Abstract
Objective
The objectives were to characterize the vitamin D status of black and white adolescents residing in the southeastern United States (latitude: 33°N) and to investigate relationships with adiposity.
Methods
Plasma 25-hydroxyvitamin D levels were measured with liquid chromatography-tandem mass spectroscopy for 559 adolescents 14 to 18 years of age (45% black and 49% female). Fat tissues, physical activity, and cardiovascular fitness also were measured.
Results
The overall prevalences of vitamin D insufficiency (<75nmol/L) and deficiency (≤50 nmol/L) were 56.4% and 28.8%, respectively. Black versus white subjects had significantly lower plasma 25-hydroxyvitamin D levels in every season (winter, 35.9±2.5 vs 77.4±2.7 nmol/L; spring, 46.4±3.5 vs 101.3±3.5 nmol/L; summer, 50.7±4.0 vs 104.3±4.0 nmol/L; autumn, 54.4± 4.0 vs 96.8±2.7 nmol/L). With adjustment for age, gender, race, season, height, and sexual maturation, there were significant inverse correlations between 25-hydroxyvitamin D levels and all adiposity measurements, including BMI percentile (P=.02), waist circumference (P<.01), total fat mass (P<.01), percentage of body fat (P <.01), visceral adipose tissue (P <.015), and subcutaneous abdominal adipose tissue (P<.039). There were significant positive associations between 25-hydroxyvitamin D levels and vigorous physical activity (P <.01) and cardiovascular fitness (P =.025).
Conclusions
Low vitamin D status is prevalent among adolescents living in a year-round sunny climate, particularly among black youths. The relationships between 25-hydroxyvitamin D levels, adiposity, physical activity, and fitness seem to be present in adolescence.
Keywords: vitamin D deficiency, 25(OH)D, adolescents, race, adiposity, physical activity, fitness
INTRODUCTION
Low vitamin D status, as indicated by circulating 25-hydroxyvitamin D concentrations, has become a common observation for the pediatric population in the United States. Several studies documented low serum 25-hydroxyvitamin D levels in children and adolescents living at northern latitudes, including Philadelphia, Pennsylvania (latitude: 40°N), New Jersey (40°N), Pittsburgh, Pennsylvania (40°N), Cleveland, Ohio (41°N), Boston, Massachusetts (42°N), and Maine (44°N).1 However, there is emerging evidence that children and adolescents residing in southern regions also have low 25-hydroxyvitamin D levels, which suggests that lower latitudes in the United States are not necessarily associated with higher serum 25-hydroxyvitamin D levels.
First, data from the National Health and Nutrition Examination Survey (NHANES) in 1988 –1994 showed that up to 47% of 12- to 19-year-old male and female youths (all races combined) from sunny locations (mean latitude: 32°N) had winter 25- hydroxyvitamin D levels of <62.5 nmol/L and up to 70% of black adolescents of the same age had winter 25- hydroxyvitamin D concentrations of <62.5 nmol/L.2 Second, the incidence of vitamin D deficiency, defined as 25-hydroxyvitamin D levels of <50 nmol/ L,3,4 was 16% in a sample of 93 children (age range: 10 –14 years), including 48 white, 13 black, 22 Hispanic, and 10 Asian children, in Houston, Texas (latitude: 30°N).5 In the same sample,1 child (1%) had a 25 hydroxyvitamin D concentration of <30 nmol/L and 68 children (73%) had levels of <80 nmol/L. Finally, 75% of 168 black or white girls 4 to 8 years of age in Athens, Georgia (latitude: 34°N), were observed to have 25-hydroxyvitamin D levels of <80 nmol/L at least once during follow-up periods of 1 to 7 years.6, 7 However, the vitamin D status, according to season and race, of adolescents in middle to late puberty (14 –18 years of age) in the southern United States remains largely undetermined.
Although adiposity is a recognized risk factor for vitamin D deficiency, the inverse relationship between adiposity and vitamin D status has been not well established in the pediatric population, particularly among adolescents.2,8,9 A direct relationship between physical activity levels and vitamin D metabolism has been recognized in adults, although the data are inconsistent.2,10–13 To date, only 1 study has investigated relationships between 25- hydroxyvitamin D levels and physical activity in adolescents. Lenders et al14 found that physical activity assessed through accelerometry was positively related to 25-hydroxyvitamin D levels in 24 obese adolescents 14.9±1.4 years of age. Additional investigations are needed to determine the relationships between 25-hydroxyvitamin D levels and physical activity in adolescents. The association of cardiovascular fitness with 25-hydroxyvitamin D levels also remains to be addressed, especially for adolescents. The present cross-sectional study of black and white adolescents residing in Augusta, Georgia (latitude:33°N), aimed (1) to characterize circulating plasma 25- hydroxyvitamin D concentrations and the prevalence of low vitamin D status according to season, gender, and race; (2) to determine the relationships between plasma 25- hydroxyvitamin D concentrations and a series of adiposity variables, including BMI, waist circumference, total fat mass, proportion of body fat, visceral adipose tissue (VAT), and subcutaneous abdominal adipose tissue (SAAT); and (3) to explore the relationships between 25- hydroxyvitamin D levels, physical activity, and cardiovascular fitness.
