Abstract
Peptides of the papillomavirus L2 minor capsid protein can induce antibodies (Ab) that neutralize a broad range of human papillomavirus (HPV) genotypes. Unfortunately, L2 is antigenically subdominant to L1 in the virus capsid. To induce a strong anti-L2 Ab response with cross-neutralizing activity to other mucosal types, chimeric virus-like particles (VLP) were generated in which HPV16 L2 neutralization epitopes (comprising L2 residues 69–81 or 108–120) are inserted within an immunodominant surface loop (between residues 133 and 134) of the L1 major capsid protein of bovine papillomavirus type 1 (BPV1). These chimeras self-assembled into pentameric capsomers, or complete VLP similar to wild type (wt) L1 protein. Immunization of rabbits with assembled particle preparations induced L2-specific serum Ab with titers 10-fold higher than those induced by cognate synthetic L2 peptides coupled to KLH. Antisera to both chimeric proteins partially neutralized HPV16 pseudovirions, confirming that both HPV16 L2 peptides define neutralization epitopes. When analyzed for the ability to cross-neutralize infection by authentic HPV11 virions, using detection of early viral RNA by RT-PCR-assays as the readout, immune serum to chimeric protein comprising L2 residues 69–81, but not 108–120, was partially neutralizing. In addition, mouse-antiserum induced by vaccinations with synthetic L2 peptide 108–120, but not 69–81, was partially neutralizing in this assay. Induction of cross-neutralization Ab by L2 epitopes displayed on chimeric VLP represents a possible strategy for the generation of broad-spectrum vaccines to protect against relevant mucosal HPV and associated neoplasia.
Keywords: HPV, L2 peptide, VLP vaccine
1. Introduction
Human papillomaviruses (HPV) comprise a heterogeneous group of species- and tissue-specific DNA tumor viruses, which exclusively infect epithelial cells. Close to 100 HPV genotypes have been identified so far that mainly cause benign epithelial papillomas on skin or mucosa [1]. So-called “low-risk” (LR) types, mostly HPV6 and 11, induce benign mucosal warts (condylomata acuminata). Persistent infection with “high-risk” (HR) types, mainly HPV16 and 18, are associated with almost all cervical cancers [2], a subset of other ano-genital cancers and cancers of the head and neck, and possibly some keratinocyte skin cancers [3–6]. The papillomavirus virion is a non-enveloped, icosahedral particle of 55–60 nm in diameter, which encloses a double stranded circular DNA of approximately 8 kilobases. The capsid consists of 72 capsomers or pentamers, each composed of 5 L1 major capsid proteins, arranged in a T = 7 icosahedral symmetry [7,8]. The second structural protein, the L2 minor capsid protein, is genetically unrelated to L1 and may be located at the capsid vertices of the virion [9].
The L1 protein alone, or L1 co-expressed with L2, is able to self-assemble into virus-like particles (VLP) that are morphologically and immunologically similar to native virions, but lack potentially oncogenic DNA [6]. Immunizations with L1 or L1 + L2 VLP induce high-titer neutralizing antisera to conformation-dependent epitopes that protect against infection both in animal models [10–15] and in human clinical trials [16–20]. Vaccine protection provided by neutralizing antibodies (Ab) to L1 VLP is mostly type-specific and may not protect against infection with heterologous types [6,19]. For example, in the seminal HPV16 L1 VLP vaccine study, 22 incident cervical intraepithelial neoplasias (CIN) related to types other than HPV16 were present in both the placebo and vaccine arms. Although HPV16 and 18 are associated with approximately 70% of anogenital cancers, at least 13 additional HR HPV types are implicated in the development of neoplasias that need consideration in designing a broadly protective HPV vaccine [21]. Such a highly multivalent vaccine combining L1 VLP of the majority or all known HR HPV may not be practical, particularly for developing nations where ~80% of cervical cancers occur.
A portion of L2 is exposed on the virion surface and accessible to Ab, a subset of which are neutralizing [22]. Antisera to L2 proteins of several papillomaviruses are cross-neutralizing to heterologous skin or mucosal types, suggesting that L2 contains type-common epitopes [23,24]. In addition, nasal administration of an HPV16 L2 peptide induced neutralizing antisera against HPV16 and HPV52 [25]. These data suggest that L2-based vaccination may confer Ab-based broad-spectrum protection against infection with multiple mucosal HPV types [22,26–28].
The minor capsid protein of papillomaviruses is incorporated into VLP, when L2 is co-expressed with L1, at a ratio of at least 1 L2 to 30 L1 proteins. However, L1 + L2 VLP do not induce significant L2 antisera, because L2 is subdominant to L1 [24]. The high degree of immunogenicity of L1 protein reflects the close-packed, regularly spaced L1 epitopes in 72 pentameric capsomers that comprise the viral capsid [29]. Even capsomers are significantly immunogenic and able to elicit neutralizing Ab [30–32]. Therefore, we attempted to develop a new type of HPV vaccine, in which L1 VLP display broadly cross-neutralizing L2 epitopes repetitively on the capsid surface in the place of the immunodominant L1 epitopes [33,34]. Kawana et al. [28] have generated a monoclonal Ab (mAb) directed to HPV16 L2 amino acids (aa) 108–120 that neutralized HPV16 and HPV6 pseudovirions. In addition, a mAb to aa 69–81 of HPV16 L2 was neutralizing for HPV16 pseudovirions. Interestingly, sera of patients with genital HPV infections contained high ELISA titers to the synthetic peptide 69–81, indicating that this epitope is a strong surface immunodeterminant. Both epitopes are highly conserved among different HPV types.
