Abstract
Background & Aims
In the enteric nervous system, neurotransmitters initiate changes in Ca2+ (Ca2+ responses) in glia, but it is not clear how this process affects intestinal function. We investigated whether Ca2+-mediated responses in enteric glial are required to maintain gastrointestinal function.
Methods
We used in situ Ca2+ imaging to monitor glial Ca2+ responses, which were manipulated with pharmacologic agents or via glia-specific disruption of the gene encoding connexin-43 (Cx43) (hGFAP::creERT2+/−/Cx43f/f mice). Gastrointestinal function was assessed based on pellet output, total gut transit, colonic bead expulsion, and muscle tension recordings. Proteins were localized and quantified by immunohistochemistry, immunoblot, and reverse transcription PCR analyses.
Results
Ca2+ responses in enteric glia of mice were mediated by Cx43 hemichannels. Cx43 immunoreactivity was confined to enteric glia within the myenteric plexus of the mouse colon; the Cx43 inhibitors carbenoxolone and 43Gap26 inhibited the ability of enteric glia to propagate Ca2+ responses. In vivo attenuation of Ca2+ responses in the enteric glial network slowed gut transit overall and delayed colonic transit—these changes are also observed during normal aging. Altered motility with increasing age was associated with reduced glial Ca2+-mediated responses and changes in glial expression of Cx43 mRNA and protein.
Conclusions
Ca2+-mediated responses in enteric glia regulate gastrointestinal function in mice. Altered intercellular signaling between enteric glia and neurons might contribute to motility disorders.
Keywords: intestinal nervous system, aging, purines, mouse model
INTRODUCTION
The enteric nervous system (ENS) acts as the principal regulator of gastrointestinal functions. Its functional output is mainly considered a product of integration between neurons but recent evidence may implicate involvement of enteric glia (EG) in this process1. EG surround neuron cell bodies and processes and “listen” to neuronal conversations2. Specifically, purines released from neurons in the myenteric plexus (MP) recruit Ca2+ responses in the surrounding EG, in vitro3 and in situ2, including during physiological patterns of gut motility4. However, the physiological significance of glial Ca2+ responses is still highly contested5 and evidence demonstrating any role of glial Ca2+ responses in gastrointestinal physiology is currently lacking.
Experimental models that specifically ablate EG6,7 or poison them with metabolic toxins8 suggest that functional EG are necessary for the maintenance of gastrointestinal function. However, these bold approaches lack the ability to specifically affect particular glial functions.
As indicted above, the importance of glial Ca2+ responses in normal gut physiology is unknown. We hypothesized that EG Ca2+ responses are necessary to maintain the fidelity of gastrointestinal function. In the present study, we found that EG express a cell surface hemichannel composed of connexin-43 (Cx43) that is necessary for the propagation of Ca2+ responses among glia. Consequently, we investigated how glial Ca2+ responses influence gut motility and intestinal transit by using an inducible, glial–specific Cx43 knock-out mouse model that blunts the ability of EG to initiate intracellular Ca2+ responses. We find that selectively hampering EG Ca2+ responses slows gut transit, creating a similar phenotype as observed during normal aging. Indeed, we also found that glial Ca2+ responses are subdued in parallel with a slowing of motility across normal adult life in mice, associated with changes in Cx43 expression/transcription. Our results suggest that EG Ca2+ responses play an essential role in the enteric regulation of gut function.
METHODS
Animals
C57Bl/6 mice of both genders, unless otherwise stated, were used (Harlan Laboratories, Inc., Indianapolis, IN). Mice were maintained in a temperature-controlled environment on a 12:12 hour light:dark cycle, with ad libitum access to food and water. Experimental protocols were approved by Institutional Animal Care and Use Committees at Michigan State University and University of Alabama at Birmingham.
Transgenic mice were generated on C57Bl/6 genetic background. The inducible and conditional knock-out (i-cKO) Cx43 mouse model (i.e. hGFAP::creERT2+/−/Cx43f/f) was generated by crossing two parental lines: (i) line with floxed exon 2 of Cx43 (Cx43f/f)9, and (ii) hGFAP::creERT2+/− line that utilizes a human glial fibrillary acidic protein (GFAP) promoter to drive the expression of a fusion protein between Cre recombinase and the mutated estrogen receptor (ERT2) responsive to the active 4-hydroxy of tamoxifen10. Additionally, we crossed the Cre reporter line Rosa26/CAG::STOPfl/fl::tdTomato11 (The Jackson Laboratory, stock number 007914) with the hGFAP::creERT2+/− line or with the icKO animals, resulting in double (hGFAP::CreERT2+/−/tdTomato+/−) and triple (hGFAP::CreERT2+/−/Cx43f/f/tdTomato+/−) transgenic animals, respectively. To induce knock-out of Cx43 from EG of male mice at 3 months of age (m.o.a). we used following induction protocols: (i) animals were fed with tamoxifen citrate (400 mg/kg) for 6 weeks12 and then 2 weeks with normal food before the in vivo experiments; (ii) to confirm reduction of Cx43 by immunohistochemistry animals were injected with tamoxifen free base (1 mg per 10 g of body weight; Sigma, Cat. No. T5648) twice a day for five days10 or fed with tamoxifen citrate (400 mg/kg) for 2 weeks. Of note, the treatments we used require tamoxifen conversion to the active 4-hydroxy form in the liver and leads to recombination in astroglia regardless of their location. Genotyping was performed in house and commercially (Transnetyx, Inc, Cordova, TN).
Ca2+ imaging
Whole–mount preparations of the MP from mouse colons were processed as described elsewhere13. Images were acquired every 1-2 s through the 40X water immersion objective [LUMPlan N, 0.8 numerical aperture (n.a.)] of an upright Olympus BX51WI fixed stage microscope (Olympus, Center Valley, PA) using IQ2 software and a Neo sCMOS camera (Andor, South Windsor, CT). Whole-mounts were continually perfused with buffer solution (~37°C) at 2-3 mL min-1.
Whole-mount immunohistochemistry
Whole-mount preparations of the colonic MP were subjected to immunohistochemistry as described previously13. Antibody details are supplied in Supplementary Table 1. Images were acquired through the 40X (PlanFluor, 0.75 n.a.) objective of an upright epifluorescence microscope (Nikon Eclipse Ni, Melville, NY) with a Retiga 2000R camera (QImaging) controlled by QCapture Pro 7.0 (QImaging) or the 20X and 60X objectives (PlanApo N, 0.85 and 1.42 n.a., oil, respectively) of a FluoView FV 300 (Olympus) confocal laser scanning microscope. Mean fluorescence intensity was measured using ImageJ (NIH) from a minimum of 10 ganglia per animal.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Total mRNA was isolated from colons following removal of mucosa and submucosal plexus using TRIzol® Reagent (Invitrogen) and reverse transcribed (Superscript® First-Strand Synthesis Kit, Invitrogen) following the manufacturer's protocol. Quantitative PCR was performed using a Taqman gene expression assay for mouse Cx43 in a 7500 Real-time PCR System (Applied Biosystems, Foster City, CA). Fold changes from 2 m.o.a. animals were calculated using the 2−ΔΔCT method14; glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used for normalization.