METHODS
Recruitment and testing protocol
In this cross-sectional study, adolescents 14 to 18 years of age (N = 559) were recruited from high schools in the vicinity of Augusta, Georgia (latitude: 33°N). Participants were asked to self-identify as white or black. The adolescents were generally healthy and free of medications and had no contraindications to any of the study procedures. Adolescents were excluded if they used any medications or had any chronic medical conditions that might affect growth and development or affect study results. Informed consent and assent were obtained from all parents and children, respectively. All procedures were approved by the human assurance committee at the Medical College of Georgia. Data collection took place between January 2001 and June 2005, and enrollment was conducted throughout the year, during winter (December through February), spring (March through May), summer (June through August), and autumn (September through November). Sexual maturation was determined by using a gender-specific questionnaire including a 5-stage scale, ranging from stage I (prepubertal) to stage V (fully mature), as described by Tanner.15 By using a gender-specific questionnaire, the subjects reported their Tanner stage by comparing their own physical development with the 5 stages in standard sets of diagrams. When an individual reported discordant stages of pubic hair and breast or genital development, the higher of the 2 stages was used. Total-body scans were assessed by using dual-energy x-ray absorptiometry (DXA) (QDR-4500W [Hologic, Waltham, MA]) to measure total-body lean mass, fat mass, and proportion of body fat.16 As described in detail elsewhere, VAT and SAAT were determined with a 1.5-T MRI system (General Electric Medical Systems, Milwaukee, WI).17
Plasma 25(OH)D measurement and vitamin D deficiency/insufficiency
Liquid chromatography-tandem mass spectroscopy was used to measure circulating plasma levels of 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3, as described previously.18 The detection limits for 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 were 10 nmol/L. The intraassay coefficients of variation for 25-hydroxyvitamin D2 and 25-hydroxyvitamin D3 were 6% to 9% and 7% to 11%, respectively; the interassay coefficients of variation for 25 hydroxyvitamin D2 and 25-hydroxyvitamin D3 were 9% to 12% and 8% to 13%, respectively. It has been proposed that the minimal optimal circulating vitamin D level should be increased from 25 to 50 nmol/L,19,20 because there is evidence that biochemical sequelae of vitamin D deficiency may manifest at cutoff levels of 75 nmol/L.3,4,21 For purposes of analyses, the prevalence of vitamin D deficiency was based on the proposed definition of 50 nmol/L, and cutoff values of 25 nmol/L and 75 nmol/L were used to describe overt vitamin D deficiency and insufficiency, respectively.3,4
Physical activity (Accelerometry)
The time (minutes) per day spent in moderate and vigorous physical activities was assessed by using MTI Actigraph monitors (model 7164 [MTI Health Services, Fort Walton Beach, FL]). With the epoch length set at 1 minute and results expressed as counts per minute, the accelerometers were to begin recording when the subject left our laboratory after the first day of testing. The subjects were instructed (1) to wear the monitor for a period of 7 days, (2) to remove the monitor for sleep and for any activity that might cause harm to either the monitor or another person, and (3) to bring the monitor back to us 1 week later. Data from days 1 and 7 were discarded because a full day of information was not available for those days. Daily and total movement counts per day were converted to minutes per day spent in moderate (3– 6 metabolic equivalents) and vigorous (>6 metabolic equivalents) physical activity by the software for the device.22
Cardiovascular fitness
Cardiovascular fitness was determined by using a multistage treadmill test. Oxygen consumption was measured with a Sensormedics Vmax 229 ardiopulmonary system (Sensormedics, Yorba Linda, CA). Our primary index of cardiovascular fitness was submaximal in nature, that is, oxygen consumption at a heart rate of 170 beats per minute per unit of body weight.23 The blood samples for 25- hydroxyvitamin D measurements were collected 1 week before the cardiovascular fitness assessments.
Statistical analyses
Descriptive statistics for raw variables are presented as means±SDs. Group differences in categorical variables were tested by using χ2 tests. Differences among the means for gender and race subgroups were examined by using 2-factor analysis of covariance (controlling for age) for all continuous independent variables. Values for plasma 25-hydroxyvitamin D levels, VAT, SAAT, and moderate and vigorous physical activities were not normally distributed; therefore, they were logarithmically transformed. This transformation yielded normal distributions, and the transformed variables were used in all subsequent analyses. Partial Pearson’s correlation coefficients were computed between plasma 25-hydroxyvitamin D levels and the anthropometric, body composition, physical activity, and cardiovascular fitness outcome variables, with controlling for age, gender, race, season, height, and sexual maturation stage. For comparisons of plasma 25-hydroxyvitamin D concentrations according to season, subjects were grouped according to race and gender for winter (December through February), spring (March through May), summer (June through August), and autumn (September through November). Three-factor analysis of covariance, with controlling for age, was used to quantify the effects of season, gender, and race on plasma 25-hydroxyvitamin D levels and to measure whether these factors interacted in this sample. Stepwise linear regression analysis was then used to identify the significant covariates of plasma 25-hydroxyvitamin D concentrations. All statistical tests used a significance level of 5% and were 2-tailed. Statistical analyses were performed by using SAS 9.1 (SAS Institute, Cary, NC).
RESULTS
Participant characteristics
A total of 559 adolescents 14 to 18 years of age (49% female, 51% male, 45% black, and 55% white) participated in this cross-sectional study. Group-specific means for height, weight, BMI percentile, waist circumference, total fat mass, proportion of body fat, VAT, and SAAT, with controlling for age, are summarized in Table 1. The majority of participants (86%) reported being in pubertal stages IV and V; however, 55 subjects reported being in pubertal stage III and 8 in stage II. Two hundred sixty-eight (97.8%) of the 274 girls reported having started menstruation.
Table 1.
