Abstract
Nicotine, the primary psychoactive component in tobacco smoke, produces its behavioral effects through interactions with neuronal nicotinic acetylcholine receptors (nAChRs). α4β2 nAChRs are the most abundant in mammalian brain, and converging evidence shows that this subtype mediates the rewarding and reinforcing effects of nicotine. A number of rare variants in the CHRNA4 gene that encode the α4 nAChR subunit have been identified in human subjects and appear to be underrepresented in a cohort of smokers. We compared three of these variants (α4R336C, α4P451L, and α4R487Q) to the common variant to determine their effects on α4β2 nAChR pharmacology. We examined [3H]epibatidine binding, interacting proteins, and phosphorylation of the α4 nAChR subunit with liquid chromatography and tandem mass spectrometry (LC-MS/MS) in HEK 293 cells and voltage-clamp electrophysiology in Xenopus laevis oocytes. We observed significant effects of the α4 variants on nAChR expression, subcellular distribution, and sensitivity to nicotine-induced receptor upregulation. Proteomic analysis of immunopurified α4β2 nAChRs incorporating the rare variants identified considerable differences in the intracellular interactomes due to these single amino acid substitutions. Electrophysiological characterization in X. laevis oocytes revealed alterations in the functional parameters of activation by nAChR agonists conferred by these α4 rare variants, as well as shifts in receptor function after incubation with nicotine. Taken together, these experiments suggest that genetic variation at CHRNA4 alters the assembly and expression of human α4β2 nAChRs, resulting in receptors that are more sensitive to nicotine exposure than those assembled with the common α4 variant. The changes in nAChR pharmacology could contribute to differences in responses to smoked nicotine in individuals harboring these rare variants.
Introduction
Nicotine, the principle psychoactive component of tobacco, exerts its effects through interactions with neuronal nicotinic acetylcholine receptors (nAChRs). The principal class of nAChRs that binds nicotine with high affinity in the mammalian central nervous system (CNS) is the α4β2*-nAChR family (Picciotto et al., 1995; Millar and Gotti, 2009) (* designates potential additional subunits) (Lukas et al., 1999). Experiments with transgenic mice lacking β2* nAChRs (Picciotto et al., 1995, 1998), cell type-selective knockout of the α4 nAChR subunit (McGranahan et al., 2011), and knock-in mice with hypersensitive α4 nAChR subunits (Tapper et al., 2004, 2007) demonstrated that activation of α4β2* nAChRs was necessary and sufficient for many behavioral effects associated with nicotine addiction. This has led recent genetic association studies to focus on nAChR subunit genes as targets of interest. A meta-analysis of nicotine dependence linkage studies supported significant genomewide linkage with a related trait at a chromosomal region overlying the CHRNA4 locus (Han et al., 2010), and association studies found significant effects of several CHRNA4 variants on nicotine dependence (Han et al., 2011; Kamens et al., 2013). A polymorphism in the α5 nAChR subunit gene (CHRNA5) (Bierut et al., 2008) has received particular attention, because it is consistently associated with nicotine dependence and produces an amino acid substitution (D397N) that results in altered function of α4β2α5 nAChRs (Bierut et al., 2008; Kuryatov et al., 2011).
Rare variants in CHRNA4 also appear to be underrepresented among smokers (Xie et al., 2011). A particular rare variant (α4P451L) has also been associated with development of amyotrophic lateral sclerosis (ALS) (Sabatelli et al., 2009, 2012), suggesting that it may be of particular functional interest. In the current study, we characterize a subset of rare variants in the CHRNA4 gene that alter amino acid sequence of the subunit. We chose polymorphisms based on a bioinformatic screen of predicted shifts in short linear interaction motifs relative to the wild-type subunit. We examined the effects of α4R336C, α4P451L, and α4R487Q on nAChR expression and protein/protein interaction in HEK 293 cells as well as receptor activation in X. laevis oocytes.
Materials and Methods
cDNA and Chemicals.
Mutations corresponding to the identified rare variants were introduced into a human α4 cDNA (hα4) construct in a psp64-polyA vector (Promega, Madison, WI) using GeneArt Site-Directed Mutagenesis (Invitrogen, Carlsbad, CA). Except where noted, all chemicals were obtained from Sigma-Aldrich (St. Louis, MO). [3H]Epibatidine (55.8 Ci/mmol) was obtained from PerkinElmer Life and Analytical Sciences (Natick, MA)
Cell Culture and Transfection.
HEK293 cells (ATCC 1573; American Type Culture Collection, Manassas, VA) were used for studies of receptor expression and identification of interacting proteins after transient transfection. Cells were allowed to reach ∼90% confluence before transfection (typically ∼96 hours) and harvested 24 hours after transfection. Details of cell culture conditions and transient transfection protocol are provided in the Supplemental Methods.
Quantitation of α4β2 nAChR Expression with [3H]Epibatidine Binding.
Measurement of [3H]epibatidine binding to cell membranes and solubilized receptors was performed as detailed in Supplemental Methods. Except where indicated, specific counts per minute were converted to femtomoles and normalized further to the total protein content present in each sample to provide units of femtomoles per milligramsprotein. Quantitation of plasma membrane α4β2 nAChRs by cell surface biotinylation was performed as described previously (Kuryatov et al., 2005) with minor modifications. A detailed description is provided in the Supplemental Materials. Measurement of [3H]epibatidine binding in subcellular compartments was achieved by fractionating transfected HEK293 cells with a subcellular fractionation kit (Pierce Biotechnology, Rockford, IL) according to the manufacturer’s instructions.
Immunocapture of nAChRs and Identification of Receptor-Associated Proteins by Liquid Chromatography and Tandem Mass Spectrometry.
Solubilized nAChRs were captured using M270 epoxy-coated Dynabeads (Invitrogen) coupled to 0.5 μg of mAb295/mg beads, which specifically recognizes β2-nAChRs (Whiting and Lindstrom, 1988), and the hα4β2 nAChR-associated proteome was identified as described in the Supplemental Methods.
Expression and Recording of α4β2 nAChRs in X. laevis Oocytes.
Expression and electrophysiological recording of hα4(RV)β2 nAChRs in X. laevis oocytes was conducted essentially as reported previously (Williams et al., 2011). Agonist application was set at 8 seconds during recording, with 241-second washes in between stimulations. Responses to agonist are reported as percent of maximum response ± S.E.M. to limit interexperimental variation or differences in receptor expression because of effects of the hα4 polymorphisms related to nAChR assembly.
Data Analysis.
For [3H]epibatidine binding, kinetic parameters were determined by fitting the measured binding to a single-site hyperbolic saturation curve of the form y = a[x]/b + [x], with measured binding (y) kd (a) and Bmax (b) at [ligand] x. Concentration-response effects on nicotine-induced nAChR upregulation were calculated with the equation y = y0 + a[x]/b + [x], where binding (y) equals initial binding at [nicotine]0 (y0), nicotine concentration (x), maximal upregulation (b), and EC50 (a).
For electrophysiological studies, normalized response values were used to generate agonist concentration-response curves. The resulting curves were fit to two- and four-parameter regular hyperbolic equations followed by F-tests to determine the statistical preference for one or two distinct activation components, respectively. Curve fit equations were: y = ax/b + x, where (y) is recorded activity with maximal activation (a) and agonist EC50 (b) for a single component (2-parameter), and y = (ax/b + x) + (cx/d + x), where (y) is recorded activity with high-sensitivity maximal activation (a), EC50 (b), and lower sensitivity (c) with EC50 (d) (4-parameter).
SPSS 21.0 and SigmaPlot 12.0 (2012 and 2011, respectively) were used for statistical analysis and data organization. Statistical significance was determined with one- or two-way analysis of variance or Student’s t test, depending on the experimental design, with a confidence interval set at 95%.
Results
Effect of hα4 Variants on α4β2 nAChR Expression.
To identify any differences in efficiency of receptor assembly due to polymorphisms in the human α4 subunit (hα4, gene symbol CHRNA4), HEK293 cells were transfected with 1 μg of hβ2 cDNA per 250,000 cells and 0.01, 0.1, or 1 μg of hα4 cDNA, and binding of 2 nM [3H]epibatidine was measured. [3H]Epibatidine binding was dependent on the amount of hα4 cDNA added (Table 1). Transient transfection with large excesses of hα4 nAChR cDNA with a fixed concentration of hβ2 cDNA results in a sharp inverted-U relationship with respect to binding sites produced. To avoid this, we confined our analysis to the ratios of α4:β2 cDNA that produced a proportional increase in binding sites per microgram α4 cDNA. The estimated 1/2 maximal cDNA concentration for expression did not vary across the α4 variants examined, but there was a significant effect of α4 variant on estimated maximal expression, with the α4P451L variant showing a 38% decrease in binding relative to control (Supplemental Fig. S1). All subsequent experiments with transient transfection of hα4 and hβ2 in HEK293 cells, including those for receptor upregulation, used a 1:1 ratio of α4:β2 subunit cDNA.
