Abstract
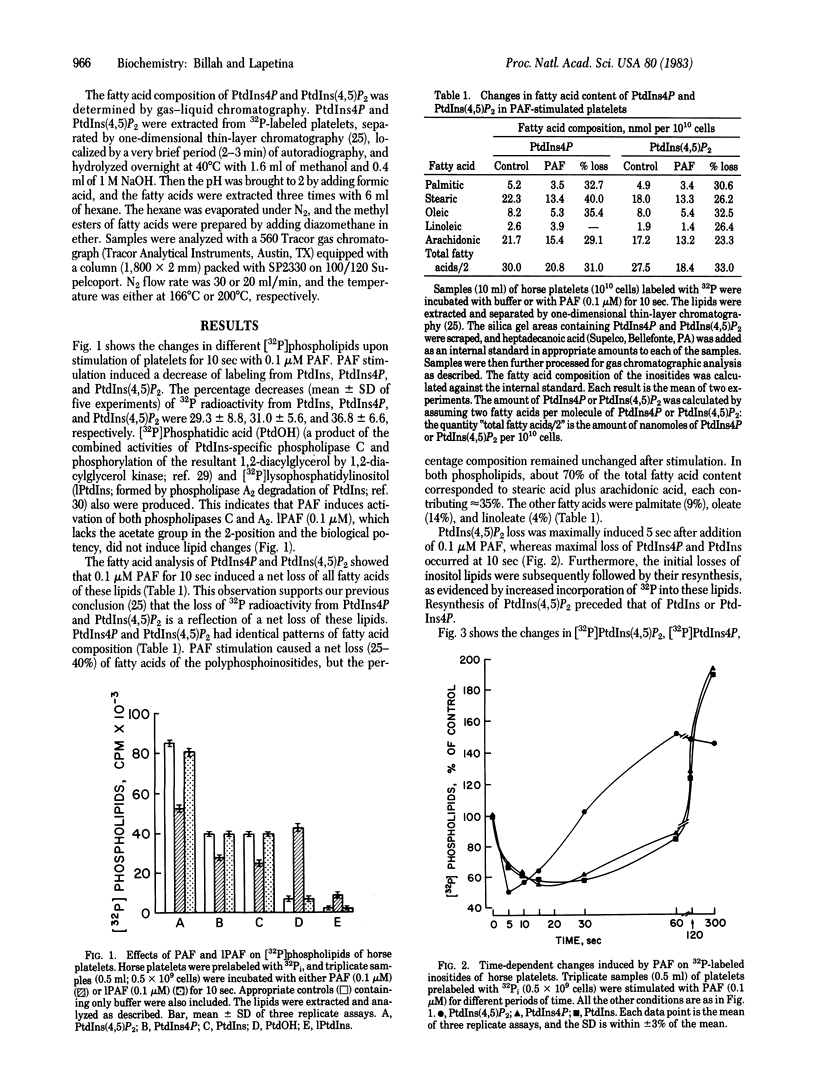
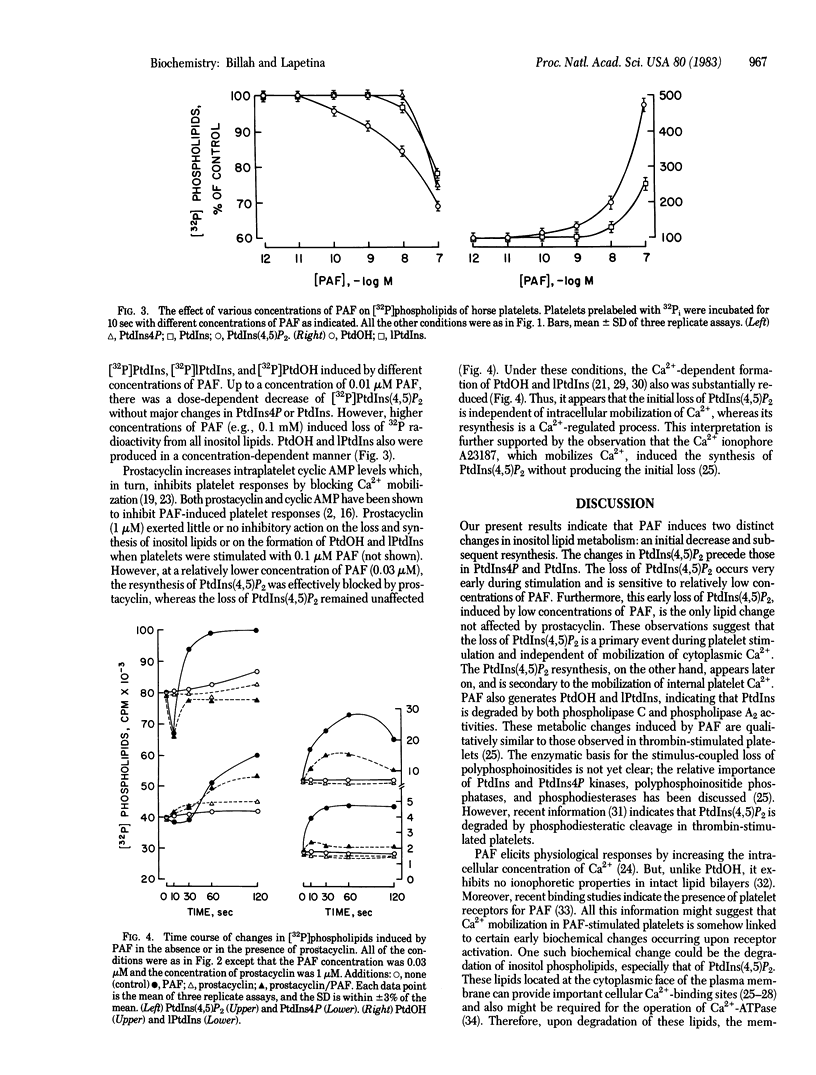
Stimulation of horse platelets with platelet-activating factor (PAF) induces a rapid degradation of phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2]. Addition of 0.1 microM PAF for 5 sec to platelets prelabeled with 32P induces a 50% loss of [32P]PtdIns(4,5)P2. 32P-Labeled phosphatidylinositol 4-monophosphate (PtdIns4P) and [32P]phosphatidylinositol (PtdIns) also are decreased, albeit at a slower rate. Loss of 32P radioactivity correlates with a net loss of fatty acids from both polyphosphoinositides. Stimulation of platelets with PAF also produces formation of [32P]phosphatidic acid and [32P]lysophosphatidylinositol. The initial disappearance of inositol lipids is subsequently followed by resynthesis, as evidenced by increased incorporation of 32P into PtdIns(4,5)P2, PtdIns4P, and PtdIns. The resynthesis of the inositides increases with time and is proportional to the concentration of PAF. Prostacyclin (1 microM) inhibits (i) the formation of phosphatidic acid and lysophosphatidylinositol and (ii) the resynthesis of polyphosphoinositides induced by 0.03 microM PAF without affecting the initial loss of PtdIns(4,5)P2. The loss of inositol lipids appears to be a primary event of platelet activation. The initial loss of polyphosphoinositides might be linked to the initiation of cellular activation by mobilizing membrane-bound Ca2+, whereas the subsequent formation of these lipids might be involved in mechanisms to prevent overstimulation of the cell.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agranoff B. W., Murthy P., Seguin E. B. Thrombin-induced phosphodiesteratic cleavage of phosphatidylinositol bisphosphate in human platelets. J Biol Chem. 1983 Feb 25;258(4):2076–2078. [PubMed] [Google Scholar]
- Benveniste J., Henson P. M., Cochrane C. G. Leukocyte-dependent histamine release from rabbit platelets. The role of IgE, basophils, and a platelet-activating factor. J Exp Med. 1972 Dec 1;136(6):1356–1377. doi: 10.1084/jem.136.6.1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Evidence for multiple metabolic pools of phosphatidylinositol in stimulated platelets. J Biol Chem. 1982 Oct 25;257(20):11856–11859. [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Formation of lysophosphatidylinositol in platelets stimulated with thrombin or ionophore A23187. J Biol Chem. 1982 May 10;257(9):5196–5200. [PubMed] [Google Scholar]
- Billah M. M., Lapetina E. G. Rapid decrease of phosphatidylinositol 4,5-bisphosphate in thrombin-stimulated platelets. J Biol Chem. 1982 Nov 10;257(21):12705–12708. [PubMed] [Google Scholar]