Descriptive characteristics of the participants
| Female | Male | ||||||
|---|---|---|---|---|---|---|---|
| Variable | All (N = 559) |
White (n = 152) |
Black (n = 122) |
White (n = 155) |
Black (n = 130) |
Gender P valueb |
Race P valueb |
| Age (yrs) | 16.2 ± 1.2 | 16.0 ± 1.1 | 16.3 ± 1.2 | 16.3 ± 1.2 | 16.0 ± 1.3 | 0.959 | 0.628 |
| Height (cm) | 168.6 ± 8.8 | 163.0 ± 5.8 | 162.2 ± 5.6 | 174.2 ± 7.1 | 174.4 ± 7.8 | < 0.01 | 0.571 |
| Weight (kg) | 65.6 ± 15.2 | 58.2 ± 10.9 | 65.0 ± 15.9 | 68.8 ± 14.6 | 70.9 ± 16.4 | <0.01 | 0.035 |
| BMI percentile | 61.6 ± 27.8 | 56.5 ± 26.0 | 68.6 ± 27.2 | 59.4 ± 28.9 | 63.7 ± 27.7 | 0.654 | 0.019 |
| Waist circumference (cm) | 74.2 ± 10.3 | 69.2 ± 8.0 | 74.0 ± 11.8 | 77.1 ± 9.8 | 75.8 ± 10.0 | < 0.01 | 0.106 |
| FFST mass (kg) | 46.4 ± 9.9 | 38.0 ± 4.4 | 41.6 ± 6.0 | 51.7 ± 7.9 | 54.6 ± 9.4 | < 0.01 | < 0.01 |
| Fat mass (kg) | 15.9 ± 9.5 | 17.4 ± 7.5 | 20.2 ± 10.8 | 13.7 ± 8.9 | 12.7 ± 9.5 | < 0.01 | 0.241 |
| Percentage body fat (%) | 23.7 ± 9.9 | 29.3 ± 7.0 | 29.9 ± 8.3 | 19.1 ± 8.2 | 16.9 ± 8.7 | < 0.01 | 0.245 |
| VAT (cm3)a | 95.6 ± 61.3 | 97.4 ± 48.6 | 114.6 ± 69.7 | 103.1 ± 67.0 | 74.1 ± 54.0 | < 0.01 | 0.031 |
| SAAT (cm3)a | 870.5 ± 734.7 | 804.8 ± 484.4 | 1374.4 ± 943.4 | 723.8 ± 647.4 | 715.6 ± 687.7 | < 0.01 | 0.020 |
| Moderate PA (hrs/d)a | 38.6 ± 24.2 | 31.1 ± 17.8 | 31.0 ± 20.2 | 46.9 ± 23.8 | 48.1 ± 26.5 | < 0.01 | 0.584 |
| Vigorous PA (hrs/d)a | 4.6 ± 6.8 | 3.0 ± 4.6 | 1.8 ± 2.7 | 6.0 ± 6.4 | 7.7 ± 11.3 | < 0.01 | 0.856 |
| CV fitness (mL/kg−1/min−1) | 27.0 ± 7.4 | 24.3 ± 5.5 | 21.8 ± 4.9 | 31.9 ± 6.7 | 29.2 ± 7.6 | < 0.01 | < 0.01 |
| 25-hydroxyvitamin D (nmol/L)a, c | 73.3 ± 38.0 | 98.7 ± 40.9 | 40.5 ± 17.9 | 91.5 ± 27.7 | 52.8 ± 22.0 | 0.020 | < 0.01 |
All values are means ± SD. FFST, fat-free soft tissue; VAT, visceral adipose tissue; SAAT, subcutaneous abdominal adipose tissue; PA, physical activity; 25(OH)D, plasma 25-hydroxyvitamin D, physical activity; CV, cardiovascular. To convert 25(OH)D levels to nanograms per milliliter, divide by 2.496.
Logarithmic transformation performed before analysis.
Tests of significance between groups were based on ANOVA.
Tests of significance between groups were based on ANCOVA, controlling for age and season.
Plasma 25-hydroxyvitamin D levels
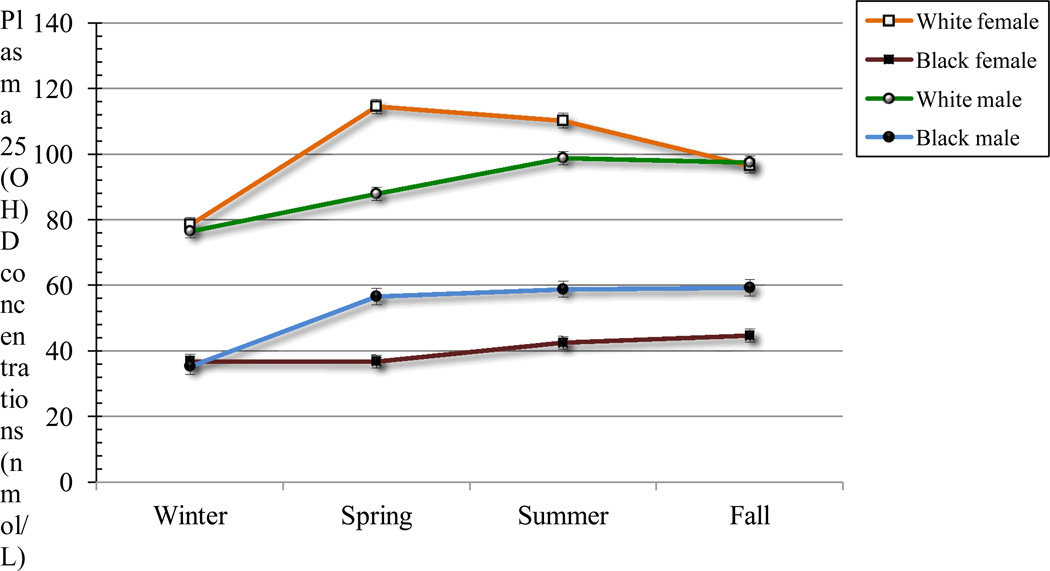
Plasma 25-hydroxyvitamin D levels differed according to race and gender (Table 1 and Fig 1). Overall, plasma 25-hydroxyvitamin D levels were higher in white subjects than in black subjects and higher in boys than in girls. Overall, mean plasma 25-hydroxyvitamin D levels were lower in girls than in boys and lower in black subjects than in white subjects (both P<.02). The 25-hydroxyvitamin D levels were lowest in winter, compared with spring, summer, and autumn, within all 4 groups (all P<.01) (Table 2). Black versus white subjects had significantly lower 25-hydroxyvitamin D levels in all seasons. Furthermore, black girls had consistently low 25- hydroxyvitamin D levels in all seasons (winter, 36.9±15.2 nmol/L; spring, 36.7±16.7 nmol/L; summer, 42.4±19.2 nmol/L; autumn, 44.7±18.7 nmol/L).