Herein, we generate chimeric particles based on the bovine papillomavirus type 1 (BPV1) L1 major capsid protein that display the HPV16 L2 peptides aa 69–81 or aa 108–120, respectively, on an immunogenic capsid surface loop [34,35] (Fig. 1). To explore their potential utility as a preventive vaccine against a broad range of HPV genotypes, we compare L2-specific neutralizing Ab titers raised by vaccination with such chimeric particles.
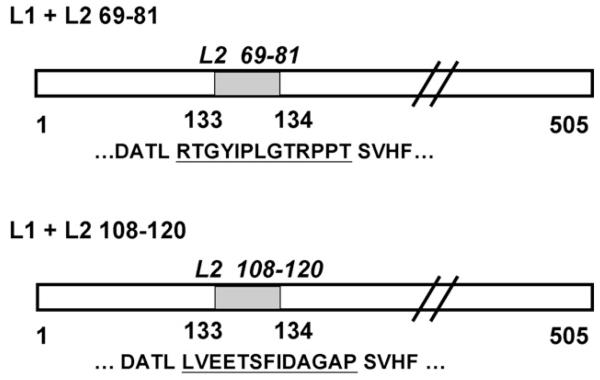
Fig. 1. Construction of recombinant BPV1-L1/HPV16-L2 baculovirus expression vectors.
Oligonucleotides encoding HPV16-L2 peptides 69–81 and 108–120 (indicated by gray bars) were engineered into the 505 amino acids encoding BPV1-L1 ORF (open bar), between residues 133 and 134. The HPV16 L2 peptides are underlined, and flanking BPV1-L1 sequences shown.
2. Materials and methods
2.1. Generation of recombinant baculoviruses
Inverse touch up polymerase chain reaction (PCR) [36] was used to insert nucleotides encoding the respective 13 aa HPV16 L2-epitopes into the BPV1 L1 open reading frame (ORF) under the control of the polyhedrin promotor of baculovirus transfer vectors. Primers complementary to the L1 gene were designed such that they were facing each other backwards on the template [34]. At their 5′ ends each primer encoded a part of the epitope to be incorporated into the L1 carrier protein. For polymerization Isis-Polymerase (Qbiogene, Heidelberg, Germany) was used to amplify the complete L1 gene plus the complete baculovirus transfer vector. Amplimers were gel purified using the NucleoSpin Extract kit (Machery-Nagel, Düren, Germany) and ethanol-precipitated. After phosphorylation and Klenow-treatment [37] amplimers were gel-purified, ethanol precipitated and ligated overnight at 4 °C. The ligation product was transformed into XL-1 supercompetent cells (Stratagene) and bacteria were plated on LB-agarose at 37 °C overnight. Colonies were screened by PCR for the presence of the inserted sequence, DNA was purified using a column procedure (Qiagen Midi-prep), and clones were verified by double-stranded DNA sequencing.
We generated two recombinant transfer vectors encoding chimeric L1 major capsid proteins for BPV1 (Fig. 1), by inserting oligonucleotides encoding 13 aa HPV16 L2-epitopes aa 69–81 RTGYIPLGTRPPT or aa 108–120 LVEETSFIDAGAP into the BPV1 L1 ORF of the plasmid BPV1 L1-pEVmod between aa residues 133/134, using primers KSL1 and 2, or KSL7 and 8 [14,34]. The clones code for the following chimeric L1-L2 epitope sequence with the added L2 aa underlined … DATLRTGYIPLGTRPPTSVHF … and … DATLLVEETSFIDAGAPSVHF ….
2.2. Oligonucleotide primers
KSL1: 5′ ACTAGTTTCTTCCACTAAGGTGACTTTTCTATTCAC 3′ (complementary to BPV1 L1 nt 382–399, coding for additional aa LVEETS = HPV16 L2 nt 322–339); KSL2: 5′ TTTATTGATGCTGGTGCACCAACCCAAACAACAGATGACAGG 3′ (complementary to BPV1 L1 nt 400–420, coding for additional aa FIDAGAP = HPV16 L2 nt 340–360); KSL7: 5′ CAATGGAATATACCCAGTGCGGGTGACTTTTCTATTCAC 3′ (complementary to BPV1 L1 nt 382–399, coding for additional aa RTGYIPL = HPV16 L2 nt 205–225); KSL8: 5′ GGAACAAGGCCTCCCACAACCCAAACAACAGATGACAGG 3′ (complementary to BPV1 L1 nt 400–420, coding for additional aa GTRPPT = HPV16 L2 nt 226–243).
Recombinant baculoviruses were generated as described previously [14]. In brief, transfer vectors were cotransfected with wild type (wt) baculovirus DNA (baculoGold, Pharmingen) into Sf-9 insect cells by lipofection. Following homologous recombination, the baculoviruses were plaque purified and amplified to high-titer. Sf-9 insect cells were infected for 3 days, lysed and analyzed by SDS-PAGE and Western blot. The expression of L1 was detected using the mouse mAb AU-1, directed against the linear BPV1 L1-epitope DTYRYI (BabCO, Berkeley Antibody, Richmond, CA), followed by peroxidase-labeled goat-anti-mouse-IgG, and visualized using the ECL chemiluminescence kit (Amer-sham). Positive clones were re-examined for HPV16 L2 epitopes using a rabbit antiserum raised against a bacterially expressed GST-HPV16 L2 fusion protein [22].