TABLE 1.
Calculated parameters for α4 cDNA-dependent [3H]epibatidine binding site production
| hα4 Variant | Max Expression | 1/2max [cDNA] |
|---|---|---|
| fmol/mg | µg/250 K cells | |
| Common | 368.80 ± 11.20 | 0.21 ± 0.02 |
| α4R336C | 408.20 ± 30.20 | 0.30 ± 0.07 |
| α4P451L | 229.90 ± 26.50* | 0.37 ± 0.13 |
| α4R487Q | 534.70 ± 22.50* | 0.41 ± 0.05 |
*P < 0.05 compared to the common variant.
HEK293 cells were transfected with hα4 and hβ2 cDNA and binding was measured across a range of [3H]epibatidine concentrations (Table 2). There was a significant effect of hα4 variant on Bmax. Dunnett’s post hoc analysis revealed that there is an approximate twofold decrease in the estimated Bmax of the α4P451L variant for [3H]epibatidine binding relative to control but no difference in the estimated affinity of any of the hα4 variants for [3H]epibatidine (Supplemental Fig. S2; statistics for all [3H]epibatidine binding experiments are listed in Supplemental Section 1).
TABLE 2.
Calculated parameters for saturation of [3H]epibatidine binding
| hα4 Variant | Bmax | Kd |
|---|---|---|
| fmol/mg | pM | |
| Common | 560.0 ± 17.0 | 26.8 ± 7.0 |
| α4R336C | 595.0 ± 57.0 | 31.0 ± 8.0 |
| α4P451L | 291.2 ± 10.9* | 16.9 ± 5.2 |
| α4R487Q | 675.0 ± 13.0 | 24.1 ± 1.4 |
*P < 0.05 compared to the common variant.
Effects of hα4 Variants on α4β2-nAChR Upregulation and Plasma Membrane Levels in Response to Nicotine Exposure.
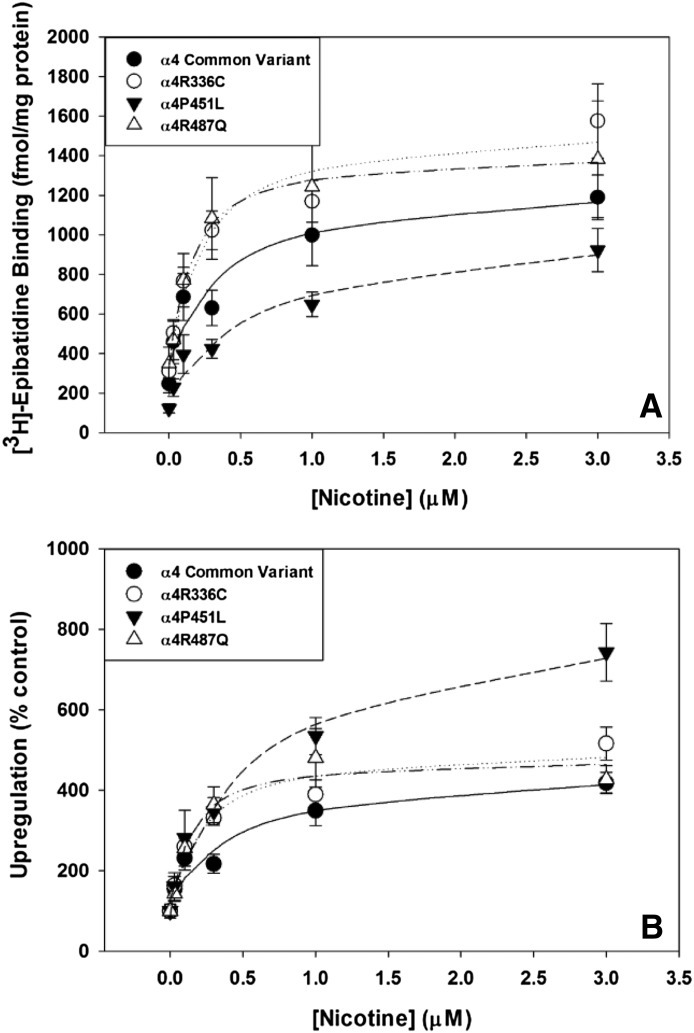
α4β2 nAChRs are upregulated by long-term exposure to nicotine (reviewed by Lester et al., 2009). To test for sensitivity and extent of nicotine-induced receptor upregulation, nicotine (0, 3, 10, 30, 100, 1000, and 3000 nM) was added to the wells with hα4 and hβ2 cDNAs, and the cells were incubated overnight. After 24-hour nicotine exposure, all hα4 variants showed concentration-dependent and saturable upregulation of [3H]epibatidine binding sites (Fig. 1A; Table 3). There was no significant difference in either maximal upregulation by nicotine or EC50. Converting the data from femtomoles per milligram to percentage of control (no nicotine) to produce a measure of fold-increase reveals significant differences in the ability of nicotine to upregulate α4β2 nAChRs depending on the hα4 variant (Fig. 1B). Dunnett’s post hoc analysis shows that nicotine upregulates [3H]epibatidine binding in α4P451Lβ2-transfected HEK293 cells to a greater extent than nAChRs, incorporating the other α4 variants examined with no significant effect on EC50 (Table 4).
Fig. 1.
Effects of nicotine on levels of nAChRs incorporating α4 variants. (A) Nicotine upregulates expression of [3H]epibatidine binding sites for the α4 common variant (●), α4R336C (○), α4P451L (▾) and α4R487Q (▵) with equivalent potency and maximal expression. (B) Nicotine-induced upregulation of [3H]epibatidine binding sites as a percent of control reveals that the level of upregulation compared with baseline is greater for α4P451L (▾) and results in greater maximal upregulation compared with the common variant (●), α4R336C (C) and α4R487Q (▵).
TABLE 3.
Calculated parameters for nicotine-induced upregulation of [3H]epibatidine binding sites
| hα4 Variant | Max Upregulation | Nicotine EC50 |
|---|---|---|
| fmol/mg | µM | |
| Common | 1262.34 ± 122.83 | 1.55 ± 0.66 |
| α4R336C | 1154.67 ± 87.67 | 0.33 ± 0.15 |
| α4P451L | 986.74 ± 218.29 | 0.77 ± 0.34 |
| α4R487Q | 1197.21 ± 233.23 | 0.20 ± 0.06 |
TABLE 4.
Nicotine-induced upregulation expressed as fold change relative to control
| hα4 Variant | Max Upregulation | Nicotine EC50 |
|---|---|---|
| Fold change | µM | |
| Common | 4.5 ± 0.5 | 1.59 ± 0.70 |
| α4R336C | 4.1 ± 0.4 | 0.34 ± 0.20 |
| α4P451L | 8.5 ± 1.3* | 1.24 ± 0.41 |
| α4R487Q | 4.0 ± 0.4 | 0.20 ± 0.06 |
*P < 0.05 compared to the common variant.
To determine the distribution of [3H]epibatidine binding sites in cells, surface receptors were biotinylated prior to cell lysis, and binding to biotinylated receptors was quantified. Measuring [3H]epibatidine binding prior to solubilization in phosphate-buffered saline supplemented with 2% Triton X-100 quantitates all available binding sites (including immature and intracellular pools of receptors), whereas avidin capture of biotinylated receptors from detergent extracts enables detection of plasma membrane nAChRs. Under control conditions, there are fewer total α4P451Lβ2 nAChRs compared with the other variants (Fig. 2A), but similar levels of plasma membrane binding sites (Fig. 2C). The deficient expression of [3H]epibatidine binding in cells transfected with α4P451L does not reflect a deficit in cell surface α4P451Lβ2 nAChRs. After treatment with 1 μM nicotine for 24 hours, significant upregulation of [3H]epibatidine binding sites was observed in both total and surface receptor pools of all α4 variants (Fig. 2C; Table 5). As observed with surface expression at baseline, the extent of upregulation measured as fold change relative to control was much greater in nAChRs containing the hα4-P451L variant (Fig. 2, B and D, statistical test results are presented in Supplemental Section 2).
Fig. 2.