Figure 1.
Age-adjusted mean ± SE plasma 25-hydroxyvitamin D[25(OH)] concentrations by season in white females (n = 36, 37, 27, and 55 for winter, spring, summer, and fall, respectively), black females (n = 18, 37, 36, and 31 for winter, spring, summer and fall, respectively), white males (n = 22, 53, 29, and 51 for winter, spring, summer and fall, respectively), and black males (n = 33, 53, 23, and 21 for winter, spring, summer and fall, respectively). Significant main effects of season, race, and sex were observed, but no significant interaction between season, race, and sex was observed. Means between seasons were significantly different for each race and marginally different when averaged over race (P < 0.05). Means between races were significantly different for winter, spring, summer, and fall and marginally different when averaged over seasons (whites > blacks; P < 0.05). Means between sex were significantly different for fall only (males > females; P = 0.03). Plasma 25(OH)D concentrations were log-transformed prior to analyses.
Table 2.
Prevalence of vitamin D severe deficiency, deficiency, and insufficiency by season, race, and sex
| Season | N | % <25 (nmol/L) |
SEa | % <50 (nmol/L) |
SEa | % <75 (nmol/L) |
SEa |
|---|---|---|---|---|---|---|---|
| All | 559 | 5.2 | 1.2 | 28.8 | 2.5 | 56.4 | 2.7 |
| White | 307 | 0.0 | 0.0 | 3.2 | 1.3 | 30.0 | 3.4 |
| Females | 152 | 0.0 | 0.0 | 2.6 | 1.7 | 29.6 | 4.8 |
| Males | 155 | 0.0 | 0.0 | 3.9 | 1.9 | 30.3 | 4.7 |
| Black | 252 | 11.5 | 2.6 | 59.9 | 4.0 | 88.5 | 2.6 |
| Females | 122 | 17.2 | 4.4 | 73.8 | 5.1 | 94.3 | 2.7 |
| Males | 130 | 6.2 | 2.7 | 46.9 | 5.6 | 83.1 | 4.2 |
| Winter | 109 | 9.2 | 3.6 | 44.0 | 6.1 | 68.8 | 5.7 |
| White | 58 | 0.0 | 0.0 | 8.6 | 4.7 | 43.1 | 8.3 |
| Females | 36 | 0.0 | 0.0 | 8.3 | 5.9 | 41.7 | 10.5 |
| Males | 22 | 0.0 | 0.0 | 9.1 | 7.8 | 45.5 | 13.6 |
| Black | 51 | 19.6 | 7.1 | 84.3 | 6.5 | 98.0 | 2.5 |
| Females | 18 | 16.7 | 11.4 | 83.3 | 11.3 | 94.4 | 7.2 |
| Males | 33 | 21.2 | 9.1 | 84.8 | 8.0 | 100.0 | 2.0 |
| Spring | 177 | 5.6 | 2.2 | 29.4 | 4.4 | 58.8 | 4.7 |
| White | 87 | 0.0 | 0.0 | 3.4 | 2.5 | 29.9 | 6.3 |
| Females | 34 | 0.0 | 0.0 | 2.9 | 3.7 | 23.5 | 9.3 |
| Males | 53 | 0.0 | 0.0 | 3.8 | 3.3 | 34.0 | 8.3 |
| Black | 90 | 11.1 | 4.3 | 54.4 | 6.7 | 86.7 | 4.6 |
| Females | 37 | 24.3 | 9.0 | 75.7 | 9.1 | 97.3 | 3.42 |
| Males | 53 | 1.9 | 2.4 | 39.6 | 8.6 | 79.2 | 7.2 |
| Summer | 115 | 4.3 | 2.4 | 28.6 | 5.4 | 54.8 | 5.9 |
| White | 56 | 0.0 | 0.0 | 0.0 | 0.0 | 19.6 | 6.8 |
| Females | 27 | 0.0 | 0.0 | 0.0 | 0.0 | 18.5 | 9.6 |
| Males | 29 | 0.0 | 0.0 | 0.0 | 0.0 | 20.7 | 9.7 |
| Black | 59 | 5.6 | 3.8 | 55.9 | 8.3 | 88.1 | 5.4 |
| Females | 36 | 13.9 | 7.4 | 69.4 | 9.9 | 94.4 | 4.9 |
| Males | 23 | 0.0 | 0.0 | 34.8 | 12.7 | 78.3 | 11.0 |
| Fall | 158 | 2.5 | 1.6 | 17.7 | 3.9 | 46.2 | 5.1 |
| White | 106 | 0.0 | 0.0 | 1.9 | 1.7 | 28.3 | 5.6 |
| Females | 55 | 0.0 | 0.0 | 0.0 | 0.0 | 30.9 | 8.0 |
| Males | 51 | 0.0 | 0.0 | 3.9 | 3.5 | 25.5 | 7.8 |
| Black | 52 | 7.7 | 4.7 | 50.0 | 8.9 | 82.7 | 6.7 |
| Females | 31 | 12.9 | 7.7 | 71.0 | 10.5 | 90.3 | 6.8 |
| Males | 21 | 0.0 | 0.0 | 19.0 | 10.9 | 71.4 | 12.6 |
Standard error of percent. Winter = December – February, Spring = March – May, Summer = June – August, and Fall = September – November
Prevalence of low vitamin D status
Detailed prevalence rates of low vitamin D status, that is, insufficiency (≤75 nmol/L), deficiency (≤50 nmol/ L), and severe deficiency (≤25 nmol/L), according to season, race, and gender are shown in Table 2. The overall prevalence rates of vitamin D insufficiency and deficiency were 56.4% and 28.8%, respectively (Table 2). Vitamin D insufficiency rates were observed to be 94.3% and 83.1% in black girls and boys, respectively, compared with 29.6% and 30.3% in white girls and boys. Vitamin D deficiency rates were found to be 73.8% and 46.9% in black girls and boys, respectively, compared with only 2.6% and 3.9% in white girls and boys. Severe vitamin D deficiency was found only in black adolescents (5.2%). In summer, no white participants were found to have 25- hydroxyvitamin D levels of ≤50 nmol/L, but 55% of black adolescents were shown to have levels indicative of vitamin D deficiency and 88.1% vitamin D insufficiency.