2.3. Expression and purification of chimeric BPV1 L1-HPV16 L2 proteins from Sf-9 insect cells
Sf-9 insect cells (5 × 108) were infected at a multiplicity of infection (MOI) of ~10 with recombinant baculoviruses and harvested after 3 days. High molecular mass structures were separated from cell lysates using a 35% sucrose cushion followed by cesium chloride (CsCl) density gradient equilibrium centrifugation as described [14]. Visible bands were collected, extensively dialysed against phosphate buffered saline (PBS)/0.5 M NaCl/0.05% NaN3, and aliquots examined by SDS-PAGE and Coomassie staining. For visualization by transmission electron microscopy (TEM), purified protein complexes were adsorbed on carbon-coated glow-discharged copper grids for 10 min, fixed on a drop of glutaraldehyde (2.5% in 0.1 M sodium cacodylate buffer, pH 7.0) for 20 min, negatively stained with 1% uranyl acetate, and investigated in a JEOL EM 1010 or a Philips CM 100 electron microscope at 80 kV, with an objective aperture of 30 μm.
2.4. Immunization protocol
New Zealand White (NZW) rabbits or Balb/c mice were each injected s.c. with 50 μg purified protein or synthetic peptides in complete Freund’s adjuvant (CFA), followed by two boosts in incomplete Freund’s adjuvant (IFA) at 4-week intervals, and one additional boost after 8 weeks. Two weeks after the last immunization, serum samples were collected, pooled, heat-inactivated at 56 °C for 1 h, and aliquots were stored at −70 °C.
2.5. Determination of serum titers by ELISA
One μg of biotinylated L2 peptide, synthesized at JPT Peptide Technologies, Berlin, was added to each well of Nunc Streptavidin coated 96-well plates (as described in the Nunc Streptavidin general coating protocol). Native chimeric VLPL2 proteins diluted in PBS were adsorbed (0.3–0.6 μg/well) to 96-well plates (Nunc MaxiSorp) at 37 °C for 1 h. For denaturation, proteins were dried onto the ELISA plate at 37 °C in NaHCO3 buffer, pH 10.6, with 0.01 M DTT. Plates were washed with PBS, blocked overnight with 0.5% non-fat dry milk (Bio Rad)/PBS at 4 °C, and incubated with indicated serial dilutions of antiserum or mAb in triplicate wells for 1 h at room temperature on an ELISA plate shaker (Dynatech). Following three washes with PBS, a 1:10,000 dilution of peroxidase-conjugated Ab was added and the plate was developed by adding the substrate ABTS (Boehringer Mannheim). The optical density (OD) at 405 nm was determined using an ELISA reader (Dynatech). Data are plotted as mean + S.D. of triplicate wells. For statistical analysis Student’s t-test was used for comparison of groups, and p-values ≤0.05 are considered significant.
2.6. HPV11 transient infectivity assay (nested RT-PCR)
A431 cells were seeded into 60 mm tissue culture plates at 3 × 105 cells [38,39]. Native HPV11 virions (purified from genital warts and typed by DNA sequencing, 1 μl of stock solution), were diluted in 1 ml RPMI w/o FCS and incubated with 20 μl pre-immune or immune sera (1:50 dilution), or neutralizing mAb H11.B2 (Chemicon) (at high concentration of 20–50 μg/ml to ensure complete neutralization) as positive control for 1 h. Culture medium was aspirated and cells were infected with the pre-incubated virus solution for 1 h at 37 °C, with gentle rocking every 15 min, and then fed with 3 ml of fresh RPMI/5% FCS medium, respectively. After 3 days of incubation, total cellular RNA was extracted using Tri Reagent (Molecular Research Center, Cincinnati, Ohio). For first-strand cDNA synthesis, oligo-p (dT)15 primers were used (Roche). Spliced HPV11 E1∧E4 mRNA was detected by two rounds of 30-cycle nested PCR as described previously [39]. The expected final size of the PCR amplimer was 628 bp for spliced HPV11 mRNA and 429 bp for spliced β-actin mRNA.
2.7. HPV11 transient infectivity assay (single round quantitative RT-PCR)
HPV11 virions were preincubated in a 1:50 dilution of serum (in DMEM/10% FCS) for 1 h at 37 °C. Controls included virus incubated with no serum, and virus incubated at vast excess with the neutralizing mAb H11.H3 (concentration of 85μg/ml) to ensure complete neutralization. At the end of the preincubation period, an additional 1 ml DMEM/10% FBS was added to the inoculum and the mixture (150 particles/cell) was spread over nearly confluent HaCaT cells, plated the previous day at 5 × 105 cells per well (6-well plate). Twenty-four hours later the medium was replaced with 3 ml fresh medium. RNA was harvested at 72 h post-infection using TRIzol (Invitrogen). Relative levels of E1∧E4 transcripts were measured using QRT-PCR as described previously [40]. Expression of “0” indicates no E1∧E4 transcripts were detected in the RNA from these wells (e.g. H11.H3). An expression level of “1” indicates that the number of E1∧E4 transcripts relative to the number of cellular TBP transcripts were found to be at the same ratio as measured in the RNA of control monolayers infected with untreated virions. Data are plotted as the mean ± S.E. of five independent experiments. Pre-immune and immune ± sera were compared within groups using Student’s t-test, and p-values ≤0.05 are considered significant.