Cell surface expression of nAChRs incorporating α4 variants. (A) Total cell membrane expression of [3H]epibatidine binding sites ±24-hour exposure to 1 µM nicotine. Under control conditions (black bars) α4P451L has significantly lower total [3H]epibatidine binding that was rescued as compared with the other variants after 24-hour incubation with 1 µM nicotine (gray bars). (B) Total cell membrane upregulation induced by 24-hour exposure to 1 µM nicotine expressed as fold change relative to control indicates that the α4P451L variant is upregulated to a greater extent than the other variants. (C) Cell surface levels of [3H]epibatidine binding sites ± 24-hour nicotine exposure shows that despite the significant difference in total binding site expression, surface levels of α4P451L are normal under control conditions (black bars) and are strongly upregulated after nicotine treatment (gray bars). (D) Cell surface upregulation induced by 24-hour 1 µM nicotine expressed as fold change relative to control indicates that α4P451L enhances surface expression of nAChRs after nicotine treatment to a greater degree than the common variant, α4R336C, and α4R487Q. *P < 0.05 compared to the common variant.
TABLE 5.
Effects of 1.0 µM nicotine on plasma membrane and total binding sites
| hα4 Variant | Control Total Binding | Control Surface Binding | Control | Nicotine Total Binding | Nicotine Surface Binding | Nicotine |
|---|---|---|---|---|---|---|
| fmol/mg | % surface binding | fmol/mg | % surface binding | |||
| Common | 1205 ± 138 | 450 ± 70 | 37.5 ± 3.7 | 3592 ± 197 | 1174 ± 136 | 32.4 ± 2.4 |
| α4R336C | 1281 ± 127 | 511 ± 142 | 43.0 ± 13.8 | 2784 ± 186* | 936 ± 54 | 34.7 ± 3.9 |
| α4P451L | 491 ± 24* | 342 ± 41 | 69.2 ± 6.3* | 2581 ± 272* | 1428 ± 77 | 57.6 ± 12.3 |
| α4R487Q | 1242 ± 97 | 636 ± 79 | 51.8 ± 5.4 | 2932 ± 204 | 1440 ± 66 | 51.2 ± 6.6 |
*P < 0.05 compared to the common variant.
Effects of hα4 Variants on Associated Proteins and Phosphorylation Patterns Identified by Tandem Mass Spectrometry.
Eluted α4β2 nAChR complexes were enriched for phosphorylated peptides prior to LC-MS/MS analysis to split each group into phospho-enriched (Phospho) and flow-through (FT) sets, and both sets were analyzed to identify phosphorylated residues of peptides in the MS/MS spectra. We hypothesize that because protein kinases and phosphatases target specific residues in the M3-M4 loop (Pollock et al., 2009), the rare variants examined in this study might affect the accessibility of these sites. From the initial lists of identified proteins for each hα4 variant, we used a protein-exclusion process to refine the apparent interactomes. Obvious contaminant proteins (keratins, dermicidins, hornerins) were excluded. Because the nAChRs were isolated from solubilized protein extracts prepared from whole cells, some nuclear and mitochondrial proteins were also identified that would normally not be expected to interact with α4β2 nAChRs, and these proteins were also excluded from further consideration. These “culled” protein lists were compared across hα4 variants to generate sets of proteins common to all α4 variants, unique to each hα4 variant, and sets where proteins were common to three of four variants and conspicuously absent from the fourth. These lists were compiled for each fraction (Phospho or FT). The complete sets of interacting proteins identified for each variant are available at http://yped.med.yale.edu/repository/. Protein discrepancies are evident for α4P451L in particular. A number of 14-3-3 chaperone protein isoforms are not identified by LC-MS/MS from immunopurified α4P451Lβ2 nAChRs that are repeatedly identified in purified α4β2 nAChRs containing the other hα4 variants. Several importin isoforms are present in α4P451Lβ2 complexes identified by LC-MS/MS that are absent in the profiled complexes of the other hα4 variants [discrepancies for each variant are listed in Supplemental Tables S1 (Phospho) and S2 (FT)]. These results provide evidence of aberrant protein/protein interactions attributed to a significant shift in the conformation of the M3-M4 loop in the α4P451L variant that disrupts its association with commonly observed nAChR chaperones and recruits importin isoforms normally associated with nuclear transport. Therefore, we examined the subcellular distribution of [3H]epibatidine binding sites in HEK 293 cells transfected with hβ2 and either hα4 or hα4P451L. Transfected cells were processed into cell membrane (plasma membranes and endoplasmic reticulum), nuclear, and cytoskeletal fractions, and [3H]epibatidine bindine was assessed in each sample. Nicotinic binding sites in the nuclear fractions of transiently transfected cells were not altered by inclusion of the α4P451L variant (Supplemental Fig. S3), indicating that the association of importin isoforms with α4P451L does not result in nuclear import of α4β2 nAChRs but instead reflects inappropriate protein/protein interactions that could be expected to influence receptor assembly and posttranslational modification.
Analysis of the 270-amino acid sequence of the M3-M4 intracellular loop of the hα4 nAChR subunit with NetPhos 2.0 (www.cbs.dtu.dk) identifies 17 potential serine phosphorylation sites. None of the α4 variants examined in this study are predicted to affect any of these 17 sites; however, there was a significant discrepancy in the phosphorylation patterns of the identifiable serine residues across the four α4 variants. Consistent with previous studies (Pollock et al., 2007), we observed phosphorylation of serine residues only on the M3-M4 loop of the α4 subunit (Table 6). Proteolysis of the hα4 subunit by trypsin and LysC prior to LC-MS/MS restricts the absolute coverage of the receptor by producing peptide fragments too small to be unambiguously assigned, and so we anticipated missing 8 of 17 possible phosphorylation sites. There was no difference in the peptide sequence coverage of the M3-M4 loop for the common hα4 variant compared with the α4R336C and α4R487Q variants, so differences in phosphorylation state of identified serine residues are not likely because of differential sequence coverage. The identification of the phosphorylation state of serines in the M3-M4 loop of α4P451L was not possible, because its coverage was insufficient for effective analysis.
TABLE 6.
Phosphorylated residues identified on α4 nAChR subunits by MS/MS
| Identified Residue | α4-Common Variant | α4 R336C | α4 P451L | α4 R487Q | Putative Kinases |
|---|---|---|---|---|---|
| Ser374 | Yes | Yes | ND | No | BARK |
| Ser467 | Yes | Yes | ND | Yes | CamKII, MAPKI, PKA |
| Ser472 | No | No | ND | Yes | CDK5, GSK3 |
| Ser473 | Yes | No | ND | No | CKII |
| Ser488 | Yes | Yes | ND | No | AKTK, MAPKI |
| Ser527 | Yes | Yes | ND | No | CDK5, GSK3 |
| Ser539 | No | Yes | ND | Yes | GSK3, MAPKII |
| Ser541 | Yes | Yes | ND | Yes | CKI, ERKI, ERKII, CDK5 |
| Ser561 | No | Yes | ND | Yes | CDK5, CKI, ERKI, ERKII, GPCRK, GSK3, MAPKII |
ND, not determined.
Electrophysiological Characterization of α4 Variants.