Correlations with 25(OH)D concentrations
Plasma 25-hydroxyvitamin D levels were not associated with age (P=.460), height (P =.139), total lean mass (P=.068), or moderate physical activities (P=.110). Controlling for age, gender, race, season, height, and Tanner pubertal stage, we found inverse relationships between 25-hydroxyvitamin D concentrations and all adiposity measures, including BMI percentile (P=.02), waist circumference (P<.01), total fat mass (P<.01), proportion of body fat (P<.01), VAT (p=0.015) and SAAT (p=0.039) (Table 3). We found positive associations between 25- hydroxyvitamin D levels and vigorous physical activity (P<.01) and maximal oxygen consumption (P =.025).
Table 3.
Plasma 25(OH)D and its relations to adiposity outcomes, fat-free soft tissue mass, physical activity, and cardiovascular fitness
| Unadjusteda | Adjustedb | |||
|---|---|---|---|---|
| Variable | r | P-value | r | P-value |
| BMI percentiles | −0.174 | < 0.01 | −0.100 | 0.020 |
| Waist circumference (cm) | −0.115 | 0.007 | −0.128 | < 0.01 |
| FFST mass | −0.077 | 0.068 | −0.018 | 0.671 |
| Fat mass (kg) | −0.152 | < 0.01 | −0.147 | < 0.01 |
| Percentage body fat (%) | −0.112 | 0.008 | −0.173 | < 0.01 |
| VAT (cm3) c | −0.004 | 0.936 | −0.130 | 0.015 |
| SAAT (cm3) c | −0.154 | 0.003 | −0.111 | 0.039 |
| Moderate PA (hrs/d) c | 0.073 | 0.110 | 0.061 | 0.188 |
| Vigorous PA (hrs/d) c | 0.139 | 0.002 | 0.132 | < 0.01 |
| CV fitness (mL/kg−1/min−1) | 0.212 | < 0.01 | 0.100 | 0.025 |
FFST indicates fat-free soft tissue; VAT, visceral adipose tissue; SAAT, subcutaneous abdominal adipose tissue; PA, physical activity; CV, cardiovascular.
Statistical analysis was conducted by using Pearson’s bivariate correlation.
Statistical analysis was conducted by using partial correlation, adjusted for age, sex, race, sexual maturation, height, and season, for relationships between loge plasma 25(OH)D and adiposity outcomes, physical activity variables, and CV fitness in this sample (N = 559).
Logarithmic transformation performed before analysis.
Independent Predictors of Plasma 25-Hydroxyvitamin D Levels
Stepwise multivariate linear regression analyses were conducted to examine the independent associations of age, gender, race, season, sexual maturation stage, height, fat mass, VAT, SAAT, vigorous physical activity, and cardiovascular fitness with plasma 25- hydroxyvitamin D levels. Race (48%), season (3%), total fat mass (1%), and vigorous physical activity (1%) explained a total of 53% of the variance in plasma 25-hydroxyvitamin D concentrations, with no contributions by age, gender, sexual maturation stage, height, VAT, SAAT, or cardiovascular fitness (Table 4).
Table 4.
Multiple linear regression model for plasma 25-hydroxyvitamin D concentrationsa
| Variable | β ± SE | R2 |
|---|---|---|
| Intercept | 1.558 ± 0.03 | |
| Age (yrs) | NS | |
| Sex | NS | |
| Race | 0.316 ± 0.02b | 0.48 |
| Season | 0.034 ± 0.009b | 0.03 |
| Sexual maturation stage (1–5) | NS | |
| Height (cm) | NS | |
| Fat mass (kg) | −0.000002 ± 0.00b | 0.01 |
| VAT (cm3)a | NS | |
| SAAT (cm3)a | NS | |
| Vigorous PA (min/d)a | 0.015 ± 0.00b | 0.01 |
| CV fitness (mL/kg−1/min−1) | NS | |
| Total R2 | 0.53 |
β, multiple regression unstandardized coefficient; SE, standard error; R2, proportion of variability in plasma 25-hydroxyvitamin D that is attributable to the regression equation.