2.8. HPV6, 16 and 18 pseudovirion neutralization assays
Pseudoviruses were generated by co-transfection of 293TT cells [41] and neutralization assays performed as described with few modifications [42]. The 293TT cells (30,000/well) were pre-plated 3–4 h in advance in 96-well non-treated sterile, polystyrene plates (Nalge-Nunc) in 100 μl neutralization buffer (DMEM/10% FCS, no phenol red). The diluted pseudovirions were incubated with different rabbit sera in triplicate wells for 1 h at 37 °C/5% CO2 and the mixture was placed on the 293TT cells. At the end of the 68–72 h incubation period, the supernatant was harvested and clarified at 1500 × g for 5 min. The secreted alkaline phosphatase (SEAP) content in the clarified supernatant was determined using p-nitrophenyl phosphate (Sigma, St. Louis, MO) dissolved in diethanolamine (Acros Organics, NJ). The resultant yellow color was measured at 405 nm. Data shown are the mean + S.D. of triplicate wells of a representative of two independent experiments. Serum neutralization titers were defined arbitrarily as the reciprocal of the highest dilution that caused at least a 50% reduction in SEAP activity (p ≤ 0.05 by Student’s t-test).
3. Results
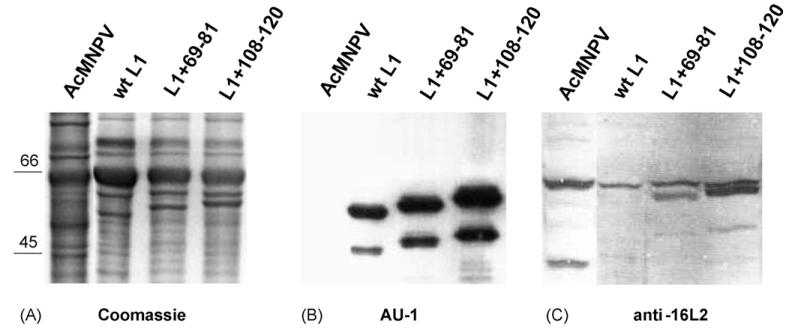
Two conserved peptides in HPV16 L2 (aa 69–81 and 108–120) have been identified that induce neutralizing antisera to homologous HPV16. Antibodies to peptide 108–120 also cross-neutralized heterologous genital HPV (types 6, 11 and 52), whereas neutralization by a mAb directed against peptide 69–81 was type-restricted [28,43,44]. Recombinant baculoviruses were generated by standard methods for expression of chimeric fusion proteins L1+69-81 and L1+108-120 (Fig. 1), or wt BPV1 L1 as a control [14]. Sf-9 insect cells were infected for 3 days, lysed, and analyzed by SDS-PAGE. Coomassie-blue staining revealed a significant 55–60 kDa band for the respective wt and chimeric proteins (Fig. 2A). Antigenicity of L1 was verified by Western blotting, using the anti-L1 mAb AU-1, which is directed against a linear BPV1 L1-epitope (DTYRYI) (Fig. 2B). Smaller bands likely represent protein degradation products. Correct translation of the inserted HPV16 L2 epitopes was detected by a polyclonal L2-antiserum [22], which recognized a specific band of the appropriate size in the cell lysates expressing chimeric proteins (Fig. 2C, lanes 3 and 4), but not in control lysates expressing wt L1 or wt baculoviruses (Fig. 2C, lanes 1 and 2).
Fig. 2. Baculovirus expression of BPV1 L1-HPV16 L2 fusion proteins.
Sf-9 insect cells were infected for 3 days with wild type AcMNPV or recombinant baculoviruses expressing L1+69-81, L1+108-120, or wild type BPV1-L1 as control, lysed, separated by SDS-Page, and analyzed by Coomassie stain (A), and Western blotting using mAb AU-1 directed against BPV1 L1 (AU-1) (B), or immune serum raised against HPV16 L2 (anti-16L2) (C). Recombinant proteins migrated at about 55–60 kDa. Smaller Coomassie-stained and immunoreactive bands that are absent in wt baculovirus (AcMNPV) infected control lanes likely represent a protein degradation product. A slower migrating band present in all four lanes of panel C is unspecifically recognized by the polyclonal anti-16L2 antiserum.