Western blot
After removing the mucosa and submucosa, colons were placed into RIPA buffer, containing a cocktail of protease inhibitors, snap frozen on dry ice and homogenized. Protein concentration was measured using a BCA protein assay kit (Bio-Rad, Hercules, CA). Aliquots containing ~40 μg of protein were resolved in 8% SDS-PAGE under reducing conditions and transferred to a nitrocellulose membrane. The membrane was blocked with 1% BSA in PBS for 1 hour and incubated with primary antibody overnight at 4°C. After washing, the membrane was incubated with secondary antibody before imaging on a Li-Cor Odyssey (Li-Cor, Lincoln, NE). Band intensity was analyzed using Image Studio (Li-Cor) and expressed as a ratio of β-actin.
Colon bead assay
Distal colonic transit time was measured using glass beads (2 mm in diameter) as described previously8.
Pellet production
Fecal pellet output was measured15 at zeitgeber +3 for 1h and fluid content was calculated16.
Whole gut transit
Whole intestinal transit time was determined as previously described17.
Contractility studies
We performed colon contractility studies as previously described13. Briefly, isometric contractions were recorded from segments of distal colon under 1 g passive tension with a force transducer (Grass Instruments, Quincy, MA) and responses charted with Labscribe (iWorx, Dover, NH). Responses were normalized to an initial carbachol-stimulated contraction. Electrical field stimulation (EFS; 20 V, 5–40 Hz) and (10 Hz, 10–40V) was applied through platinum concentric electrodes to evoke neurogenic contractions/relaxations. Maximum relaxations were stimulated by sodium nitroprusside (SNP, 10 μM) and tetrodotoxin (TTX, 0.3 μM) was used to confirm EFS-evoked responses were neurogenic.
Solutions
Modified Krebs buffer contained (in mmol/L): 121 NaCl, 5.9 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 10 HEPES, 21.2 NaHCO3, 1 pyruvic acid, 8 glucose (pH adjusted to 7.4 with NaOH). 3 μmol/L nicardipine and 1 μmol/L scopolamine were added to inhibit muscle contractions during Ca2+ imaging and whole-mount dissections.
RESULTS
Enteric glia express connexin-43
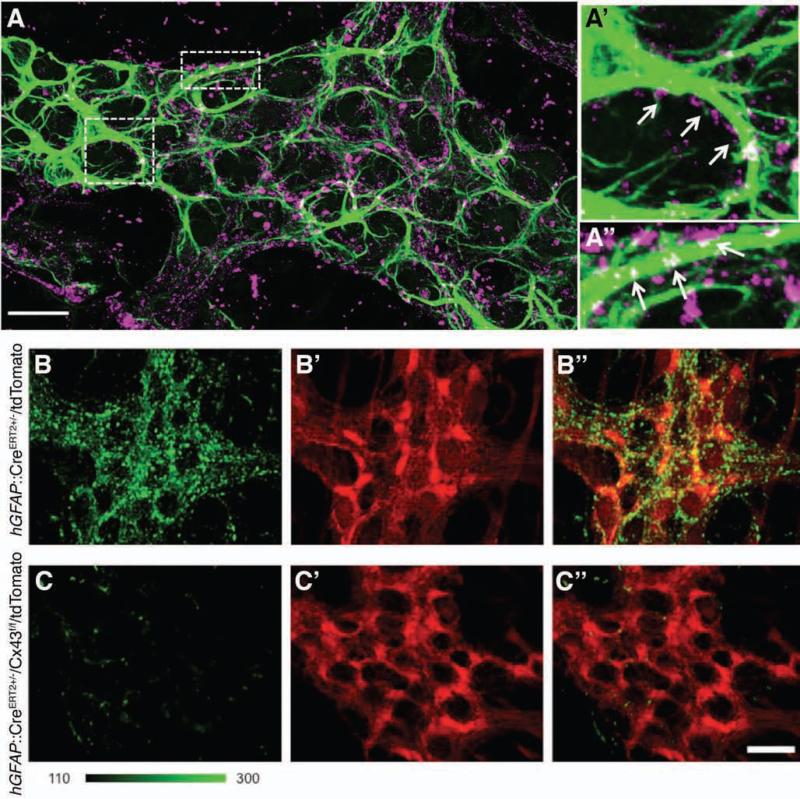
Propagation of Ca2+ responses between EG depends on their ATP release through hemichannels18. Given that Cx43 hemichannels are the most prominent form expressed by astrocytes and participate in purine release19-21, we hypothesized that Cx43 hemichannels are responsible for propagated Ca2+ responses between EG. Because Cx43 expression within the ENS is uncharacterized, we began by evaluating the distribution of Cx43 within the MP. We found that Cx43 is distributed throughout the mouse colon MP and localizes to the cell bodies and processes of EG (Fig. 1A-A”). Cx43-immunoreactive plaques colocalize with GFAP–immunoreactive glial processes and are distributed across most, if not all myenteric glia. Thus, the pattern of Cx43 immunoreactivity and localization to EG within the MP is consistent with the localization of Cx43 on astrocytes in the CNS22,23.
Figure 1.
EG express Cx43. (A) GFAP (green) and Cx43 (magenta) immunoreactivity (ir) in the mouse colon myenteric plexus (scale bar = 20 μm). (A’–A”) Panels at right show enlarged view of boxed areas in A. Note connexin-43–ir puncta in near vicinity of (A’) of or colocalized with (A”) GFAP–ir EG (arrows). Images are representative of labeling in a minimum of 4 animals. Tamoxifen treatment induces tdTomato reporter expression in EG (red, B’) without altering Cx43–ir (green, B) in the hGFAP::CreERT2+/−/tdTomato+/− reporter mouse. (B””) Overlay of B-B”. (C) Cx43-ir (green) is diminished following tamoxifen–mediated specific glial ablation of Cx43 in hGFAP::CreERT2+/−/Cx43f/f/tdTomato+/− mice. (C’) tdTomato reporter expression in EG (red). (C”) Overlay of C-C’. B and C are presented using the same fluorescence dynamic scale (a linear green scale ranging 100-300 fluorescence intensity units). Scale bar in C” = 10 μm and applies to B-C”.
The above approach could not discount the possibility that enteric neurons also express Cx43. To determine the breadth of Cx43 expression throughout the ENS, we used an inducible and conditional knock out (i-cKO), hGFAP::CreERT2+/−/Cx43f/f, mouse model10 (see also material and methods). Tamoxifen administration to i-cKO animals selectively knocks out Cx43 gene in GFAP positive EG. We also crossed i-cKO with a reporter line where Cre recombinase drives expression of tdTomato to facilitate the assessment of the efficiency and specificity of Cre-mediated recombination (triple cross line denoted as hGFAP::CreERT2+/−/Cx43f/f/tdTomato+/−). As controls, we used hGFAP::CreERT2+/−/tdTomato+/− mice to rule out confounding effects of transgene expression or tamoxifen treatment. Induction of Cre recombinase with tamoxifen in hGFAP::CreERT2+/−/tdTomato+/− mice drove robust expression of tdTomato in myenteric glia of the mouse colon (Supplementary Fig. 1) and immunolabeling for Cx43 remained comparable to wild type in both intensity and distribution (Fig. 1B-B”). In contrast, tamoxifen treatment induced a loss of Cx43 immunoreactivity within the MP in hGFAP::CreERT2+/−/Cx43f/f/tdTomato+/− mice without overtly altering glial morphology (Fig. 1C-C”). From these results, we conclude that Cx43 is expressed predominantly, if not exclusively, by EG within the mouse MP and that our transgenic mouse strategy efficiently removes EG Cx43.