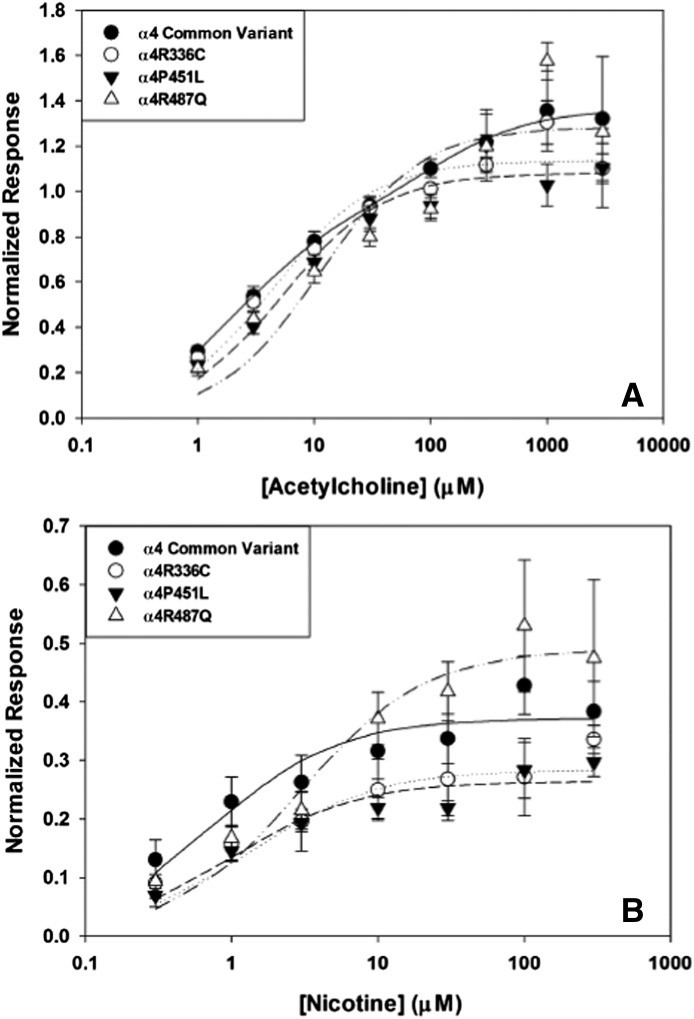
We expressed hα4 variants α4R336C, α4P451L, and α4R487Q with human β2 in X. laevis oocytes and recorded currents elicited by application of nAChR agonists. With equal amounts of hα4 and hβ2 cRNA, activation of common variant α4β2 with ACh produced the predicted biphasic concentration-response curve (Table 7), indicating the presence of nAChRs with high sensitivity to ACh (HS) and a lower sensitivity component (LS), as has been described previously (Marks et al., 2010). The other hα4 variants (α4R336C, α4P451L, α4R487Q) all had ACh concentration-response curves that favored the activation of a single HS component. The calculated potency of ACh for HS α4β2 activation was similar across variants, indicating that these variants in the α4 subunit do not appreciably affect sensitivity to activation by ACh but limit the stoichiometry of assembled nAChRs to the HS form of α42β23. Nicotine produces similar concentration-response relationships as ACh (Table 8), with the exception that the HS form of common variant α4β2 is more prominently activated, with no change in potency, when nicotine is used as the agonist. (Calculated activation parameters are found in Tables 7 and 8; Fig. 3.)
TABLE 7.
Calculated parameters for acetylcholine concentration-response profiles
| hα4 Variant | HS EC50 | HS Max Response | LS EC50 | LS Max Response |
|---|---|---|---|---|
| µM | µM | |||
| Common | 2.12 ± 0.34 | 0.89 ± 0.06 | 109.0 ± 41.0 | 0.47 ± 0.05 |
| α4R336C | 4.34 ± 1.06 | 1.14 ± 0.04 | — | — |
| α4P451L | 5.30 ± 1.20 | 1.08 ± 0.04 | — | — |
| α4R487Q | 11.2 ± 5.1 | 1.28 ± 0.10 | — | — |
TABLE 8.
Calculated parameters for nicotine concentration-response profiles
| hα4 Variant | HS EC50 | HS Max Response | LS EC50 | LS Max Response |
|---|---|---|---|---|
| µM | µM | |||
| Common | 0.73 ± 0.24 | 0.37 ± 0.02 | — | — |
| α4R336C | 0.93 ± 0.29 | 0.29 ± 0.02 | — | — |
| α4P451L | 0.97 ± 0.32 | 0.26 ± 0.01 | — | — |
| α4R487Q | 2.88 ± 0.82 | 0.49 ± 0.03 | — | — |
Fig. 3.
Electrophysiological recordings of α4β2 nAChRs expressed in X. laevis ooctyes and activated by acetylcholine. (A) The common variant of α4 (●) demonstrates a biphasic concentration-response curve, indicative of high- and low-sensitivity activation components. α4R336C (○), α4P451L (▾), and α4R487Q (▵) all show monophasic, high-sensitivity activation by acetylcholine. (B) Electrophysiological recordings of α4β2 nAChRs expressed in X. laevis oocytes activated by nicotine. The common variant of α4 (●) has a monophasic, high-sensitivity concentration-response relationship with nicotine. Similarly, α4R336C (○), α4P451L (▾), and α4R487Q (▵) also show monophasic high-sensitivity activation by nicotine.
Because the subunit composition and stoichiometry of α4β2 nAChRs affects their functional profiles, we expressed the α4 variants as concatamers (α4-β2 dimers) to isolate the two defined stoichiometries and determine whether any effects of α4 variant were apparent when receptor assembly is experimentally constrained. Agonist-evoked responses from α4β2 nAChRs assembled with concatenated subunits (as described in Zhou et al., 2003) largely confirmed our hypothesis that hα4 rare variants will affect receptor assembly. In particular, α4P451L shows a reduced ability to assemble as an LS receptor [α43β22] even when introduced into a concatamer, and α4R336C forces assembly of both HS and LS stoichiometries under every condition examined (full concentration-response curves and parameters of concatenated receptor experiments are found in Supplemental Figs. S4 and S5, and Table S3, respectively; a detailed description of receptor concatamer experiments is presented in Supplemental Materials).
Effects of 24-hour Nicotine Exposure on Activation Parameters of α4 Variants.
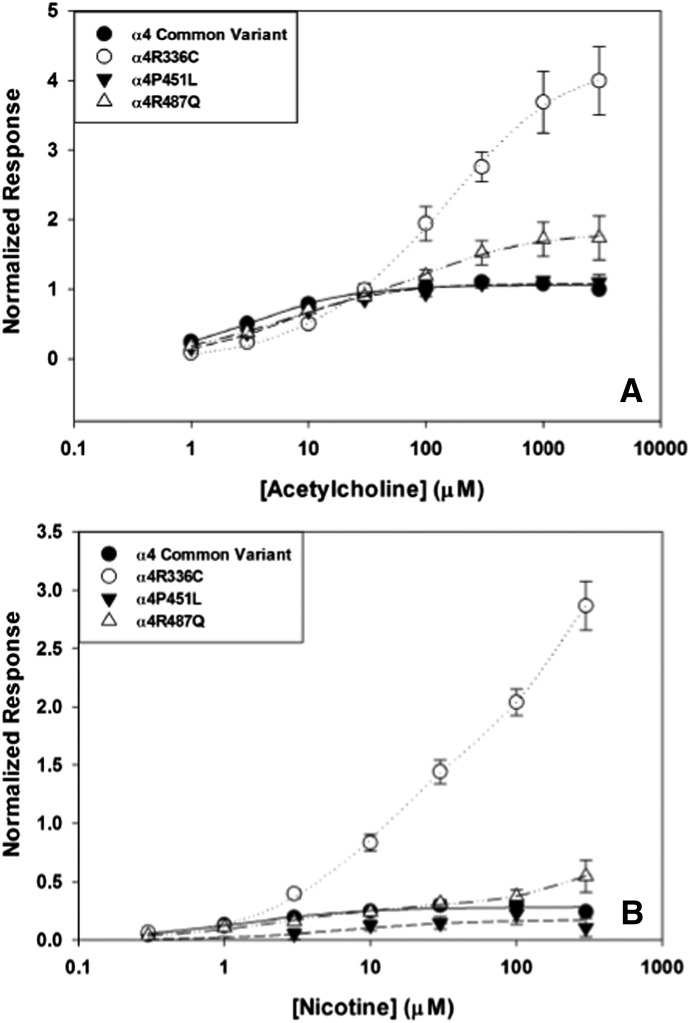
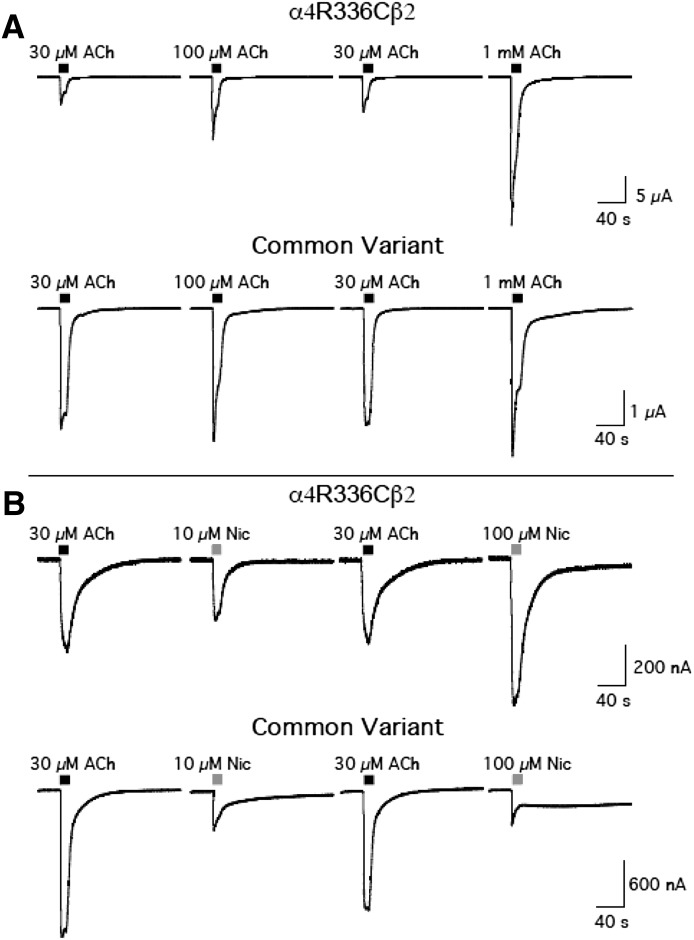
Prolonged nicotine exposure promotes the production of HS nAChRs in heterologous systems expressing human α4β2 nAChRs (Kuryatov et al., 2005). Because the α4 variants appear to alter the stoichiometry of α4β2 nAChRs, we examined α4β2 nAChR activation by ACh and nicotine after oocytes were incubated with 1 μM nicotine for 24 hours. The effects of nicotine exposure were strikingly divergent according to hα4 variant (Fig. 4A; Tables 9 and 10). Consistent with previous reports, nicotine incubation shifted the concentration-response curve for ACh to a single HS component for common variant α4β2 nAChRs, indicating that exposure to nicotine (1 μM) favors the production of α4β2 nAChRs with the α42β23 stoichiometry. In contrast, nicotine exposure shifted the potency of ACh to a significant degree in the α4R336C variants, resulting in the appearance of a substantial LS response profile. This effect appears to reflect the preferential assembly of LS α4R336Cβ2 nAChRs after prolonged nicotine exposure, an effect in striking contrast to what is routinely observed with the common variant of hα4. Nicotine exposure did not affect the ACh concentration-response profile for α4P451L, apart from a slight decrease in the overall magnitude of the response (likely due to nicotine-mediated desensitization). The α4R487Q variant retained both HS and LS components after 24-hour nicotine exposure, indicating a decrease in the preferential assembly of HS α4β2 nAChRs in the presence of nicotine. Both control and P451L α4 variants maintained a single HS concentration-response profile in response to acute nicotine challenge after nicotine exposure, whereas the R336C and R487Q α4 variants showed the same biphasic responses as observed before 24-hour nicotine exposure (Fig. 4B). Incubation of oocytes transfected with the α4R336C variant with nicotine for 24 hours had the same effect on nicotine-evoked currents as observed with ACh, revealing a shift in agonist potency that produced a large LS response. Representative traces for agonist-evoked inward currents through α4R336Cβ2 and common variant α4β2 nAChRs after 24-hour nicotine exposure are shown in Fig. 5. These results show that whether ACh (Fig. 5A) or nicotine (Fig. 5B) is used as the agonist, α4R336Cβ2 nAChRs are substantially less sensitive to agonist activation after 24-hour nicotine exposure than common variant α4β2 nAChRs.