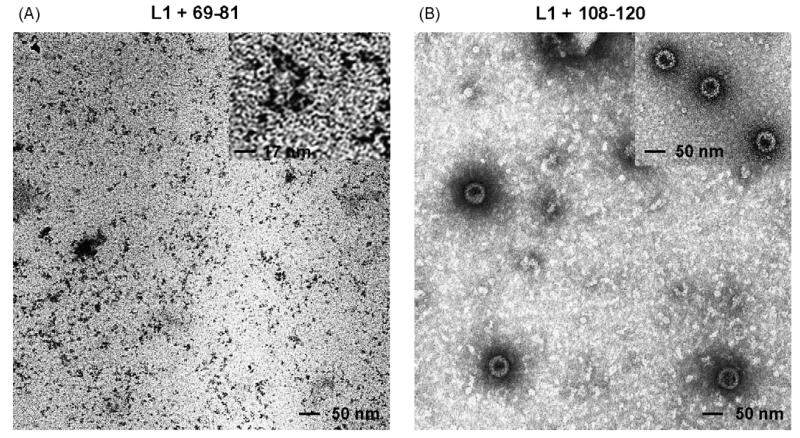
To determine if chimeric proteins self-assemble into viral particles or capsomer subunits similar to parental wt L1 protein, high-molecular weight complexes were purified by density gradient centrifugation, and preparations were negatively stained and analyzed by TEM [34]. Electron micrographs demonstrated that L1+69-81 assembled into capsomers, the pentameric L1 subunit of VLP (Fig. 3A and inset). In contrast, TEM of the L1+108-120 preparation showed spherical particles with a diameter of approximately 55 nm (Fig. 3B) and the morphology similar to that of wt L1 VLP (Fig. 3B, inset). In addition, smaller particles, and pentamers either singly or in aggregates, were observed (Fig. 3B).
Fig. 3. Transmission electron microscopy of baculovirus expressed fusion proteins.
Chimeric L1+69-81 (A) and L1+108-120 (B) fusion proteins were expressed in Sf-9 insect cells, purified on density gradients, negatively stained and analyzed by transmission electron microscopy at 30,000 magnification. (A) Inset shows L1+69-81 pentamers at three-fold higher magnification. (B) Inset shows BPV1 wild type L1 VLP for comparison.
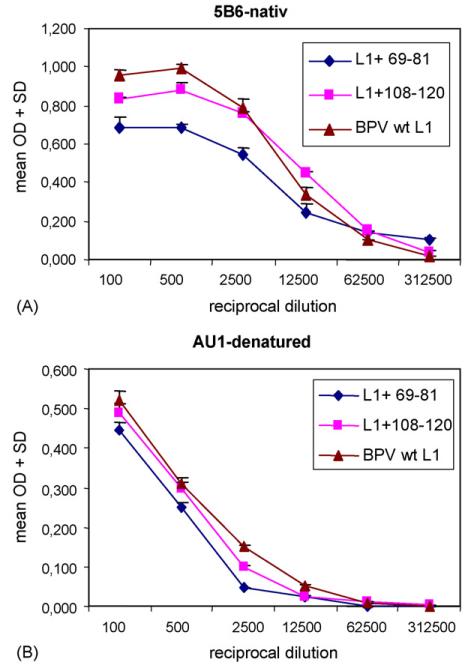
As compared to denatured linear L1 proteins, papillomavirus L1 VLP and capsomers are highly immunogenic due to their multimeric structure, and display conformational neutralization epitopes [6,45]. We thus examined whether chimeric proteins retained the ability to self-assemble into a multimeric platform, capable of presenting the L2 epitopes in a highly immunogenic form. As a surrogate marker for self-assembly, we examined reactivity with the BPV1-neutralizing mAb 5B6, which is directed to a conformation-dependent L1 epitope [22]. Chimeric L1+69-81 and L1+108-120 proteins, and wt BPV- L1 VLP as a reference, were analyzed by ELISA under native conditions (Fig. 4A). The mAb 5B6 recognized both fusion proteins, as well as wt VLP, indicating assembly into potentially immunogenic multimeric structures. OD values were considerably reduced for chimeric protein L1+69-81 (p≤0.05 for 5B6 dilutions up to 1:12,500), and less prominent for L1+108-120 (p≤0.05 for 5B6 dilutions up to 1:500), as compared to wt VLP. Impaired binding of mAb 5B6 to L1+69-81 and L1+108-120 might be explained by steric hindrance, or slight distortion of the conformational epitope by the inserted peptide resulting in lower Ab affinity. As a negative control, reactivity to 5B6 was completely abolished when wt and chimeric particles were applied to the ELISA plate under denaturing conditions (pH 10.8) (data not shown). To rule out the possibility that different protein concentrations might account for differences in mAb 5B6 binding, in a parallel control experiment denatured antigens were tested with mAb AU-1, which is directed against a linear L1 epitope.Fig. 4B demonstrates similar OD values of AU-1 bound to L1+69-81, L1+108-120 and wt L1 VLP. These data infer that similar amounts of chimeric and wt L1 VLP proteins had been attached to the ELISA plate. Thus reduced reactivity of 5B6 to native L1+69-81 and L1+108-120 (Fig. 4A) was not due to lower amount of antigen applied to the ELISA plate.
Fig. 4. Analysis of baculovirus expressed chimeric L1+69-81 and L1+108-120 proteins by ELISA.
BPV1 wild type L1 (triangles), and chimeric L1+108-120 (squares) or L1+69-81 (diamonds) proteins were analyzed under native conditions by ELISA, using the indicated serial dilutions of mAb 5B6 directed to a conformation-dependent neutralizing epitope (A), or mAb AU-1 directed to a linear BPV1 L1 epitope under denaturing conditions (B). OD, absorbance at 405 nm, mean + S.D. of triplicate wells.