Purine-evoked enteric glial network responses require connexin-43
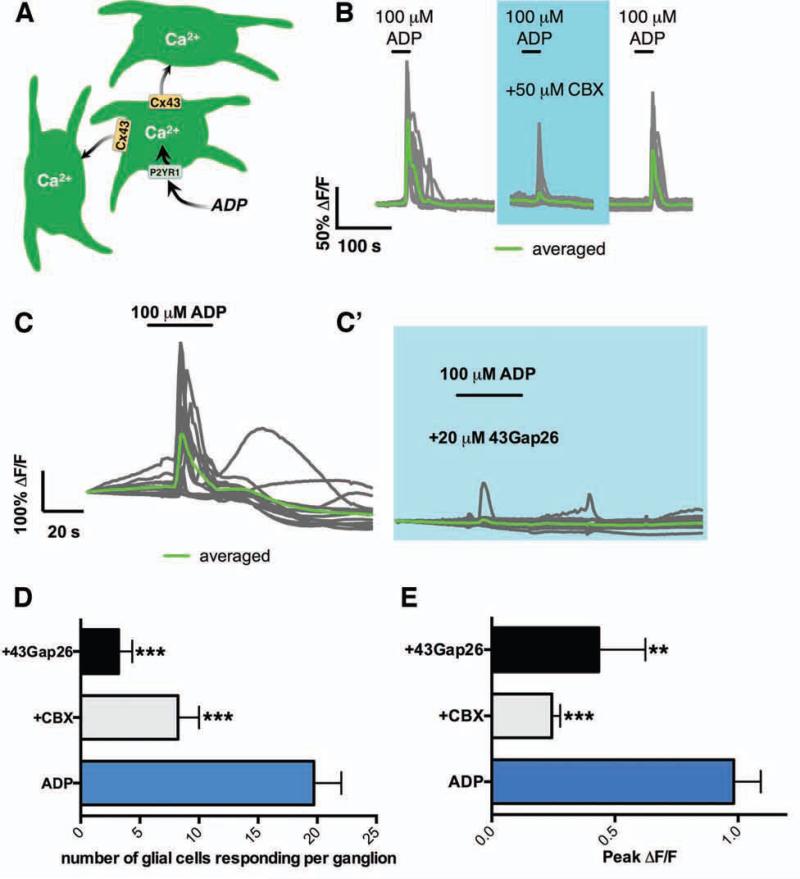
We next tested if EG Cx43 hemichannels are necessary for propagated Ca2+ responses among EG (Fig. 2a). We began by directly assessing how the pharmacological inhibition of Cx43 with carbenoxolone (CBX; 50 μM) affects the ability of glia to respond to purines and propagate Ca2+ responses. Stimulation of glial P2Y1 receptors with the agonist ADP (100 μM) elicited robust Ca2+ responses (average peak ΔF/F = 0.9825 ± 0.1; Fig. 2B, E) in an average of 20 ± 2 glia per myenteric ganglion (Fig. 2B, D). CBX reduced the number of responding glia per ganglion by 60% (8 ± 2 glia per myenteric ganglion) (Fig. 2B, D). Peak Ca2+ responses in glia still exhibiting responses in the presence of CBX were reduced by 75% (Fig. 2E).
Figure 2.
Purine–evoked Ca2+ waves between EG depend on Cx43. (A) Model of purine–evoked Ca2+ responses through the EG network. (B) Representative traces of Ca2+ response evoked by ADP (30 s, 100 μM) in the presence or absence of the Cx43 antagonist, carbenoxolone (CBX; 50 μM; blue shaded area) in 21 EG within a myenteric ganglion (gray traces, average in green). Note that CBX limits the number of responding glia and on average, the Ca2+ response is lost. Glial responses recover upon washout of drug. (C) Representative Ca2+ response evoked by ADP in 13 EG within a myenteric ganglion (gray traces, average in green) in the presence or absence of the specific Cx43 mimetic peptide, 43Gap26 (20 μM). Effect of CBX and 43Gap26 on (D) the number of glia responding to ADP per ganglion and (E) peak glial Ca2+ responses (n=5-14, ***P=.0001, ANOVA).
Although CBX at 50 μM is relatively selective for Cx4324, unintended effects mediated by modulation of other channels are possible. Consequently, we repeated the above experiments with a selective, small molecule mimetic peptide inhibitor of Cx43 hemichannels (43Gap26; 20 μM40). Similar to CBX, 43Gap26 significantly reduced both the number of glia per ganglion responding to ADP by 85% (3 ± 1 glia per ganglion; Fig. 2C-C’ and D) and peak Ca2+ responses in those glia by 56% (Fig. 2E). Importantly, glial responses in the presence of 43Gap26 were not significantly different from those in the presence of CBX. Thus, Cx43 hemichannels are necessary for propagated Ca2+ responses through the EG network initiated by exogenous agonists.
Purine-evoked enteric glial network responses require connexin-43
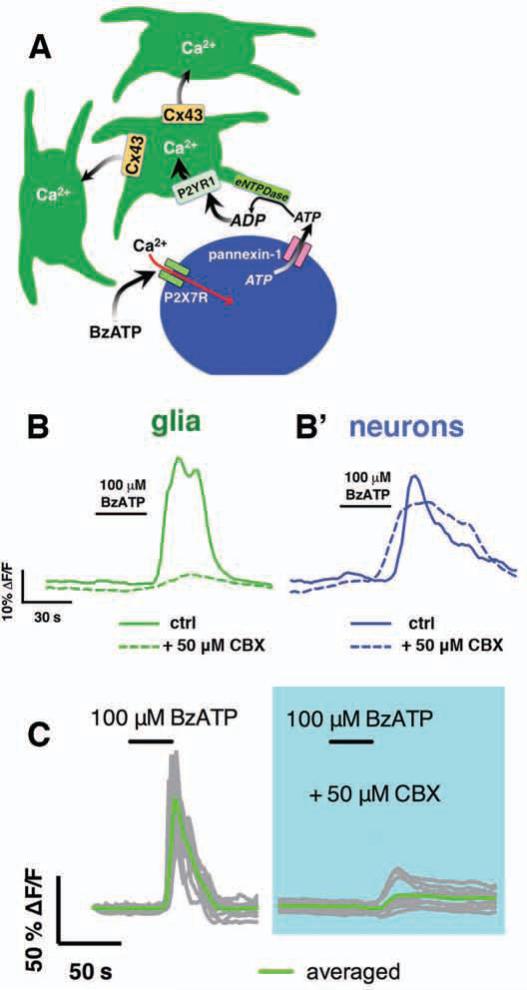
To determine if Cx43 is required for glial responses initiated by endogenous agonists, we stimulated enteric neuron–to–glia communication with the neuronal P2X7 selective agonist BzATP (100 μM) and monitored glial Ca2+ responses (generated neuron released purines) in the presence or absence of CBX. As we previously described13, BzATP elicited robust Ca2+ responses in enteric neurons followed by Ca2+ responses in the surrounding EG (Fig. 3B). Blockade of Cx43 channels with CBX did not affect the ability of enteric neurons to respond to BzATP (Fig. 3B’) but attenuated subsequent glial responses by 91.4 ± 4% (Fig. 3B). The remaining minority of responsive glia, however, failed to recruit Ca2+ responses in surrounding glia (Fig. 3C). These results suggest that Cx43 function is necessary for the propagation of responses through the EG network initiated by endogenous neurotransmitters.
Figure 3.