Fig. 4.
Electrophysiological recordings of α4β2 nAChRs expressed in X. laevis oocytes activated by acetylcholine following 24 hour exposure to 1 µM nicotine. (A) The concentration-response curve for the common α4 variant (●) shifts from biphasic to monophasic, high-sensitivity activation by acetylcholine. The concentration-response curve for α4P451L (▾) remains monophasic and high-sensitivity activation. The curves for α4R336C (○) and α4R487Q (▵) shift from a monophasic, high-sensitivity relationship to biphasic activation, with both variants gaining low-sensitivity activation components. (B) Electrophysiological recordings of α4β2 nAChRs expressed in X. laevis oocytes activated by nicotine after 24-hour exposure to 1 µM nicotine. The concentration-response curve for the common α4 variant (●) remains a monophasic, high-agonist sensitivity relationship, as does the curve established for α4P451L (▾). The curves for α4R336C (○) and α4R487Q (▵) shift from a monophasic, high-sensitivity component to biphasic curves with added low-sensitivity components. The size of the LS component is particularly large for α4R336C.
TABLE 9.
Calculated acetylcholine concentration-response parameters after 24-hour nicotine
| hα4 Variant | HS EC50 | HS Max Response | LS EC50 | LS Max Response |
|---|---|---|---|---|
| µM | µM | |||
| Common | 3.36 ± 0.40 | 1.05 ± 0.02 | — | — |
| α4R336C | 18.3 ± 12.3 | 1.05 ± 0.54 | 222.0 ± 65.0 | 3.2 ± 0.50 |
| α4P451L | 6.26 ± 0.79 | 1.08 ± 0.02 | — | — |
| α4R487Q | 3.5 ± 1.0 | 0.80 ± 0.10 | 128.0 ± 35.0 | 1.0 ± 0.09 |
TABLE 10.
Calculated nicotine concentration-response parameters after 24-hour nicotine
| hα4 Variant | HS EC50 | HS Max Response | LS EC50 | LS Max Response |
|---|---|---|---|---|
| µM | µM | |||
| Common | 1.30 ± 0.36 | 0.28 ± 0.01 | — | — |
| α4R336C | 13.2 ± 2.20 | 1.90 ± 0.20 | 5493 ± 4.6 × 104 | 20.2 ± 158.8 |
| α4P451L | 0.97 ± 0.32 | 0.26 ± 0.01 | — | — |
| α4R487Q | 2.23 ± 0.50 | 0.29 ± 0.03 | 3623 ± 2.0 × 104 | 3.30 ± 17.0 |
Fig. 5.
Representative traces of inward currents elicited by nAChR agonists for α4R336Cβ2 and common variant α4β2 nAChRs expressed in X. laevis oocytes after 24-hour incubation with nicotine. (A) Inward currents elicited by acute application of ACh after 24-hour nicotine treatment. (B) Inward currents elicited by acute application of nicotine after 24-hour nicotine treatment. Both agonists reveal the presence of a distinct LS component in the α4R336C variant that is absent in oocytes injected with cRNA for the common hα4 variant after 24-hour nicotine exposure.
Discussion
We examined the effects of rare CHRNA4 variants on expression, protein/protein interactions, and activation parameters of α4β2 nAChRs. We observed significant effects of these rare α4 variants on nAChR binding site expression and upregulation by nicotine. Most importantly, the α4 variants also altered the nAChR interactome and the parameters of α4β2 nAChR activation by ACh and nicotine and resulted in significant shifts in concentration-response profiles both under control conditions and after a 24-hour exposure to nicotine, indicating that smoking could alter the properties of α4β2 nAChRs in humans carrying these rare genetic variants.
nAChR subunits all possess a large intracellular loop between the M3 and M4 transmembrane domains. The α4 nAChR subunit has the largest (270-amino acids long) M3-M4 loop, and this domain is pivotal in nAChR assembly and functional modulation (Harkness and Millar, 2002; Kuo et al., 2005; Kracun et al., 2008; Tsetlin et al., 2011). The loop is predicted to be largely unfolded, existing as a series of linear interaction motifs (Kukhtina et al., 2006). The α4 polymorphisms examined in this study (R336C, P451L, R487Q) all occur within the M3-M4 loop at amino acid residues that are 100% conserved across humans, mice, rats, and chickens, suggesting that these rare variants will affect one or more elements of α4β2 nAChR maturation, expression, and function.
Maximal nAChR production was significantly affected by α4 variant: the α4R487Q variant produced significantly more [3H]epibatidine binding sites when cDNA concentration was maximal, and α4P451L produced significantly fewer nicotinic binding sites. The α4R336C polymorphism was equivalent to the common variant under these conditions. The estimates for α4 cDNA-dependent α4β2 nAChR production were confined to ratios of α4:β2 that yielded concentration-dependent increases in binding sites, as extreme ratios of α4:β2 can produce sharp declines in receptor assembly. Although this restricted curve does not show the absolute saturation of binding site production as a function of α4 cDNA concentration, it does provide accurate estimates of the differences in binding sites produced across the α4 variants examined. Eliminating ER retention motifs in the β2 nAChR enhances nAChR transport from the ER, and the effect of the α4R487Q variant is consistent with accelerated nAChR assembly because of enhanced ER transport (Srinivasan et al., 2011), considering the proximity of this variant to an established RxR retention motif in the hα4 subunit. The α4P451L variant introduces a peroxisome targeting motif (PTS) and eliminates a highly conserved (100% across humans, rats, mice, and chickens) diproline motif. The decrease in [3H]epibatidine binding sites observed with α4P451L is consistent with the possibility that introducing a PTS results in mistargeting of α4 subunits, limiting the total number of pentameric α4β2 nAChRs produced. Although other interpretations are possible, the current observations provide a context for future studies. The α4R336C variant lost a putative 14-3-3 binding motif, but had no effect on the concentration dependence for cDNA effects on nAChR production, which was somewhat unexpected, given the role of 14-3-3 binding in nAChR trafficking (Jeanclos et al., 2001). Interestingly, despite these differences in assembly across variants, the number of α4β2 nAChRs that were expressed on the cell surface was not affected by any of these α4 variants.