We further analyzed if chimeric particles could induce an immune response specific for the surface-displayed L2 epitopes. Furthermore, we sought to compare immunogenicity of the multimeric antigens to that of synthetic L2 peptides, which were conventionally coupled to KLH. Therefore, rabbits were immunized four times with chimeric fusion proteins L1+69-81 or L1+108-120, using Freund’s as the adjuvant. In addition, mice were immunized with synthetic L2 peptides aa 69–81 and 108–120 coupled to KLH, using Freund’s as adjuvant as well. Sera were analyzed by ELISA, using the respective cognate L2-peptide as the antigen.
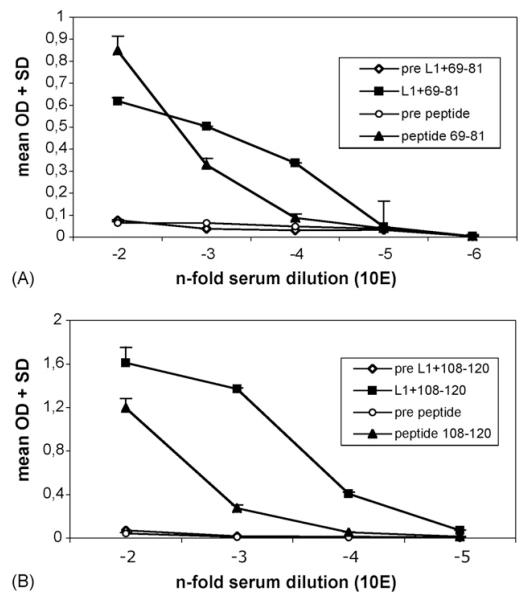
As shown in Fig. 5, both L1 + L2 fusion proteins induced antisera to L2 with titers of 10,000, and chimeric pentamers (L1+69-81) were as efficient as chimeric VLP (L1+108-120). ELISA titers were 10-fold higher for antisera induced by chimeric proteins than that induced by the respective synthetic peptides (p≤0.05). As expected, pre-immune sera did not react in the ELISA. These results indicated that multimeric display of L2-peptides on chimeric particles was more efficient in immunizations than the standard method of coupling peptides to KLH.
Fig. 5. Analysis of antisera raised against chimeric proteins L1+69-81 and L1+108-120, or synthetic peptides by ELISA.
Streptavidin-coated ELISA plates were satured with biotinylated synthetic HPV16 L2 peptides 69–81 (A) or 108–120 (B). Pre-immune and immune sera raised against multimeric chimeric proteins L1+69-81 (A) or L1+108-120 (B), or raised against synthetic KLH-coupled peptides were serially diluted as indicated and analyzed by ELISA. OD, absorbance at 405 nm, mean + S.D. of triplicate wells.
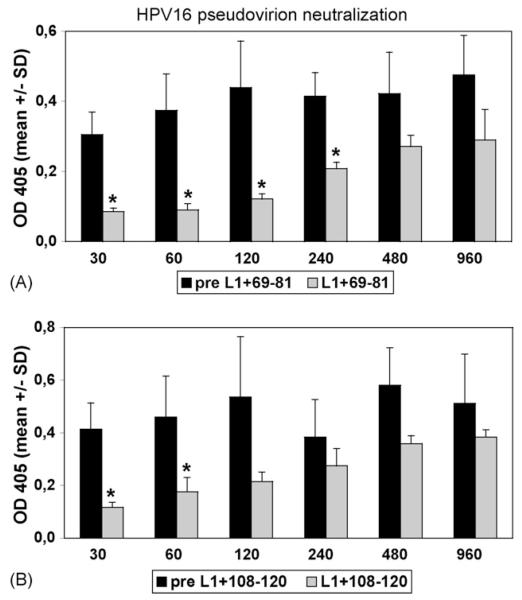
To determine if L2 peptide binding translates into neutralizing activity, immune sera were analyzed in HPV pseudovirion neutralization assays [42,46]. Diluted pseudovirus was incubated with serial dilutions of antisera in triplicate wells for 1 h and the mixture was placed on 293TT cells. Following a 3 days incubation period supernatants were harvested, and the secreted alkaline phosphatase (SEAP) content in the clarified supernatant determined as a readout. Serum neutralization titers were defined as the reciprocal of the highest dilution that caused at least 50% reduction in SEAP activity (p≤0.05 by Student’s t-test). In HPV16 pseudovirion assays, the antiserum to L1+69-81 was neutralizing with a titer of 240 (Fig. 6A). Antiserum against L1+108-120 neutralized HPV16 with a titer of 60 (Fig. 6B). In contrast, antisera against both synthetic peptides did not neutralize any of the pseudovirions (not shown), supporting the concept that chimeric particles are more immunogenic than conjugated L2-peptides for induction of (cross)-neutralizing Ab.
Fig. 6. Neutralization of HPV16 pseudovirions by antisera to chimeric VLP and pentamers.
HPV16 pseudovirions were incubated in the presence of indicated dilutions of pre-immune (black bars) or immune sera (grey bars) raised against chimeric proteins. (A) Antisera raised against fusion protein L1+69-81 neutralized with a titer of 240. (B) Antiserum raised against L1+108-120 neutralized with a titer of 60 (stars indicate a p-value 0.05 by Student’s t-test). Antisera raised against synthetic peptides 69–81≤or 108–120 did not neutralize (not shown).