EG Ca2+ waves evoked by enteric neuron–to–glia communication depend on Cx43. (A) Model depicting how pharmacological activation of enteric neurons initiates Ca2+ responses in EG. (B) Representative traces of average Ca2+ response in glia (green traces) and neurons (blue traces) within a myenteric ganglion following stimulation of enteric neuron–to–glia communication with the P2X7 agonist, BzATP (100 μM). Note that both neurons and glia respond in control (solid traces) but glial responses are lost when Cx43 is inhibited with CBX (50 μM; dashed traces). (C) Representative Ca2+ wave evoked through 12 glia within a myenteric ganglion (gray traces, average in green) by stimulating enteric neuron–glia communication with BzATP in the presence or absence of CBX.
Selective ablation of glial connexin-43 alters gastrointestinal motility
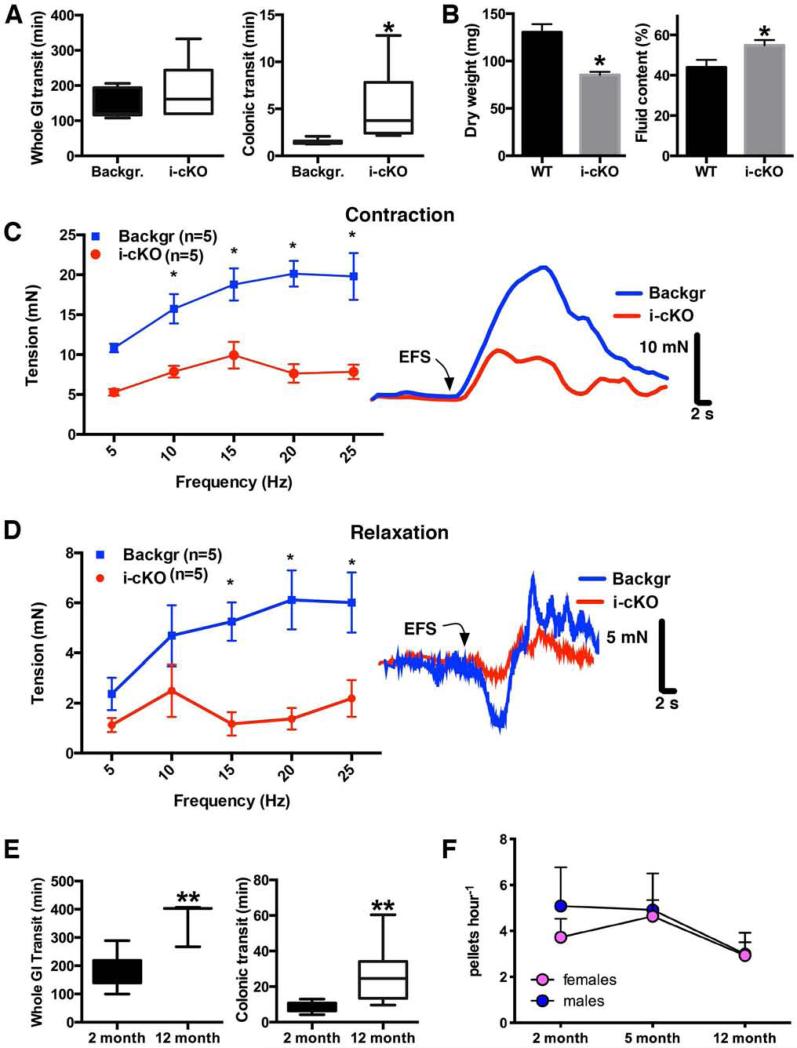
We hypothesize that glial Ca2+ responses modulate mechanisms that control gut motility. Given that inhibition of Cx43 hemichannels limited glial Ca2+ responses, we hypothesized that selectively ablating glial Cx43, and thus Ca2+ responses, would impair gut function. We tested our hypothesis by assessing in vivo gut function in our i-cKO mouse line (hGFAP::CreERT2+/−/Cx43f/f). Whole gut transit time tended to be prolonged in mice following excision of Cx43 from EG, but on average, this trend was not significant (Fig. 4A). Specifically assessing colonic function with the colon bead assay revealed a significant delay in colonic transit time in mice lacking glial Cx43 (Fig. 4A). Likewise, the number and total wet mass of endogenous fecal pellets produced per hour was not significantly altered in mice lacking glial Cx43 (data not shown) but fecal pellets produced contained significantly less fecal matter and an elevated fluid content (Fig. 4B).
Figure 4.
Specific deletion of glial Cx43 impairs gastrointestinal transit to a similar extent as physiological aging. Whole gut and colonic transit times (A; n=6-8, median ± interquartile range, *P=.0097, Mann-Whitney U-Test), fecal pellet composition analysis (B; n=8-9, mean ± SEM, *P<0.05, Student's t-test) and isometric muscle tension recordings (summary data on left for contractions in C, relaxations in D; representative traces at right show the effect of 20 Hz (500 milliamps) EFS on contractile and relaxation responses; n=5, *P<0.05, two-way ANOVA) measured in tamoxifen–treated background (Backgr: pooled WT and Cx43f/f) and inducible and conditional, glial–specific, Cx43 knockout (i-cKO) mice. Whole gut and colonic transit (E; n=3-8, mean ± SEM, **P=.0041, two-tailed t test and *P=.0011, Mann-Whitney U-Test, respectively) and endogenous fecal pellet output (F; n=4, mean ± SEM, P=.793, two-way ANOVA) measured in mice at from 2–12 m.o.a.
To more accurately assess colonic function, we performed isometric muscle strip recordings in i-cKO mice. In these experiments we utilized mice hemizygous for floxed Cx43 (hGFAP::CreERT2+/−/Cx43f/wt) to induce a partial glial defect while circumventing possible compensatory actions by glia. EFS evoked biphasic muscle responses characterized by an initial relaxation followed by contraction in colons from both control and i-cKO mice. The amplitude of both relaxations and contractions were significantly diminished in mice with reduced levels of glial Cx43 (Fig. 4C, D). Carbachol (1-100 μM) produced concentration-dependent increases in the contractile force in mice of both genotypes yet the amplitude of contractions to carbachol were rightward shifted in the i-cKO mice (7.9 ± 0.7 mN vs. 16 ± 2 mN at 10 μM and 9.1 ± 1 mN vs. 17 ± 2 mN at 30 μM; n=5). Relaxations induced by the nitric oxide (NO) donor SNP (10 μM) measured after treatment with carbachol (30 μM) were similar in both i-cKO and controls. These results indicate that Ca2+ responses through the EG network mediated by Cx43 are necessary to maintain the normal fidelity of gut motility and that a loss of glial Cx43 impairs both excitatory and inhibitory neuromuscular transmission in the mouse colon.
Selective ablation of glial connexin-43 mirrors functional gastrointestinal changes with age
The above selective ablation of glial Cx43 in adulthood resulted in a phenotype highly reminiscent of functional changes in gut motility with advancing age25,26. Thus, we hypothesized that glial alterations contribute to the slowing of motility in aging adult mice.
In agreement with previous findings in humans25 and rats26, we find that gastrointestinal motility declines throughout adulthood in mice. Whole gut transit and latency of colonic bead expulsion (Fig. 4E) were both prolonged in 12 m.o.a. mice compared to 2 m.o.a. mice and fecal pellet output declined between 5 – 12 m.o.a. (Fig. 4F). Thus, our results suggest that a loss of glial Cx43 produces similar functional changes in gastrointestinal transit as observed in aging.