Logarithmic transformation performed before analysis.
Tests of significance were determined by stepwise linear regression at P < 0.05. NS, nonsignificant.
DISCUSSION
The present study is one of the few studies investigating vitamin D status in the pediatric population in the southern region of the United States. In particular, this study is the first to address 25-hydroxyvitamin D levels over 4 seasons in a cohort of black and white adolescents living at southern US latitudes. Our data demonstrate that low vitamin D status is common among adolescents residing in the southeastern region and is related to various adiposity and lifestyle factors.
From NHANES III (1988 –1994)2 to NHANES 2000 –2004,2, 24 the prevalence of vitamin D deficiency (25-hydroxyvitamin D levels of ≤ 50 nmol/L) in black and white adolescents increased from 28% to 48% and that of vitamin D insufficiency (25-hydroxyvitamin D levels of ≤ 75nmol/L) increased from 66% to 81%. More importantly, ~70% of the black adolescents in NHANES 2000 –2004 had winter serum vitamin D deficiency, which might have important implications for known health disparities.24 The NHANES provided key information regarding the vitamin D status of adolescents; however, data collection from the southern US region was limited to the winter season. Consequently, the effect of seasonality on vitamin D status in adolescents living in the southern US region remained unknown. We observed a high prevalence of low 25-hydroxyvitamin D levels in data collected during all seasons among black and white adolescents living in the southeastern United States. According to the proposed definitions of vitamin D deficiency (≤ 50 nmol/L) and insufficiency (≤ 75 nmol/L),3,4,19,20 one-third (28.8%) of the adolescents were identified as having vitamin D deficiency and more than one-half (56.4%) as having vitamin D insufficiency. The high prevalence of low vitamin D status observed in our adolescent population may reflect, in general, the trend of decreasing circulating vitamin D levels observed over the past 10 to 15 years in the nationally representative sample of adolescents.2,24 Taken together, these findings suggest that low vitamin D status is a growing national problem for adolescents in the United States, regardless of latitude.
One of the key findings in our study is that a substantial proportion of black adolescents may be at risk for low vitamin D status not only in winter but throughout the year. At all ages, black subjects have been shown to have lower plasma 25-hydroxyvitamin D concentrations than do white subjects.6,8,25,26 As expected, we found significantly lower plasma 25-hydroxyvitamin D concentrations in black adolescents, compared with white adolescents, and the circulating vitamin D concentrations in black subjects were half of those in white subjects in every season. Accordingly, the prevalence of vitamin D deficiency was higher among black subjects, compared with white subjects, in winter (84% vs 9%), spring (54% vs 3%), summer (56% vs 0%), and autumn (50% vs 1.9%). Because of the study design, NHANES 2000 –2004 was not able to present seasonal variations.2,24 However, the combined northern latitude (blood samples collected in the summer) and southern latitude (blood samples collected in the winter) US data confirmed that a large proportion (73%) of black adolescents potentially have vitamin D deficiencies.2,24 It is important to note from our study that the mean plasma 25-hydroxyvitamin D concentrations of black girls never reached 50 nmol/L in any season. Low vitamin D status in black individuals has been attributed mainly to reduced skin synthesis of vitamin D because of their greater skin pigmentation.27 Some concerns suggest, however, that the high prevalence of low vitamin D status also may be attributable to the high rates of obesity in black populations.2,24,28
BMI-based categorization of obesity and/or body fatness was shown to be associated with decreased 25-hydroxyvitamin D levels in childhood investigations.2,8,9 In contrast, other studies did not find any associations of BMI and/or fat mass with 25-hydroxyvitamin D levels in the pediatric population.6,29 Discrepancies in these findings may be attributed, in part, to BMI-based categorization of obesity and the variations associated with growth and development. To account for these issues, we determined fat content and fat distribution comprehensively by using a variety of measures, including BMI, waist circumference, DXA, and MRI, and we took into consideration, in the statistical analyses, various factors associated with growth and development, such as age, gender, race, season, height, and sexual maturation stage. We found plasma 25- hydroxyvitamin D concentrations to be consistently and inversely related to all of the adiposity variables, including BMI percentiles, DXA measures for the whole body, waist circumference for central fat tissue in general, and MRI measures specifically for VAT and SAAT. Therefore, as an easy, inexpensive, adiposity measure, BMI percentiles could potentially be used alone for study of the relationship between adiposity and vitamin D levels in future studies. Our data suggest that the relationship between 25-hydroxyvitamin D levels and adiposity in adolescents is independent of the site of fat content and fat distribution. Similar to our results, Looker2 found that the proportion of body fat, measured through bioelectrical impedance, was negatively related to 25-hydroxyvitamin D levels in white and black female subjects 12 to 49 years of age. To our knowledge, only 1 study previously determined relationships between circulating vitamin D concentrations and VAT and SAAT in youths. For 90 white and Hispanic female subjects 16 to 22 years of age residing in California, Kremer et al30 found that 25-hydroxyvitamin D levels were significantly and inversely related to visceral and subcutaneous fat assessed through computed tomography and total fat mass assessed through DXA. The mechanism by which obesity affects low vitamin D status is not known; however, some hypotheses include 25- hydroxyvitamin D being sequestered in adipose tissue,31 negative 25-hydroxyvitamin D feedback from higher circulating 1,25-dihydroxyvitamin D3 levels in obese individuals,32 and decreased sun exposure because of reduced outdoor activity.33
In addition to the lack of available data on serum parathyroid hormone and 1,25-dihydroxyvitamin D levels, other limitations include a lack of information on sun exposure, including use of sunscreen, and dietary intake of vitamin D and calcium. Another weakness of this study is the use of a cross-sectional study design. To determine precisely the vitamin D status of adolescents, longitudinal studies are required. In addition, the seasonal variations of plasma 25-hydroxyvitamin D levels would be better characterized by monitoring each participant individually across all 4 seasons. The self-assessment of Tanner pubertal stages, which was not verified by a physician, also might be a limitation.