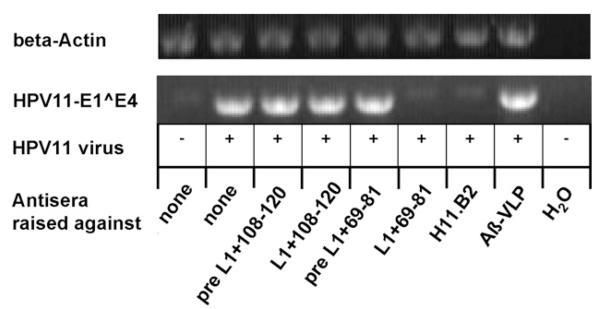
It has been shown previously that HPV6, 16, and 18 L2 proteins contain cross-neutralization epitopes [24,28,43,44]. To determine if antisera induced by chimeric proteins are able to cross-neutralize heterologous mucosal HPV types, an HPV11 infectivity assay was performed using authentic virions isolated from genital warts. A431 monolayer cell cultures were transiently infected for 3 days, and analyzed by nested reverse transcriptase (RT)-PCR, detecting spliced early viral transcripts in infected cells as readout. HPV11 virions were incubated with antisera at 1:50 dilution for 1 h, or left untreated, prior to infecting cells. Antiserum raised against L1+69-81 (but not pre-immune sera) neutralized HPV11 virions as shown by the absence of early transcripts (Fig. 7), indicating that HPV16 L2 peptide 69–81 can induce cross-clade neutralizing antisera to the distantly related HPV11. Unexpectedly, antiserum raised against L1+108-120 was non-neutralizing. In addition, immune sera raised against the KLH-coupled synthetic peptides 69–81 and 108–120, and antiserum to wt L1 VLP (not shown), or Aβ-VLP (a chimeric BPV1 L1 VLP containing an irrelevant 9 aa peptide of beta amyloid) (Fig. 7) [47], did not neutralize HPV11 infection. Neutralization was further determined for HPV6 and HPV18 pseudovirions, but neither were neutralized by the immune sera. As expected, both sera raised against the chimeric proteins neutralized BPV1 pseudovirions with a titer of at least 2560 (the highest serum dilution tested), indicating a vigorous immune response to the BPV1 L1 carrier protein.
Fig. 7. Cross-neutralization of HPV11 virions by RT-PCR assay.
Authentic HPV11 virions were incubated with sera at 1:50 dilution or left untreated for 1 h prior to infecting A431 cells. Transient HPV11-infection of A431 cells was analyzed by detection of spliced viral E1∧E4 mRNA by nested RT-PCR. Antisera raised against L1+69-81, L1+108-120, or pre-immune sera are indicated. H11.B2 is a neutralizing mAb raised against HPV11 virions. The negative control serum was raised against chimeric BPV1 L1 VLP containing an irrelevant beta-amyloid nona-peptide (Aβ-VLP).
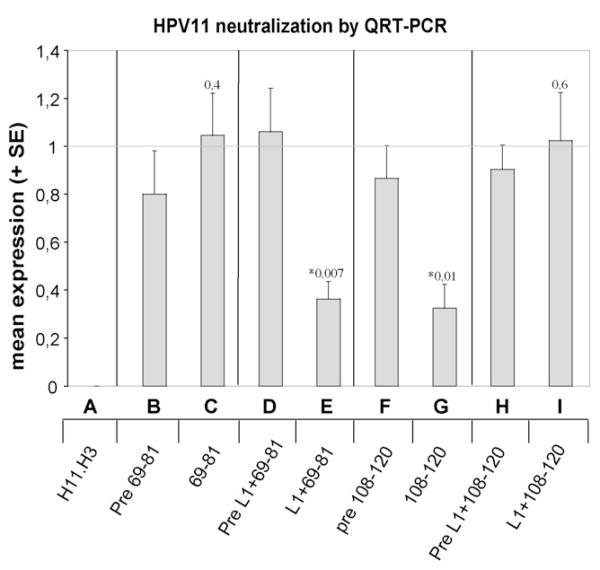
Neutralizing activity of the antisera induced by chimeric proteins and synthetic L2 peptides was further assayed in a more sensitive HPV11 infectivity assay, using single-round quantitative RT-PCR to detect HPV 11 E1∧E4 spliced transcripts as a readout [40]. Quantification of this transcript provided a direct measure of the ability of antiserum to neutralize viral infection. HaCaT cells were infected with HPV11 virions pre-incubated with 1:50 diluted antisera induced by L1+69-81, L1+108-120, pre-immune sera, or sera raised against synthetic peptides 69–81 and 108–120, respectively. Similar to the results obtained by nested RT-PCR infectivity assay, antiserum raised against L1+69-81 displayed neutralizing activity, as demonstrated by a 66% (p = 0.007) inhibition of RNA expression compared to pre-immune serum (Fig. 8, D and E). In agreement with the previous experiment, antiserum raised against L1+108-120 did not neutralize HPV 11 (p = 0.6) (Fig. 8, H and I). Similar to the nested RT-PCR results, antiserum raised against the synthetic peptide 69–81 was non-neutralizing (p = 0.4) (Fig. 8, B and C), whereas antiserum against the synthetic 108–120 peptide was partially neutralizing in the assay (p = 0.01) (Fig. 7, F and G). The latter discrepant result may be explained by the higher sensitivity of the quantitative amplification method (as compared to nested RT-PCR) to detect incomplete neutralization of HPV11 virions.