Slowing of gut transit with age is associated with a decline in enteric glial Ca2+ responses
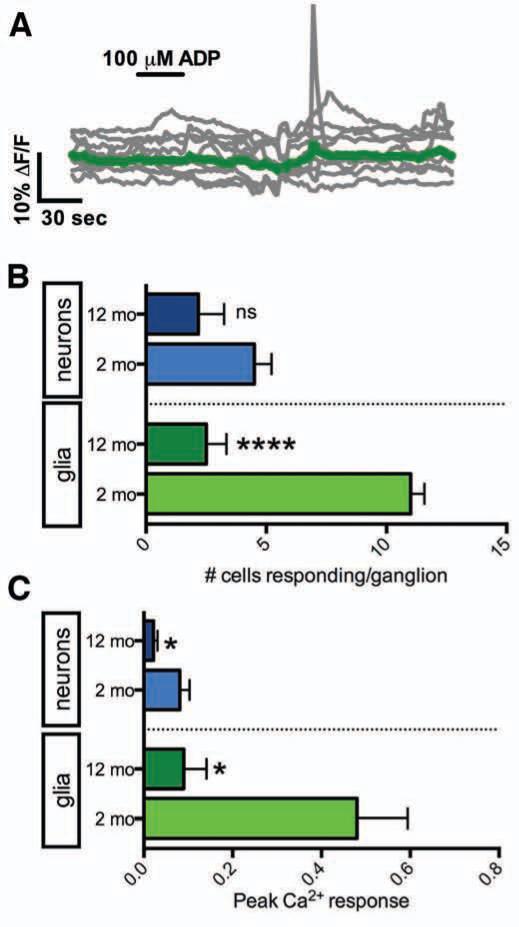
Because limiting glial Ca2+ responses produced a similar functional deficit in gut motility as observed during aging, we postulated that altered glial Ca2+ responses are a contributing mechanism underlying functional gastrointestinal changes with age. We tested this notion by assessing the maintenance of glial Ca2+ responses across adult lifespan initiated by directly or indirectly challenging EG, either by ADP (100 μM) or by stimulating enteric neuron–to–glia transmission with BzATP (100 μM), respectively, in tissue from 2 and 12 m.o.a. animals. When compared to 2 m.o.a. animals (Fig. 5B), fewer number of EG mobilized Ca2+ in response to ADP in 12 m.o.a. animals (Fig. 5A-B) and peak Ca2+ responses in those glia exhibiting responses were reduced by 81 ± 11% (Fig. 5C). In contrast, similar numbers of enteric neurons responded to ADP with small Ca2+ responses in both 2 and 12 m.o.a. animals (Fig. 5B), but peak neuron Ca2+ responses to ADP were reduced by 73 ± 11% (Fig. 5C) in 12 m.o.a. animals.
Figure 5.
Purine–evoked Ca2+ waves between EG are lost with aging. (A) Representative Ca2+ response evoked by ADP in 9 EG (gray traces, average in green) within a myenteric ganglion from a 12 m.o.a. mouse. (B) Quantification of the number of neurons (blue) or glia (green) responding per myenteric ganglion to ADP in 2 (light shaded bars) and 12 m.o.a. mice (dark shaded bars; n = 6, mean ± SEM; ****P<.0001 between 2 and 12 m.o.a. glia, P=.1018 between 2 and 12 m.o.a. neurons, two-tailed t test). (C) Quantification of peak Ca2+ responses initiated by ADP in neurons (blue) or glia (green) in 2 (light shaded bars) and 12 (dark shaded bars) m.o.a. mice (n = 6, mean ± SEM; *P=.011 between 2 and 12 m.o.a. glia, *P=.0352 between 2 and 12 m.o.a. neurons, two-tailed t test).
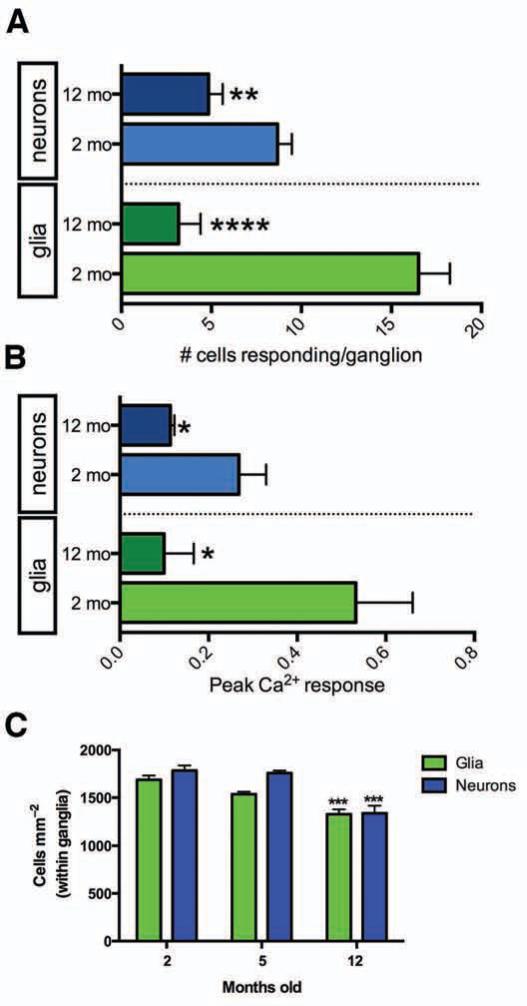
To assess if glial Ca2+ responses initiated by endogenous purines are altered during adulthood, we stimulated purine release from enteric neurons with BzATP13 and examined glial Ca2+ responses. Glial Ca2+ responses to the BzATP stimulus were rare in 12 m.o.a. animals (Fig. 6A) and peak Ca2+ responses were reduced by 81 ± 12% (Fig. 6B). The 82% reduction in the number of glia responding is far greater than the 21% reduction in glial packing density within ganglia observed over the same time period (Fig. 6C). Therefore, reduced glial numbers cannot account for the reduction in glial responsiveness.
Figure 6.
EG Ca2+ waves evoked by enteric neuron–to–glia communication are lost with aging. (A) Numbers of neurons (blue) or glia (green) responding per myenteric ganglion to BzATP (100 μM) at 2 (light shaded bars) and 12 (dark shaded bars) m.o.a. (n = 6, mean ± SEM; ****P<.0001 between 2 and 12 m.o.a. glia, **P=.0068 between 2 and 12 m.o.a. neurons, two-tailed t test). (B) Peak Ca2+ response magnitude initiated by ADP in neurons (blue) or glia (green) at 2 (light shaded bars) and 12 (dark shaded bars) m.o.a. (n = 6, mean ± SEM; *P=.0136 between 2 and 12 m.o.a. glia, *P=.0219 between 2 and 12 m.o.a. neurons, two-tailed t test). (C) Neuron (blue) and glial (green) packing density within myenteric ganglia with age (n = 4, mean ± SEM; glia ***P=.0007, neurons ***P=.0006, ANOVA).
Interestingly, the number of neurons responding to BzATP in 12 m.o.a. animals was reduced to 44% (Fig. 6A). Fewer enteric neurons may respond because humans27 and experimental animals28 experience an age related decline in preponderance of myenteric neurons. Consistent with this notion, the packing density of enteric neurons declined by 25% in 12 m.o.a. mice (Fig. 6C).
Expression and localization of crucial signaling components involved in the neuronal generation of ATP and the extracellular conversion of ATP to ADP, including P2X7 receptors, pannexin-1 and NTPDase2 and NTPDase3 were comparable between 2 to 12 m.o.a. suggesting that evoked responses in neurons maintain the potential to activate glial Ca2+ responses (Supplementary Fig. 2).