- Blank M. L., Snyder F., Byers L. W., Brooks B., Muirhead E. E. Antihypertensive activity of an alkyl ether analog of phosphatidylcholine. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1194–1200. doi: 10.1016/0006-291x(79)91163-x. [DOI] [PubMed] [Google Scholar]
- Buckley J. T., Hawthorne J. N. Erythrocyte membrane polyphosphoinositide metabolism and the regulation of calcium binding. J Biol Chem. 1972 Nov 25;247(22):7218–7223. [PubMed] [Google Scholar]
- Cazenave J. P., Benveniste J., Mustard J. F. Aggregation of rabbit platelets by platelet-activating factor is independent of the release reaction and the arachidonate pathway and inhibited by membrane-active drugs. Lab Invest. 1979 Sep;41(3):275–285. [PubMed] [Google Scholar]
- Dawson R. M. 'Phosphatido-peptide'-like complexes formed by the interaction of calcium triphosphoinositide with protein. Biochem J. 1965 Oct;97(1):134–138. doi: 10.1042/bj0970134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein M. B. Release of intracellular membrane-bound calcium precedes the onset of stimulus-induced exocytosis in platelets. Biochem Biophys Res Commun. 1980 Mar 28;93(2):593–600. doi: 10.1016/0006-291x(80)91119-5. [DOI] [PubMed] [Google Scholar]
- Gerrard J. M., Butler A. M., Peterson D. A., White J. G. Phosphatidic acid releases calcium from a platelet membrane fraction in vitro. Prostaglandins Med. 1978 Nov;1(5):387–396. doi: 10.1016/0161-4630(78)90125-8. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Derian C. K., Tauber A. I., Valone F. H. Novel effects of 1-O-hexadecyl-2-acyl-sn-glycero-3-phosphorycholine mediators on human leukocyte function: delineation of the specific roles of the acyl substituents. Biochem Biophys Res Commun. 1980 Jun 16;94(3):881–888. doi: 10.1016/0006-291x(80)91317-0. [DOI] [PubMed] [Google Scholar]
- Hanahan D. J., Demopoulos C. A., Liehr J., Pinckard R. N. Identification of platelet activating factor isolated from rabbit basophils as acetyl glyceryl ether phosphorylcholine. J Biol Chem. 1980 Jun 25;255(12):5514–5516. [PubMed] [Google Scholar]
- Harrison D. G., Long C. The calcium content of human erythrocytes. J Physiol. 1968 Dec;199(2):367–381. doi: 10.1113/jphysiol.1968.sp008658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson H. S., Reinertsen J. L. Comparison of metal-binding properties of trans-1,2-cyclohexanediol diphosphate and deacylated phosphoinositides. Biochemistry. 1969 Dec;8(12):4855–4858. doi: 10.1021/bi00840a031. [DOI] [PubMed] [Google Scholar]
- Ingraham L. M., Coates T. D., Allen J. M., Higgins C. P., Baehner R. L., Boxer L. A. Metabolic, membrane, and functional responses of human polymorphonuclear leukocytes to platelet-activating factor. Blood. 1982 Jun;59(6):1259–1266. [PubMed] [Google Scholar]