Upregulation of α4β2* nAChRs by long-term exposure to nicotine has been suggested to play a role in the development/maintenance of nicotine dependence and has been observed repeatedly both in vitro and in vivo in rodents and human smokers (Staley et al., 2006; Lester et al., 2009). Nicotine increased [3H]epibatidine binding to nAChRs containing all α4 variants examined; however, although production of α4β2 nAChRs is deficient for α4P451L under control conditions, incubation with nicotine effectively “normalized” its ability to produce [3H]epibatidine binding sites with identical parameters as the other α4 variants.
Upregulation of nAChRs has been attributed to the ability of nicotine to bind to and stabilize nascent nAChRs in the process of assembly and maturation in the ER and reduce nAChR degradation after insertion into the plasma membrane (Kuryatov et al., 2005; Srinivasan et al., 2011). This process of pharmacological chaperoning is thought to involve selective stabilization of fully folded nAChR intermediates, rather than to promote the folding of primarily linear peptides (Lester et al., 2009). The observation that the α4P451L variant produces substantially fewer nAChRs, but that pharmacological chaperoning with nicotine boosts α4P451L assembly with β2 to levels consistent with the α4 common variant is particularly interesting because the P451L substitution removes a highly conserved diproline residue and introduces a novel PTS motif that may mistarget the receptor to the peroxisome or other intracellular compartments. It is possible that a conformational change produced when nascent α4P451Lβ2 receptors bind nicotine obscures the recognition of the PTS motif and allows assembly to proceed normally, without mistargeting or preferentially degrading the α4P451Lβ2 receptor intermediates. An alternate possibility is that elimination of the diproline residue produces an α4 subunit with more conformational flexibility in the large M3-M4 loop than the common variant and may trigger ER-resident chaperoning in a manner that favors rapid degradation in the absence of a stabilized conformation (as would be the case with pharmacological chaperoning by nicotine). Pro-pro motifs tend to produce segments of polypeptides with a constrained conformation (Saha and Shamala, 2012), so eliminating a strong structural constituent motif would be expected to radically alter the position and accessibility of several linear interaction motifs both C- and N-terminal of the disrupted sequence.
The possibility that the α4P451L variant results in ER-dependent nAChR degradation is supported by studies that identified a significant association between several rare variants in the α4 nAChR subunit gene, in particular the α4P451L polymorphism, and ALS (Sabatelli et al., 2009, 2012). A study examining an ALS-risk variant of the β4 nAChR subunit (β4R348C) found decreased expression of nAChRs when this variant was coexpressed with hα4, an effect primarily due to decreased ER export of α4β4R348C nAChRs (Richards et al., 2011). In that study, submicromolar nicotine did not alter the amount of α4β4 plasma membrane expression, so the deficient nAChR production resulting from expression of the β4R348C ALS-risk variant could not be rescued by concentrations of nicotine that normally produce upregulation of nAChRs and typically observed in smoker’s blood (Benowitz, 1996). Unlike the resistance of α4β4 nAChRs to nicotine-initiated upregulation in vitro, we find that nicotine reliably upregulates α4P451Lβ2 receptors to levels consistent with the common variant of α4. The α4R487Q variant was also identified as a risk variant for sporadic ALS (Sabatelli et al., 2012), but with lower frequency than observed for α4P451L. The results presented here demonstrate altered expression of these ALS-risk variants of the α4 nAChR subunit and suggest that this polymorphism clearly demands additional study, both for its effects on nicotine addiction and as a contributor to cellular stress and neurodegenerative disease.
The effects of the rare CHRNA4 variants on nAChR binding site production, plasma membrane trafficking, and upregulation by nicotine are further supported by proteomic studies. The α4 subunit of the nAChR is phosphorylated on several sites within the M3-M4 loop (Wecker et al., 2001; Pacheco et al., 2003; Pollock et al., 2007, 2009), and we augmented the proteomic study by enriching for phosphopeptides prior to LC-MS/MS to identify and profile potential shifts in the phosphorylation state of nAChR subunits and other putatively associated proteins attributable to the presence of the α4 rare variants. The degree of difference relative to the common variant may identify interesting targets for future mechanistic studies and is an indicator of the severity of effect of a single amino acid substitution on the function/assembly/trafficking of α4β2 nAChRs conferred by these rare α4 variants. The α4P451L and α4R336C variants have as many proteins that are unique or missing as are shared with the common α4 variant, whereas the α4R487Q variant is less divergent and only differs by 30%. The interactome of α4P451L in particular highlights the disproportionately large effect this single amino acid substitution has on its associated proteins. The α4P451L variant binds importin subunits without any evidence of nuclear transport, fails to associate with several isoforms of 14-3-3 (a known nAChR chaperone), in the Phospho fraction, and lacks association with the transport protein VAPB in the FT fraction. This lost interaction with VAPB is important, because mutations in VAPB are strongly associated with familial ALS (Nishimura et al., 2004), adding further support to the potential involvement of the α4P451L variant in the etiology of this neurodegenerative disease.
Electrophysiological measurements of heteromeric nAChRs have demonstrated that functional properties of these receptors are strongly influenced by receptor stoichiometry (Papke et al., 1989). Experiments that alter the relative expression of individual subunits (Zwart and Vijverberg 1998; Nelson et al., 2003; Moroni and Bermudez, 2006; Gotti et al., 2008) or use concatenated α4 and β2 subunits to force expression of defined subunit arrangements (Zhou et al., 2003), have demonstrated that functional parameters of α4β2 nAChRs are largely dependent on the position of individual subunits in the pentamer. Overall, α42β23 nAChRs are more sensitive to agonists than α43β22 nAChRs (Nelson et al., 2003), and both stoichiometries of α4β2 can be observed in the mammalian CNS (Marks et al., 1999, 2007). The current studies indicate that α4 polymorphisms may shift the ratio of α4β2 nAChR assembly to favor the production of HS receptors. Consistent with this possibility, we see that ACh concentration-response curves for the common α4 variant show both HS and LS components, indicative of the presence of both α4β2 stoichiometries, whereas α4 rare variants produced monophasic, high-sensitivity ACh concentration-response curves when expressed in X. laevis oocytes. Both X. laevis oocyte and HEK 293 experiments suggest that α4P451L preferentially assumes the α42β23 HS stoichiometry, likely due to decreased efficiency of α4 subunit incorporation. We did not, however, expect the same result for α4R336C and α4R487Q, which showed little difference relative to the common variant of α4 in nAChR expression or subcellular distribution when measured in transfected HEK293 cells. When expressing the receptor subunits as concatamers to restrict stoichiometry, the α4P451L variant produced HS receptors even when conditions strictly supported the assembly of LS α4β2 nAChRs, supporting the assertion that incorporation of this α4 variant is inefficient. The effects of α4R336C as a concatamer are more difficult to interpret, because both α4β2 stoichiometries were produced regardless of experiment conditions.
Upregulation of α4β2 nAChRs by nicotine exposure preferentially produces the HS form of the receptor (Kuryatov et al., 2005). After 24-hour nicotine, the common variant produced an ACh concentration-response curve with a monophasic, HS component. The α4P451L variant only produced HS nAChRs under baseline conditions and continued to produce only HS receptors after nicotine treatment. The α4R336C and α4R487Q variants, by contrast, responded to nicotine exposure by producing LS components in response to both ACh and nicotine. This effect opposes the actions of those variants on receptor assembly in the absence of nicotine. A confounding factor comes from the observation that X. laevis oocytes act as “nicotine sponges” during incubation with nicotine and release desensitizing concentrations of nicotine into superfusion buffer during recording (Jia et al., 2003). However, HS and LS forms of α4β2 nAChRs do not differ in their sensitivity to steady-state desensitization with subactivating concentrations of nicotine (Marks et al., 2010), so any differences in function observed after 24-hour nicotine across the α4 variants are due to effects conferred by the variants themselves.