CONCLUSIONS
Low vitamin D status seems to be prevalent among adolescents living in a sunny climate. Adiposity, physical activity, and fitness are associated with plasma 25-hydroxyvitamin D concentrations. Additional studies are needed to investigate the implications of low vitamin D status and appropriate treatments.
ACKNOWLEDGMENT
Funding was provided by National Institutes of Health grant HL077230 and HL64157.
ABRRIVATIONS
- 25(OH)D
25-hydroxyvitamin D
- 1,25-(OH)2D
1,25-dihydroxyvitamin D3
- DXA
Dual-energy X-ray absorptiometry
- HR
Heart rate
- LC-MS/MS
Liquid chromatography tandem mass spectroscopy
- METs
Metabolic equivalents
- PA
Physical activities
- PTH
Parathyroid hormone
- SAAT
Subcutaneous abdominal adipose tissue
Footnotes
Conflict of interest: None
Contributor Information
Norman Pollock, Email: NPOLLOCK@mail.mcg.edu.
Inger Susanne Stallmann-Jorgensen, Email: ISTALLMAN@mail.mcg.edu.
Bernard Gutin, Email: BGUTIN@mail.mcg.edu.
Ling Lan, Email: LLAN@mail.mcg.edu.
Tai C Chen, Email: taichen@bu.edu.
Daniel Keeton, Email: DKEETON@mail.mcg.edu.
Karen Petty, Email: KPETTY@mail.mcg.edu.
Michael F Holick, Email: mfholick@bu.edu.
Haidong Zhu, Email: HZHU@mail.mcg.edu.
REFERNCES
- 1.Rovner AJ, O'Brien KO. Hypovitaminosis D among healthy children in the United States: a review of the current evidence. Arch Pediatr Adolesc Med. 2008;162(6):513–519. doi: 10.1001/archpedi.162.6.513. [DOI] [PubMed] [Google Scholar]
- 2.Looker AC. Body fat and vitamin D status in black versus white women. J Clin Endocrinol Metab. 2005;90(2):635–640. doi: 10.1210/jc.2004-1765. [DOI] [PubMed] [Google Scholar]
- 3.Malabanan A, Veronikis IE, Holick MF. Redefining vitamin D insufficiency. Lancet. 1998;351(9105):805–806. doi: 10.1016/s0140-6736(05)78933-9. [DOI] [PubMed] [Google Scholar]
- 4.Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr. 2006;84(1):18–28. doi: 10.1093/ajcn/84.1.18. [DOI] [PubMed] [Google Scholar]
- 5.Abrams SA, Griffin IJ, Hawthorne KM, Gunn SK, Gundberg CM, Carpenter TO. Relationships among vitamin D levels, parathyroid hormone, and calcium absorption in young adolescents. J Clin Endocrinol Metab. 2005;90(10):5576–5581. doi: 10.1210/jc.2005-1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jago R, Harrell JS, McMurray RG, Edelstein S, El Ghormli L, Bassin S. Prevalence of abnormal lipid and blood pressure values among an ethnically diverse population of eighth-grade adolescents and screening implications. Pediatrics. 2006;117(6):2065–2073. doi: 10.1542/peds.2005-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Willis CM, Laing EM, Hall DB, Hausman DB, Lewis RD. A prospective analysis of plasma 25-hydroxyvitamin D concentrations in white and black prepubertal females in the southeastern United States. Am J Clin Nutr. 2007;85(1):124–130. doi: 10.1093/ajcn/85.1.124. [DOI] [PubMed] [Google Scholar]
- 8.Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ. Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med. 2004;158(6):531–537. doi: 10.1001/archpedi.158.6.531. [DOI] [PubMed] [Google Scholar]
- 9.Saintonge S, Bang H, Gerber LM. Implications of a new definition of vitamin D deficiency in a multiracial us adolescent population: the National Health and Nutrition Examination Survey III. Pediatrics. 2009;123(3):797–803. doi: 10.1542/peds.2008-1195. [DOI] [PubMed] [Google Scholar]
- 10.Bell NH, Godsen RN, Henry DP, Shary J, Epstein S. The effects of muscle-building exercise on vitamin D and mineral metabolism. J Bone Miner Res. 1988;3(4):369–373. doi: 10.1002/jbmr.5650030402. [DOI] [PubMed] [Google Scholar]
- 11.Scragg R, Holdaway I, Jackson R, Lim T. Plasma 25-hydroxyvitamin D3 and its relation to physical activity and other heart disease risk factors in the general population. Ann Epidemiol. 1992;2(5):697–703. doi: 10.1016/1047-2797(92)90014-h. [DOI] [PubMed] [Google Scholar]
- 12.Klausen T, Breum L, Sorensen HA, Schifter S, Sonne B. Plasma levels of parathyroid hormone, vitamin D, calcitonin, and calcium in association with endurance exercise. Calcif Tissue Int. 1993;52(3):205–208. doi: 10.1007/BF00298719. [DOI] [PubMed] [Google Scholar]
