOI] [PubMed] [Google Scholar]
- 29. Kirkpatrick RB, Parveen Z, Martin PF (1994) Isolation of silencer-containing sequences causing a tissue-specific position effect on alcohol dehydrogenase expression in Drosophila melanogaster . Dev Genet 15: 188–200. [DOI] [PubMed] [Google Scholar]
- 30. Castronuevo P, Martin PF (2002) Localization of a position effect element that affects alcohol dehydrogenase transgene expression in Drosophila melanogaster . DNA Cell Biol 21: 535–540. [DOI] [PubMed] [Google Scholar]
- 31. Handler AM, Harrell RA (1999) Germline transformation of Drosophila melanogaster with the piggyBac transposon vector. Insect Mol Biol 8: 449–457. [DOI] [PubMed] [Google Scholar]
- 32. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]