In all, these observations suggest that altered glia mechanisms limit purine–initiated Ca2+ responses through the EG network of the mouse colon in adult life and that these changes parallel functional changes in gut motility.
Enteric glial connexin-43 expression is altered by aging
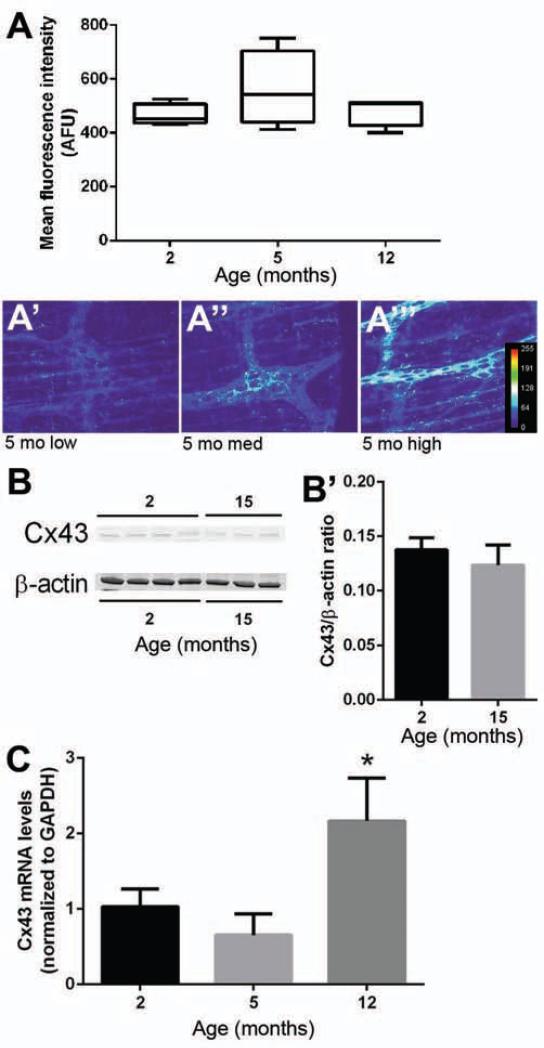
Our results indicate that altered Cx43 function limits EG Ca2+ responses (Figs. 2-3) and that glial Ca2+ responses decline with advancing age (Figs. 5-6). Thus, we hypothesized that glial Cx43 expression is altered during aging. Mean immunofluorescence intensity (Fig. 7A) and protein expression (Fig. 7B-B’) of Cx43 remained relatively stable between 2-12 m.o.a. (Fig. 7A-B) but immunoreactivity for Cx43 increased in variability at 5 m.o.a. (Fig. 7A’-A’”). Analysis by rtPCR revealed a near doubling of Cx43 mRNA expression in 12 m.o.a. animals as compared to 2 m.o.a. animals (Fig. 7C). Taken together, these data indicate that glial expression of Cx43 becomes dysregulated with age.
Figure 7.
Cx43 expression with age. (A) Mean Cx43-ir intensity with age (n = 4, mean ± SEM). (A’-A’”) Representative ganglia showing variable Cx43-ir (transformed to heatmap images to depict intensity, arbitrary fluorescence units [AFU]) at 5 m.o.a. (scale bar = 60 μm). (B) Representative Western blots of Cx43 and β-actin (loading control) from the colons of 2 and 15 m.o.a. mice. (B’) Cx43 protein expression at 2 and 15 m.o.a. expressed as the ratio of Cx43 to β-actin (mean ± SEM). (C) Cx43 mRNA levels (normalized to GAPDH) at 2, 5 and 12 m.o.a. (n = 4, mean ± SEM, ANOVA, *P≤.5).
DISCUSSION
Our observations provide the first evidence that EG Ca2+ responses regulate gastrointestinal function. We found that Cx43 expression is confined to EG within the ENS of the mouse colon and our data show that Cx43 is required for EG to propagate Ca2+ responses. Further, our data show that blunting glial Ca2+ responses by interfering with Cx43 hemichannels slows colonic transit. Thus, our data support the hypothesis that Ca2+ signaling amongst EG acts to regulate gastrointestinal motility.
EG are increasingly recognized as active participants within enteric circuits. Hence, it has been demonstrated that neuronal activity initiates glial Ca2+ responses2, which can occur during physiological patterns of ENS activity that underlie gut motility4. Our present data suggest that these glial Ca2+ responses do in fact play an essential role in modulating gastrointestinal motility and that a loss of glial communication impairs colonic function. Similar to aging mice, Cx43 i-cKO mice had no significant reduction in the number of pellets and wet mass produced per hour. However, we found that the composition of fecal pellets in Cx43 i-cKO mice is significantly altered. Pellets from these mice contain less matter and higher fluid content (Fig. 4B). This result is in contrast to the retarded colonic transit of i-cKO animals (Fig. 4A), which would provide more time for water removal. This novel finding suggests that enteric glia at the level of the submucosal plexus are necessary for the coordination of water reabsorption/secretion in the colon.
We find that the ability of endogenous and exogenous purines to initiate Ca2+ responses in EG declines concomitantly with a slowing of colonic transit in adult life. Our observations suggest that Cx43 dysregulation contributes to limited glial Ca2+ responses with age. Our results support previous findings showing that Cx43 function declines with age in astrocytes29. In our hands, inhibition of Cx43 in 2 m.o.a. animals recapitulates the limited glial signaling observed in 12 m.o.a. animals by constraining the number of responding glia and diminishing the peak Ca2+ responses of glia. Further, our analyses of Cx43 mRNA and protein expression suggest that Cx43 expression is altered with advancing age in the adult gut. Of note, Cx43 gene, Gja1, mutations lead to oculodigitodental dysplasia syndrome; patients have variable bowel disturbances30, which is expected as some mutations lead to a decrease (relevant in the proposed study), while others to an increase in the activity of Cx43 channel31.
Many bioactive molecules exert effects on Cx43 function and/or expression32. Our current data suggest that post-translational modifications of Cx43 may contribute to low function with age because we found no overall change in Cx43 protein expression. Alternatively, increased Cx43 internalization and degradation could explain our results as we observed elevated Cx43 mRNA expression with no change in protein. However, the present expression data should be interpreted with caution as expression of Cx43 by intestinal smooth muscle cells may mask subtle changes specific to glia33. Our current functional data demonstrates that even a partial defect in glial Cx43 is sufficient to induce organ dysfunction because an excision of one copy of glial Cx43 resulted in abnormal colonic contractility. Thus, our current methods of assessing Cx43 expression in tissue may lack the sensitivity needed to observe small, cell-type-specific alterations in Cx43 expression within glia, which appears to be more accurately reflected by Ca2+ imaging.
While the factors that alter glial Cx43 in adult life are currently unclear, we speculate that glial changes reflect an increasingly pro–inflammatory environment in the gut wall34. Cx43 expression32 and function35 are modulated by inflammation36,37 and inflammatory stimuli differentially regulate Cx43 expression in astrocytes depending on the type, duration and distance from the inflammatory stimulus38. Inflammation34 and oxidative stress39 increase with age in the gastrointestinal tract while neuron survival declines40. Increasing inflammatory mediators41,42 and excitotoxic neuron death43,44 downregulate Cx43 in astrocytes and may act to alter Cx43 expression or function in EG.