- 13.Scragg R, Camargo CA., Jr Frequency of leisure-time physical activity and serum 25-hydroxyvitamin D levels in the US population: results from the Third National Health and Nutrition Examination Survey. Am J Epidemiol. 2008;168(6):577–586. doi: 10.1093/aje/kwn163. discussion 587–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lenders CM, Feldman HA, Von Scheven E, Merewood A, Sweeney C, Wilson DM, Lee PD, Abrams SH, Gitelman SE, Wertz MS, Klish WJ, Taylor GA, Chen TC, Holick MF. Relation of body fat indexes to vitamin D status and deficiency among obese adolescents. Am J Clin Nutr. 2009;90(3):459–467. doi: 10.3945/ajcn.2008.27275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tanner J. Growth and Adolescence. 2nd Edition. Oxford, UK: Blackwell Scientific Publications; 1962. [Google Scholar]
- 16.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. Jama. 2003;289(19):2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 17.Owens S, Gutin B, Allison J, Riggs S, Ferguson M, Litaker M, Thompson W. Effect of physical training on total and visceral fat in obese children. Med Sci Sports Exerc. 1999;31(1):143–148. doi: 10.1097/00005768-199901000-00022. [DOI] [PubMed] [Google Scholar]
- 18.Chen TC, Turner AK, Holick MF. Methods for the determination of the circulating concentration of 25-hydroxyvitamin D. J Nutr Biochem. 1990;1(6):315–319. doi: 10.1016/0955-2863(90)90067-u. [DOI] [PubMed] [Google Scholar]
- 19.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 20.Rebsamen MC, Sun J, Norman AW, Liao JK. 1alpha, 25-dihydroxyvitamin D3 induces vascular smooth muscle cell migration via activation of phosphatidylinositol 3-kinase. Circ Res. 2002;91(1):17–24. doi: 10.1161/01.res.0000025269.60668.0f. [DOI] [PubMed] [Google Scholar]
- 21.Maalouf J, Nabulsi M, Vieth R, Kimball S, El-Rassi R, Mahfoud Z, El-Hajj Fuleihan G. Short- and long-term safety of weekly high-dose vitamin D3 supplementation in school children. J Clin Endocrinol Metab. 2008;93(7):2693–2701. doi: 10.1210/jc.2007-2530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutin B, Yin Z, Humphries MC, Barbeau P. Relations of moderate and vigorous physical activity to fitness and fatness in adolescents. Am J Clin Nutr. 2005;81(4):746–750. doi: 10.1093/ajcn/81.4.746. [DOI] [PubMed] [Google Scholar]
- 23.Martin SB, Morrow JR, Jackson AW, Dunn AL. Variables related to meeting the CDC/ACSM physical activity guidelines. Med Sci Sports Exerc. 2000;32(12):2087–2092. doi: 10.1097/00005768-200012000-00019. [DOI] [PubMed] [Google Scholar]
- 24.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169(6):626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harkness L, Cromer B. Low levels of 25-hydroxy vitamin D are associated with elevated parathyroid hormone in healthy adolescent females. Osteoporos Int. 2005;16(1):109–113. doi: 10.1007/s00198-004-1656-8. [DOI] [PubMed] [Google Scholar]
- 26.Rajakumar K, Fernstrom JD, Janosky JE, Greenspan SL. Vitamin D insufficiency in preadolescent African-American children. Clin Pediatr (Phila) 2005;44(8):683–692. doi: 10.1177/000992280504400806. [DOI] [PubMed] [Google Scholar]
- 27.Matsuoka LY, Wortsman J, Haddad JG, Kolm P, Hollis BW. Racial pigmentation and the cutaneous synthesis of vitamin D. Arch Dermatol. 1991;127(4):536–538. [PubMed] [Google Scholar]
- 28.Yanoff LB, Parikh SJ, Spitalnik A, Denkinger B, Sebring NG, Slaughter P, McHugh T, Remaley AT, Yanovski JA. The prevalence of hypovitaminosis D and secondary hyperparathyroidism in obese Black Americans. Clin Endocrinol (Oxf) 2006;64(5):523–529. doi: 10.1111/j.1365-2265.2006.02502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weng FL, Shults J, Leonard MB, Stallings VA, Zemel BS. Risk factors for low serum 25-hydroxyvitamin D concentrations in otherwise healthy children and adolescents. Am J Clin Nutr. 2007;86(1):150–158. doi: 10.1093/ajcn/86.1.150. [DOI] [PubMed] [Google Scholar]
- 30.Kremer R, Campbell PP, Reinhardt T, Gilsanz V. Vitamin D status and its relationship to body fat, final height, and peak bone mass in young women. J Clin Endocrinol Metab. 2009;94(1):67–73. doi: 10.1210/jc.2008-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 32.Bell NH, Epstein S, Greene A, Shary J, Oexmann MJ, Shaw S. Evidence for alteration of the vitamin D-endocrine system in obese subjects. J Clin Invest. 1985;76(1):370–373. doi: 10.1172/JCI111971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Compston JE, Vedi S, Ledger JE, Webb A, Gazet JC, Pilkington TR. Vitamin D status and bone histomorphometry in gross obesity. Am J Clin Nutr. 1981;34(11):2359–2363. doi: 10.1093/ajcn/34.11.2359. [DOI] [PubMed] [Google Scholar]