- Kirk C. J., Creba J. A., Downes C. P., Michell R. H. Hormone-stimulated metabolism of inositol lipids and its relationship to hepatic receptor function. Biochem Soc Trans. 1981 Oct;9(5):377–379. doi: 10.1042/bst0090377. [DOI] [PubMed] [Google Scholar]
- Lapetina E. G., Billah M. M., Cuatrecasas P. The phosphatidylinositol cycle and the regulation of arachidonic acid production. Nature. 1981 Jul 23;292(5821):367–369. doi: 10.1038/292367a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapetina E. G. Platelet-activating factor stimulates the phosphatidylinositol cycle. Appearance of phosphatidic acid is associated with the release of serotonin in horse platelets. J Biol Chem. 1982 Jul 10;257(13):7314–7317. [PubMed] [Google Scholar]
- Lee T. C., Malone B., Blank M. L., Snyder F. 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine (platelet-activating factor) stimulates calcium influx in rabbit platelets. Biochem Biophys Res Commun. 1981 Oct 30;102(4):1262–1268. doi: 10.1016/s0006-291x(81)80147-7. [DOI] [PubMed] [Google Scholar]
- Lloyd J. V., Mustard J. F. Changes in 32P-content of phosphatidic acid and the phosphoinositides of rabbit platelets during aggregation induced by collagen or thrombin. Br J Haematol. 1974 Feb;26(2):243–253. doi: 10.1111/j.1365-2141.1974.tb00469.x. [DOI] [PubMed] [Google Scholar]
- Lynch J. M., Lotner G. Z., Betz S. J., Henson P. M. The release of a platelet-activating factor by stimulated rabbit neutrophils. J Immunol. 1979 Sep;123(3):1219–1226. [PubMed] [Google Scholar]
- Marcus A. J., Safier L. B., Ullman H. L., Wong K. T., Broekman M. J., Weksler B. B., Kaplan K. L. Effects of acetyl glyceryl ether phosphorylcholine on human platelet function in vitro. Blood. 1981 Nov;58(5):1027–1031. [PubMed] [Google Scholar]
- McManus L. M., Hanahan D. J., Demopoulos C. A., Pinckard R. N. Pathobiology of the intravenous infusion of acetyl glyceryl ether phosphorylcholine (AGEPC), a synthetic platelet-activating factor (PAF), in the rabbit. J Immunol. 1980 Jun;124(6):2919–2924. [PubMed] [Google Scholar]
- McManus L. M., Hanahan D. J., Pinckard R. N. Human platelet stimulation by acetyl glyceryl ether phosphorylcholine. J Clin Invest. 1981 Mar;67(3):903–906. doi: 10.1172/JCI110108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Flaherty J. T., Wykle R. L., Miller C. H., Lewis J. C., Waite M., Bass D. A., McCall C. E., DeChatelet L. R. 1-O-Alkyl-sn-glyceryl-3-phosphorylcholines: a novel class of neutrophil stimulants. Am J Pathol. 1981 Apr;103(1):70–78. [PMC free article] [PubMed] [Google Scholar]