Downregulation of purinergic receptors in astrocytes during adulthood reduces their ability to initiate Ca2+ responses45. Our data does not support a similar situation in the ENS. Specifically, inhibiting Cx43 function in 2 m.o.a. mice restricted the population of responding glia to a similar size as observed in 12 m.o.a. animals. We interpret this finding as indicating that only a limited number of EG are normally responsive to external P2Y1 agonists. Because the size of this population of “initial responders” did not change with age, we speculate that constrained glial responses result from an impairment to propagate responses to surrounding cells rather than the ability of P2Y1 responsive cells to respond.
Exactly how EG Ca2+ responses influence gastrointestinal function through Cx43 is currently unknown but glial effects are likely mediated through modulation of enteric neuron activity. Although the role of astroglial Ca2+ responses is still highly contested in the CNS5,46, Ca2+–dependent mechanisms in astrocytes47 and astrocytic gliotransmitter release through Cx43 hemichannels48 are known to modulate neural network function. Given that intracellular Ca2+ responses in astrocytes trigger ATP release through Cx43 hemichannels19,21 and ATP is a potent modulator of enteric neuron activity49, we speculate that EG–mediated neuromodulation may occur via glial purine release. We aim to address this hypothesis in future work.
In summary, our work shows that EG Cx43 hemichannels are necessary for intercellular communication between EG. Further, our data provide the first evidence that interfering with EG communication alters gastrointestinal function and we propose that altered glial signaling may contribute to gastrointestinal motility disorders.
Supplementary Material
Acknowledgements
We are especially thankful for the generosity of Dr. Keith Sharkey (University of Calgary) who shared equipment, reagents and time in support of preliminary data. We thank Dr. K. D. McCarthy (University of North Carolina) for providing Cx43f/fand hGFAP::creERT2+/− lines, Dr. Lucas Pozzo-Miller and Dr. Linda Overstreet Wadiche (University of Alabama at Birmingham) for access to a confocal microscope and Dr. James Galligan (Michigan State University) for assistance and equipment to perform contractility studies. RAGS experiments were performed during his fellowship in Pediatric Gastroenterology at the Department of Pediatrics Division of Pediatric Gastroenterology Hepatology and Nutrition, University of Alabama at Birmingham
Grant Support:
We are grateful to the Michigan State University Neuroscience Program and College of Natural Science for start-up funds (B. Gulbransen, G. Leinninger) and grants from the American Neurogastroenterology and Motility Society (ANMS, to B. Gulbransen) and National Institutes of Health (NIH; BIRCWH) grant K12 HD065879 (B. Gulbransen) that supported this work. VP acknowledges the support of this work by a National Science Foundation Award CBET 0943343. JS was a recipient of a senior Scholarship from the Fonds de recherche du Québec – Santé (FRQS).
Abbreviations
- Ca2+
calcium
- Cx43
connexin-43
- EG
enteric glia
- ENS
enteric nervous system
- moa
month of age
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions: BDG and VP were responsible for the overall project conception, design and supervision. Experiments performed by BDG, JM, VG, DF and RAGS. JS contributed unpublished reagents and GML provided technical and material support. Manuscript written by BDG with all authors contributing to revisions and editing.
Disclosures: The authors declare no competing financial interests.
REFERENCES
- 1.Gulbransen BD, Sharkey KA. Novel functional roles for enteric glia in the gastrointestinal tract. Nat Rev Gastroenterol Hepatol. 2012;9:625–632. doi: 10.1038/nrgastro.2012.138. [DOI] [PubMed] [Google Scholar]
- 2.Gulbransen BD, Sharkey KA. Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology. 2009;136:1349–1358. doi: 10.1053/j.gastro.2008.12.058. [DOI] [PubMed] [Google Scholar]
- 3.Gomes P, Chevalier J, Boesmans W, et al. ATP-dependent paracrine communication between enteric neurons and glia in a primary cell culture derived from embryonic mice. Neurogastroenterol. Motil. 2009;21:870–e62. doi: 10.1111/j.1365-2982.2009.01302.x. [DOI] [PubMed] [Google Scholar]
- 4.Broadhead MJ, Bayguinov PO, Okamoto T, et al. Ca2+ transients in myenteric glial cells during the colonic migrating motor complex in the isolated murine large intestine. J. Physiol. 2012;590:335–350. doi: 10.1113/jphysiol.2011.219519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fiacco TA, Agulhon C, Taves SR, et al. Selective stimulation of astrocyte calcium in situ does not affect neuronal excitatory synaptic activity. Neuron. 2007;54:611–626. doi: 10.1016/j.neuron.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 6.Bush TG, Savidge TC, Freeman TC, et al. Fulminant Jejuno-Ileitis following Ablation of Enteric Glia in Adult Transgenic Mice. Cell. 1998;93:13–13. doi: 10.1016/s0092-8674(00)81571-8. [DOI] [PubMed] [Google Scholar]
- 7.Cornet AA, Savidge TCT, Cabarrocas JJ, et al. Enterocolitis induced by autoimmune targeting of enteric glial cells: a possible mechanism in Crohn's disease? PNAS. 2001;98:13306–13311. doi: 10.1073/pnas.231474098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nasser Y, Fernandez E, Keenan CM, et al. Role of enteric glia in intestinal physiology: effects of the gliotoxin fluorocitrate on motor and secretory function. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;291:912–927. doi: 10.1152/ajpgi.00067.2006. [DOI] [PubMed] [Google Scholar]
- 9.Liao Y, Day KH, Damon DN, et al. Endothelial cell-specific knockout of connexin 43 causes hypotension and bradycardia in mice. PNAS. 2001;98:9989–9994. doi: 10.1073/pnas.171305298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casper KB, Jones K, McCarthy KD. Characterization of astrocyte-specific conditional knockouts. Genesis. 2007;45:292–299. doi: 10.1002/dvg.20287. [DOI] [PubMed] [Google Scholar]
- 11.Madisen L, Zwingman TA, Sunkin SM, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiermayer C, Conrad M, Schneider M, et al. Optimization of spatiotemporal gene inactivation in mouse heart by oral application of tamoxifen citrate. Genesis. 2007;45:11–16. doi: 10.1002/dvg.20244. [DOI] [PubMed] [Google Scholar]
- 13.Gulbransen BD, Bashashati MM, Hirota SAS, et al. Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat. Med. 2012;18:600–604. doi: 10.1038/nm.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 15.France M, Bhattarai Y, Galligan JJ, et al. Impaired propulsive motility in the distal but not proximal colon of BK channel β1-subunit knockout mice. Neurogastroenterol. Motil. 2012;24:e450–9. doi: 10.1111/j.1365-2982.2012.01981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L, Martínez V, Kimura H, et al. 5-Hydroxytryptophan activates colonic myenteric neurons and propulsive motor function through 5-HT4 receptors in conscious mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G419–28. doi: 10.1152/ajpgi.00289.2006. [DOI] [PubMed] [Google Scholar]
- 17.Nagakura Y, Naitoh Y, Kamato T, et al. Compounds possessing 5-HT3 receptor antagonistic activity inhibit intestinal propulsion in mice. Eur. J. Pharmacol. 1996;311:67–72. doi: 10.1016/0014-2999(96)00403-7. [DOI] [PubMed] [Google Scholar]