A growing body of epidemiologic evidence suggests nicotine sensitivity is a potent determinant of smoking liability (Pomerleau et al., 2003; Hu et al., 2006). Furthermore, investigations with mouse strains led to the discovery of naturally occurring polymorphisms in nAChR subunits that influenced sensitivity to nicotine (Stitzel et al., 2000). One of these naturally occurring mouse polymorphisms was found in the intracellular loop of the α4 subunit (α4A529T) (Stitzel et al., 2001). Expression of the α4A529 variant increases sensitivity to nicotine, eliminates nicotine conditioned place preference, and produces increased HS α4β2 nAChRs in the midbrain (Wilking et al., 2010). A smoking risk-associated nAChR polymorphism that occurs in the α5 nAChR subunit (Bierut et al., 2008) introduces an amino acid substitution in the intracellular loop of α5 (α5D397N; D398N for mouse sequence) and results in decreased function of α4β2 and α3β4 nAChRs (Bierut et al., 2008; Kuryatov et al., 2011). Thus, expression of nAChRs in the CNS that favor high-agonist sensitivity assemblies appear to be associated with a decrease in risk for nicotine dependence, whereas expression of lower-sensitivity receptors increases risk of nicotine dependence.
Conclusions
The current observations that certain rare α4 nAChR subunit variants that are apparently underrepresented among dependent smokers are more likely to assemble in the high sensitivity conformation supports the link between agonist sensitivity of neuronal nAChRs with the likelihood of nicotine dependence and addiction-related behaviors. We have shown that single amino acid substitutions in the large M3-M4 loop of the α4 nAChR subunit can result in large changes in receptor number, stoichiometry, and associated proteins. These studies also suggest potential molecular mechanisms by which these effects are produced by the individual mutations. Although our mechanistic grasp of the functional and expression differences for α4R336C and α4R487Q is not complete, changes in intracellular interactions with associated proteins or posttranslational modifications (phosphorylation, glycosylation) may be involved in the effects of these rare α4 nAChR subunit variants. For α4P451L, considerable evidence suggests that deficient assembly in the early maturational stages of the receptor results in the preferential exclusion of α4P451L from the fifth position, resulting in constrained expression of α42β23 nAChRs. This mechanism is consistent with reports indicating that α4P451L is a risk variant for sporadic ALS, and such association may result from enhanced ER stress due to deficient receptor assembly.
Supplementary Material
Abbreviations
- ACh
acetylcholine
- ALS
amyotrophic lateral sclerosis
- CNS
central nervous system
- FT
flow through
- HS
high sensitivity
- LC-MS/MS
liquid chromatography and tandem mass spectrometry
- LS
lower sensitivity
- nAChR
nicotinic acetylcholine receptor
- Phospho
phospho enriched
- VAPB
vesicle-associated-protein-B
Authorship Contributions
Participated in research design: McClure-Begley, Papke, Gelernter, Xie, Picciotto.
Conducted experiments: McClure-Begley, Papke, Stone, Stokes, Levy.
Contributed new reagents or analytic tools: Stone, Lindstrom, Xie.
Performed data analysis: McClure-Begley, Papke, Stone, Stokes, Levy.
Wrote or contributed to the writing of the manuscript: McClure-Begley, Papke, Gelernter, Picciotto.
Footnotes
This work was supported by the National Institutes of Health National Institute on Drug Abuse (NIDA) [Grants DA14241, DA018343 (NIDA Proteomics Center at Yale University)]; National Institutes of Health National Institute of Mental Health [Grant MH077681]; National Institutes of Health National Center for Advancing Translational Sciences [Grant UL1-TR000142 (Yale Clinical and Translational Science Award)]; National Institutes of Health National Institute on Drug Abuse [Grants RC2-DA028909, R01-DA12690, and R01-DA12849]; and the State of Connecticut Department of Mental Health and Addiction Services. T.D.M. was supported by National Institutes of Health National Institute of Mental Health [Grant T32-MH014276] and National Institutes of Health National Institute on Alcohol and Alcoholism [Grant T32-AA07464].
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Bierut LJ, Stitzel JA, Wang JC, Hinrichs AL, Grucza RA, Xuei X, Saccone NL, Saccone SF, Bertelsen S, Fox L, et al. (2008) Variants in nicotinic receptors and risk for nicotine dependence. Am J Psychiatry 165:1163–1171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL. (1996) Pharmacology of nicotine: addiction and therapeutics. Annu Rev Pharmacol Toxicol 36:597–613 [DOI] [PubMed] [Google Scholar]
- Gotti C, Moretti M, Meinerz NM, Clementi F, Gaimarri A, Collins AC, Marks MJ. (2008) Partial deletion of the nicotinic cholinergic receptor alpha 4 or beta 2 subunit genes changes the acetylcholine sensitivity of receptor-mediated 86Rb+ efflux in cortex and thalamus and alters relative expression of alpha 4 and beta 2 subunits. Mol Pharmacol 73:1796–1807 [DOI] [PubMed] [Google Scholar]
- Han S, Gelernter J, Luo X, Yang BZ. (2010) Meta-analysis of 15 genome-wide linkage scans of smoking behavior. Biol Psychiatry 67:12–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Yang BZ, Kranzler HR, Oslin D, Anton R, Gelernter J. (2011) Association of CHRNA4 polymorphisms with smoking behavior in two populations. Am J Med Genet B Neuropsychiatr Genet 156B:421–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness PC, Millar NS. (2002) Changes in conformation and subcellular distribution of alpha4beta2 nicotinic acetylcholine receptors revealed by chronic nicotine treatment and expression of subunit chimeras. J Neurosci 22:10172–10181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu MC, Davies M, Kandel DB. (2006) Epidemiology and correlates of daily smoking and nicotine dependence among young adults in the United States. Am J Public Health 96:299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanclos EM, Lin L, Treuil MW, Rao J, DeCoster MA, Anand R. (2001) The chaperone protein 14-3-3eta interacts with the nicotinic acetylcholine receptor alpha 4 subunit. Evidence for a dynamic role in subunit stabilization. J Biol Chem 276:28281–28290 [DOI] [PubMed] [Google Scholar]
- Jia L, Flotildes K, Li M, Cohen BN. (2003) Nicotine trapping causes the persistent desensitization of alpha4beta2 nicotinic receptors expressed in oocytes. J Neurochem 84:753–766 [DOI] [PubMed] [Google Scholar]
- Kamens HM, Corley RP, McQueen MB, Stallings MC, Hopfer CJ, Crowley TJ, Brown SA, Hewitt JK, Ehringer MA. (2013) Nominal association with CHRNA4 variants and nicotine dependence. Genes Brain Behav 12:297–304 Epub ahead of print [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kracun S, Harkness PC, Gibb AJ, Millar NS. (2008) Influence of the M3-M4 intracellular domain upon nicotinic acetylcholine receptor assembly, targeting and function. Br J Pharmacol 153:1474–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukhtina V, Kottwitz D, Strauss H, Heise B, Chebotareva N, Tsetlin V, Hucho F. (2006) Intracellular domain of nicotinic acetylcholine receptor: the importance of being unfolded. J Neurochem 97 (Suppl 1):63–67 [DOI] [PubMed] [Google Scholar]
- Kuo YP, Xu L, Eaton JB, Zhao L, Wu J, Lukas RJ. (2005) Roles for nicotinic acetylcholine receptor subunit large cytoplasmic loop sequences in receptor expression and function. J Pharmacol Exp Ther 314:455–466 [DOI] [PubMed] [Google Scholar]
- Kuryatov A, Berrettini W, Lindstrom J. (2011) Acetylcholine receptor (AChR) α5 subunit variant associated with risk for nicotine dependence and lung cancer reduces (α4β2)₂α5 AChR function. Mol Pharmacol 79:119–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Luo J, Cooper J, Lindstrom J. (2005) Nicotine acts as a pharmacological chaperone to up-regulate human alpha4beta2 acetylcholine receptors. Mol Pharmacol 68:1839–1851 [DOI] [PubMed] [Google Scholar]
- Lester HA, Xiao C, Srinivasan R, Son CD, Miwa J, Pantoja R, Banghart MR, Dougherty DA, Goate AM, Wang JC. (2009) Nicotine is a selective pharmacological chaperone of acetylcholine receptor number and stoichiometry. Implications for drug discovery. AAPS J 11:167–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas RJ, Changeux JP, Le Novère N, Albuquerque EX, Balfour DJ, Berg DK, Bertrand D, Chiappinelli VA, Clarke PB, Collins AC, et al. (1999) International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol Rev 51:397–401 [PubMed] [Google Scholar]