- Owen N. E., Le Breton G. C. Ca2+ mobilization in blood platelets as visualized by chlortetracycline fluorescence. Am J Physiol. 1981 Oct;241(4):H613–H619. doi: 10.1152/ajpheart.1981.241.4.H613. [DOI] [PubMed] [Google Scholar]
- Polonsky J., Tencé M., Varenne P., Das B. C., Lunel J., Benveniste J. Release of 1-O-alkylglyceryl 3-phosphorylcholine, O-deacetyl platelet-activating factor, from leukocytes: chemical ionization mass spectrometry of phospholipids. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7019–7023. doi: 10.1073/pnas.77.12.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serhan C., Anderson P., Goodman E., Dunham P., Weissmann G. Phosphatidate and oxidized fatty acids are calcium ionophores. Studies employing arsenazo III in liposomes. J Biol Chem. 1981 Mar 25;256(6):2736–2741. [PubMed] [Google Scholar]
- Shaw J. O., Klusick S. J., Hanahan D. J. Activation of rabbit platelet phospholipase and thromboxane synthesis by 1-O-hexadecyl/octadecyl-2-acetyl-sn-glyceryl-3-phosphorylcholine (platelet activating factor). Biochim Biophys Acta. 1981 Jan 26;663(1):222–229. doi: 10.1016/0005-2760(81)90208-3. [DOI] [PubMed] [Google Scholar]
- Sheetz M. P., Febbroriello P., Koppel D. E. Triphosphoinositide increases glycoprotein lateral mobility in erythrocyte membranes. Nature. 1982 Mar 4;296(5852):91–93. doi: 10.1038/296091a0. [DOI] [PubMed] [Google Scholar]
- Shukla S. D., Hanahan D. J. AGEPC (platelet activating factor) induced stimulation of rabbit platelets: effects on phosphatidylinositol, di- and triphosphoinositides and phosphatidic acid metabolism. Biochem Biophys Res Commun. 1982 Jun 15;106(3):697–703. doi: 10.1016/0006-291x(82)91767-3. [DOI] [PubMed] [Google Scholar]
- Takai Y., Kaibuchi K., Matsubara T., Nishizuka Y. Inhibitory action of guanosine 3', 5'-monophosphate on thrombin-induced phosphatidylinositol turnover and protein phosphorylation in human platelets. Biochem Biophys Res Commun. 1981 Jul 16;101(1):61–67. doi: 10.1016/s0006-291x(81)80010-1. [DOI] [PubMed] [Google Scholar]
- Vargaftig B. B., Lefort J., Chignard M., Benveniste J. Platelet-activating factor induces a platelet-dependent bronchoconstriction unrelated to the formation of prostaglandin derivatives. Eur J Pharmacol. 1980 Jul 25;65(2-3):185–192. doi: 10.1016/0014-2999(80)90391-x. [DOI] [PubMed] [Google Scholar]
- Weiss S. J., McKinney J. S., Putney J. W., Jr Receptor-mediated net breakdown of phosphatidylinositol 4,5-bisphosphate in parotid acinar cells. Biochem J. 1982 Sep 15;206(3):555–560. doi: 10.1042/bj2060555. [DOI] [PMC free article] [PubMed] [Google Scholar]