- 18.Zhang W, Segura BJ, Lin TR, et al. Intercellular calcium waves in cultured enteric glia from neonatal guinea pig. Glia. 2003;42:252–262. doi: 10.1002/glia.10215. [DOI] [PubMed] [Google Scholar]
- 19.Kang J, Kang N, Lovatt D, et al. Connexin 43 hemichannels are permeable to ATP. J. Neurosci. 2008;28:4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Orellana JA, Froger N, Ezan P, et al. ATP and glutamate released via astroglial connexin 43 hemichannels mediate neuronal death through activation of pannexin 1 hemichannels. J. Neurochem. 2011;118:826–840. doi: 10.1111/j.1471-4159.2011.07210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cotrina ML, Lin JH, Alves-Rodrigues A, et al. Connexins regulate calcium signaling by controlling ATP release. PNAS. 1998;95:15735–15740. doi: 10.1073/pnas.95.26.15735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dermietzel R, Hertberg EL, Kessler JA, et al. Gap junctions between cultured astrocytes: immunocytochemical, molecular, and electrophysiological analysis. J. Neurosci. 1991;11:1421–1432. doi: 10.1523/JNEUROSCI.11-05-01421.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altevogt BM, Paul DL. Four classes of intercellular channels between glial cells in the CNS. J. Neurosci. 2004;24:4313–4323. doi: 10.1523/JNEUROSCI.3303-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torres A, Wang F, Xu Q, et al. Extracellular Ca2+ acts as a mediator of communication from neurons to glia. Sci Signal. 2012;5:ra8. doi: 10.1126/scisignal.2002160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madsen JL, Graff J. Effects of ageing on gastrointestinal motor function. Age and ageing. 2004 doi: 10.1093/ageing/afh040. [DOI] [PubMed] [Google Scholar]
- 26.McDougal JN, Miller MS, Burks TF, et al. Age-related changes in colonic function in rats. Am. J. Physiol. 1984;247:G542–6. doi: 10.1152/ajpgi.1984.247.5.G542. [DOI] [PubMed] [Google Scholar]
- 27.Bernard CE, Gibbons SJ, Gomez-Pinilla PJ, et al. Effect of age on the enteric nervous system of the human colon. Neurogastroenterol. Motil. 2009;21:746–e46. doi: 10.1111/j.1365-2982.2008.01245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van Ginneken C, Schäfer K-H, Van Dam D, et al. Morphological changes in the enteric nervous system of aging and APP23 transgenic mice. Brain Res. 2011;1378:43–53. doi: 10.1016/j.brainres.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 29.Cotrina ML, Gao Q, Lin JH, et al. Expression and function of astrocytic gap junctions in aging. Brain Res. 2001;901:55–61. doi: 10.1016/s0006-8993(01)02258-2. [DOI] [PubMed] [Google Scholar]
- 30.Loddenkemper T, Grote K, Evers S, et al. Neurological manifestations of the oculodentodigital dysplasia syndrome. J. Neurol. 2002;249:584–595. doi: 10.1007/s004150200068. [DOI] [PubMed] [Google Scholar]
- 31.Dobrowolski R, Sasse P, Schrickel JW, et al. The conditional connexin43G138R mouse mutant represents a new model of hereditary oculodentodigital dysplasia in humans. Hum. Mol. Genet. 2008;17:539–554. doi: 10.1093/hmg/ddm329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rouach N, Avignone E, Même W, et al. Gap junctions and connexin expression in the normal and pathological central nervous system. Biol. Cell. 2002;94:457–475. doi: 10.1016/s0248-4900(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 33.Döring B, Pfitzer G, Adam B, et al. Ablation of connexin43 in smooth muscle cells of the mouse intestine: functional insights into physiology and morphology. Cell Tissue Res. 2007;327:333–342. doi: 10.1007/s00441-006-0281-6. [DOI] [PubMed] [Google Scholar]
- 34.Biagi E, Nylund L, Candela M, et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE. 2010;5:e10667–e10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Karpuk N, Burkovetskaya M, Fritz T, et al. Neuroinflammation Leads to Region-Dependent Alterations in Astrocyte Gap Junction Communication and Hemichannel Activity. J. Neurosci. 2011;31:414–425. doi: 10.1523/JNEUROSCI.5247-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamby ME, Coppola G, Ao Y, et al. Inflammatory mediators alter the astrocyte transcriptome and calcium signaling elicited by multiple g-protein-coupled receptors. J. Neurosci. 2012;32:14489–14510. doi: 10.1523/JNEUROSCI.1256-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bennett MVL, Garré JM, Orellana JA, et al. Connexin and pannexin hemichannels in inflammatory responses of glia and neurons. Brain Res. 2012 doi: 10.1016/j.brainres.2012.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giaume CC, Koulakoff AA, Roux LL, et al. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat. Rev. Neurosci. 2010;11:87–99. doi: 10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- 39.Thrasivoulou C, Soubeyre V, Ridha H, et al. Reactive oxygen species, dietary restriction and neurotrophic factors in age-related loss of myenteric neurons. Aging Cell. 2006;5:247–257. doi: 10.1111/j.1474-9726.2006.00214.x. [DOI] [PubMed] [Google Scholar]
- 40.Camilleri M, Cowen T, koch TR. Enteric neurodegeneration in ageing. Neurogastroenterol. Motil. 2008;20:185–196. doi: 10.1111/j.1365-2982.2007.01072.x. [DOI] [PubMed] [Google Scholar]
- 41.Même W, Calvo C-F, Froger N, et al. Proinflammatory cytokines released from microglia inhibit gap junctions in astrocytes: potentiation by beta-amyloid. FASEB J. 2006;20:494–496. doi: 10.1096/fj.05-4297fje. [DOI] [PubMed] [Google Scholar]
- 42.Zhao Y, Rivieccio MA, Lutz S, et al. The TLR3 ligand polyI:C downregulates connexin 43 expression and function in astrocytes by a mechanism involving the NF-κB and PI3 kinase pathways. Glia. 2006;54:775–785. doi: 10.1002/glia.20418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koulakoff A, Ezan P, Giaume C. Neurons control the expression of connexin 30 and connexin 43 in mouse cortical astrocytes. Glia. 2008;56:1299–1311. doi: 10.1002/glia.20698. [DOI] [PubMed] [Google Scholar]
- 44.Gangoso E, Ezan P, Valle-Casuso JC, et al. Reduced connexin43 expression correlates with c-Src activation, proliferation, and glucose uptake in reactive astrocytes after an excitotoxic insult. Glia. 2012;60:2040–2049. doi: 10.1002/glia.22418. [DOI] [PubMed] [Google Scholar]
- 45.Sun W, McConnell E, Pare JF, et al. Glutamate-Dependent Neuroglial Calcium Signaling Differs Between Young and Adult Brain. Science. 2013;339:197–200. doi: 10.1126/science.1226740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rouach N, Koulakoff A, Abudara V, et al. Astroglial metabolic networks sustain hippocampal synaptic transmission. Science. 2008;322:1551–1555. doi: 10.1126/science.1164022. [DOI] [PubMed] [Google Scholar]
- 47.Wang F, Smith NA, Xu Q, et al. Astrocytes modulate neural network activity by Ca²+-dependent uptake of extracellular K+. Sci Signal. 2012;5:ra26. doi: 10.1126/scisignal.2002334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stehberg J, Moraga-Amaro R, Salazar C, et al. Release of gliotransmitters through astroglial connexin 43 hemichannels is necessary for fear memory consolidation in the basolateral amygdala. FASEB J. 2012;26:3649–3657. doi: 10.1096/fj.11-198416. [DOI] [PubMed] [Google Scholar]
- 49.Galligan JJ, Bertrand PP. ATP mediates fast synaptic potentials in enteric neurons. J. Neurosci. 1994;14:7563–7571. doi: 10.1523/JNEUROSCI.14-12-07563.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.