- Marks MJ, Meinerz NM, Brown RW, Collins AC. (2010) 86Rb+ efflux mediated by alpha4beta2*-nicotinic acetylcholine receptors with high and low-sensitivity to stimulation by acetylcholine display similar agonist-induced desensitization. Biochem Pharmacol 80:1238–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Meinerz NM, Drago J, Collins AC. (2007) Gene targeting demonstrates that alpha4 nicotinic acetylcholine receptor subunits contribute to expression of diverse [3H]epibatidine binding sites and components of biphasic 86Rb+ efflux with high and low sensitivity to stimulation by acetylcholine. Neuropharmacology 53:390–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MJ, Whiteaker P, Calcaterra J, Stitzel JA, Bullock AE, Grady SR, Picciotto MR, Changeux JP, Collins AC. (1999) Two pharmacologically distinct components of nicotinic receptor-mediated rubidium efflux in mouse brain require the beta2 subunit. J Pharmacol Exp Ther 289:1090–1103 [PubMed] [Google Scholar]
- McGranahan TM, Patzlaff NE, Grady SR, Heinemann SF, Booker TK. (2011) α4β2 nicotinic acetylcholine receptors on dopaminergic neurons mediate nicotine reward and anxiety relief. J Neurosci 31:10891–10902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar NS, Gotti C. (2009) Diversity of vertebrate nicotinic acetylcholine receptors. Neuropharmacology 56:237–246 [DOI] [PubMed] [Google Scholar]
- Moroni M, Bermudez I. (2006) Stoichiometry and pharmacology of two human alpha4beta2 nicotinic receptor types. J Mol Neurosci 30:95–96 [DOI] [PubMed] [Google Scholar]
- Nelson ME, Kuryatov A, Choi CH, Zhou Y, Lindstrom J. (2003) Alternate stoichiometries of alpha4beta2 nicotinic acetylcholine receptors. Mol Pharmacol 63:332–341 [DOI] [PubMed] [Google Scholar]
- Nishimura AL, Mitne-Neto M, Silva HC, Richieri-Costa A, Middleton S, Cascio D, Kok F, Oliveira JR, Gillingwater T, Webb J, et al. (2004) A mutation in the vesicle-trafficking protein VAPB causes late-onset spinal muscular atrophy and amyotrophic lateral sclerosis. Am J Hum Genet 75:822–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco MA, Pastoor TE, Wecker L. (2003) Phosphorylation of the alpha4 subunit of human alpha4beta2 nicotinic receptors: role of cAMP-dependent protein kinase (PKA) and protein kinase C (PKC). Brain Res Mol Brain Res 114:65–72 [DOI] [PubMed] [Google Scholar]
- Papke RL, Boulter J, Patrick J, Heinemann S. (1989) Single-channel currents of rat neuronal nicotinic acetylcholine receptors expressed in Xenopus oocytes. Neuron 3:589–596 [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Léna C, Bessis A, Lallemand Y, Le Novère N, Vincent P, Pich EM, Brûlet P, Changeux JP. (1995) Abnormal avoidance learning in mice lacking functional high-affinity nicotine receptor in the brain. Nature 374:65–67 [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Léna C, Marubio LM, Pich EM, Fuxe K, Changeux JP. (1998) Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature 391:173–177 [DOI] [PubMed] [Google Scholar]
- Pollock VV, Pastoor T, Katnik C, Cuevas J, Wecker L. (2009) Cyclic AMP-dependent protein kinase A and protein kinase C phosphorylate alpha4beta2 nicotinic receptor subunits at distinct stages of receptor formation and maturation. Neuroscience 158:1311–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock VV, Pastoor TE, Wecker L. (2007) Cyclic AMP-dependent protein kinase (PKA) phosphorylates Ser362 and 467 and protein kinase C phosphorylates Ser550 within the M3/M4 cytoplasmic domain of human nicotinic receptor alpha4 subunits. J Neurochem 103:456–466 [DOI] [PubMed] [Google Scholar]
- Pomerleau OF, Fagerström KO, Marks JL, Tate JC, Pomerleau CS. (2003) Development and validation of a self-rating scale for positive- and negative-reinforcement smoking: The Michigan Nicotine Reinforcement Questionnaire. Nicotine Tob Res 5:711–718 [DOI] [PubMed] [Google Scholar]
- Richards CI, Srinivasan R, Xiao C, Mackey ED, Miwa JM, Lester HA. (2011) Trafficking of alpha4* nicotinic receptors revealed by superecliptic phluorin: effects of a beta4 amyotrophic lateral sclerosis-associated mutation and chronic exposure to nicotine. J Biol Chem 286:31241–31249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatelli M, Eusebi F, Al-Chalabi A, Conte A, Madia F, Luigetti M, Mancuso I, Limatola C, Trettel F, Sobrero F, et al. (2009) Rare missense variants of neuronal nicotinic acetylcholine receptor altering receptor function are associated with sporadic amyotrophic lateral sclerosis. Hum Mol Genet 18:3997–4006 [DOI] [PubMed] [Google Scholar]
- Sabatelli M, Lattante S, Conte A, Marangi G, Luigetti M, Del Grande A, Chiò A, Corbo M, Giannini F, Mandrioli J, et al. (2012) Replication of association of CHRNA4 rare variants with sporadic amyotrophic lateral sclerosis: the Italian multicentre study. Amyotroph Lateral Scler 13:580–584 [DOI] [PubMed] [Google Scholar]
- Saha I, Shamala N. (2012) Investigating diproline segments in proteins: occurrences, conformation and classification. Biopolymers 97:54–64 [DOI] [PubMed] [Google Scholar]
- Srinivasan R, Pantoja R, Moss FJ, Mackey ED, Son CD, Miwa J, Lester HA. (2011) Nicotine up-regulates alpha4beta2 nicotinic receptors and ER exit sites via stoichiometry-dependent chaperoning. J Gen Physiol 137:59–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E, Dubin JA, Estok K, Brenner E, Baldwin RM, et al. (2006) Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. J Neurosci 26:8707–8714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitzel JA, Dobelis P, Jimenez M, Collins AC. (2001) Long sleep and short sleep mice differ in nicotine-stimulated 86Rb+ efflux and alpha4 nicotinic receptor subunit cDNA sequence. Pharmacogenetics 11:331–339 [DOI] [PubMed] [Google Scholar]
- Stitzel JA, Jimenez M, Marks MJ, Tritto T, Collins AC. (2000) Potential role of the alpha4 and alpha6 nicotinic receptor subunits in regulating nicotine-induced seizures. J Pharmacol Exp Ther 293:67–74 [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Marks MJ, Lester HA. (2007) Nicotine responses in hypersensitive and knockout alpha 4 mice account for tolerance to both hypothermia and locomotor suppression in wild-type mice. Physiol Genomics 31:422–428 [DOI] [PubMed] [Google Scholar]
- Tapper AR, McKinney SL, Nashmi R, Schwarz J, Deshpande P, Labarca C, Whiteaker P, Marks MJ, Collins AC, Lester HA. (2004) Nicotine activation of alpha4* receptors: sufficient for reward, tolerance, and sensitization. Science 306:1029–1032 [DOI] [PubMed] [Google Scholar]
- Tsetlin V, Kuzmin D, Kasheverov I. (2011) Assembly of nicotinic and other Cys-loop receptors. J Neurochem 116:734–741 [DOI] [PubMed] [Google Scholar]
- Wecker L, Guo X, Rycerz AM, Edwards SC. (2001) Cyclic AMP-dependent protein kinase (PKA) and protein kinase C phosphorylate sites in the amino acid sequence corresponding to the M3/M4 cytoplasmic domain of alpha4 neuronal nicotinic receptor subunits. J Neurochem 76:711–720 [DOI] [PubMed] [Google Scholar]
- Whiting PJ, Lindstrom JM. (1988) Characterization of bovine and human neuronal nicotinic acetylcholine receptors using monoclonal antibodies. J Neurosci 8:3395–3404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilking JA, Hesterberg KG, Crouch EL, Homanics GE, Stitzel JA. (2010) Chrna4 A529 knock-in mice exhibit altered nicotine sensitivity. Pharmacogenet Genomics 20:121–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DK, Stokes C, Horenstein NA, Papke RL. (2011) The effective opening of nicotinic acetylcholine receptors with single agonist binding sites. J Gen Physiol 137:369–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie P, Kranzler HR, Krauthammer M, Cosgrove KP, Oslin D, Anton RF, Farrer LA, Picciotto MR, Krystal JH, Zhao H, et al. (2011) Rare nonsynonymous variants in alpha-4 nicotinic acetylcholine receptor gene protect against nicotine dependence. Biol Psychiatry 70:528–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Nelson ME, Kuryatov A, Choi C, Cooper J, Lindstrom J. (2003) Human alpha4beta2 acetylcholine receptors formed from linked subunits. J Neurosci 23:9004–9015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart R, Vijverberg HP. (1998) Four pharmacologically distinct subtypes of alpha4beta2 nicotinic acetylcholine receptor expressed in Xenopus laevis oocytes. Mol Pharmacol 54:1124–1131 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.