Fig. 8. HPV11 virion neutralization assay by quantitative RT-PCR.
Authentic HPV11 virions were incubated with indicated pre-immune or immune sera at dilution 1:50 prior to infecting HaCaT cells. The ranges 69–81 and 108–120 indicate mouse (pre-)immune sera raised against synthetic L2 peptides; L1+69-81 and L1+108-120 indicate rabbit (pre-)immune sera raised against the respective chimeric proteins. H11.H3 is a neutralizing mAb raised against HPV11 virions that was used at excess concentration to ensure complete neutralization. Data are plotted as the mean + S.E. of five independent experiments as described in materials and methods. Pre-immune and immune sera were compared within groups using Student’s t-test, and p-values ≤ 0.05 (indicated by stars) are considered significant.
4. Discussion
Neutralizing Ab to papillomaviruses are mainly type-specific and directed against the immunodominant L1 capsid protein. In addition, the N-terminus of L2 can induce low-titer neutralization of homologous and heterologous types [23]. However, immunization with co-assembled L1 + L2 VLP does not provide cross-neutralizing Ab to L2 [24], perhaps because the relevant epitopes of L2 are masked by the capsid, resulting in poor presentation to the immune system [48]. Alternatively the highly immunogenic nature of the closely packed L1 epitopes might dominate the humoral response because L2 is more distantly spaced [49]. To overcome immunological subdominance of the L2 protein we have generated two BPV1 L1 proteins that display a short HPV16 L2 epitope repetitively and closely spaced on the assembled L1 particles. The respective epitope was incorporated between L1 aa 133 and 134 into the D-E loop, as previous studies have shown that the site is both immunogenic and tolerant for insertion of relatively short peptides, without preventing self-assembly into particles [8,34,35]. It is noteworthy that despite insertion within this loop, the chimeric VLP still induced high titers of BPV1 neutralizing Ab that is presumably specific to neutralizing epitopes on other loops. This suggests that HPV16 L1 may represent a suitable carrier for clinical introduction.
The close-packed, ordered structure of assembled L1 pentamers or VLP is a key factor in their immunogenicity [14,31,49]. Papillomavirus VLP directly bind and activate dendritic cells [50,51] as well as B-cells [52]. In addition, immunization with HPV16 VLP induces potent cytokine and T helper 1 (Th1)-biased immune responses via a toll-like receptor recognition mechanism [53–55]. To determine their state of assembly, we analyzed chimeric vaccine preparations using morphology by TEM, and immunologically using the conformation-dependent neutralizing mAb 5B6 to probe the chimeric VLP surface. Correct assembly is supported by TEM of negatively stained vaccine preparations, demonstrating assembly into pentamers (for L1+69-81 protein), or complete VLP (for L1+108-120 protein), respectively. Monoclonal Ab 5B6 recognizes infectious BPV virions, VLP and pentamers self-assembled from wt L1, but not denatured L1 protein. Under native conditions using chimeric VLP preparations as the ELISA antigen, mAb 5B6 reacted with both chimeric proteins, although demonstrating reduced OD values for L1+69-81 and L1+108-120 as compared to wt L1 VLP (Fig. 4A). Furthermore, vaccination with these chimeric VLPs also induces high titers of BPV1 neutralizing Ab suggesting correct conformation. Thus, despite L2 epitope insertion into the DE surface loop, BPV1 L1 assembled of into particles that correctly present conformational and immunogenic BPV1-neutralization epitopes [34].
We next determined immunogenicity of L2 in our L1 carrier-based vaccine preparations. Immunization of rabbits with chimeric proteins induced antisera that recognized the cognate HPV16 L2 by ELISA, with a titer that was one order of magnitude higher than that induced by synthetic L2 peptides coupled to KLH. This indicates that epitope display on the surface of L1 particles is an efficient strategy to boost immunity to L2 peptides (Fig. 5). Our results are consistent with previous studies using VLP as vaccine carrier to induce antisera to autologous (TNF-alpha, CCR5, amyloid-beta) [47,56,57], or heterologous viral (gp 41 env) antigens [34].
It has been reported that a mAb directed to, and antisera raised against, aa 108–120 of HPV16 L2 neutralized homologous HPV16, and in addition heterologous HPV6, HPV11, and HPV52 pseudoinfections, whereas neutralization by a mAb to aa 69–81 of HPV16 L2 was HPV16-specific [25,28,44]. Both antisera raised herein against chimeric L1-L2 proteins neutralized homologous HPV16 pseudovirions at low titer (60–240) (Fig. 6), whereas antisera raised against the cognate synthetic peptides were non-neutralizing by nested RT-PCR assay. Our ability to detect HPV16-neutralization only when chimeric proteins, but not peptides were used for immunization are in agreement with a previous report [58], and may reflect differences in immunization procedures, or the use of different systems for production of pseudovirions used in neutralization assays.
The use of authentic virions is important for HPV neutralization assays, but limited by the very few available types. Importantly, antiserum to chimeric L1+69-81 proteins cross-neutralized HPV11 in both nested and single-round quantitative RT-PCR assays, whereas sera to L1+108-120 were non-neutralizing. In addition, neutralization of HPV11 virions by antisera to synthetic peptides was not detected by nested RT-PCR. However, an antiserum to synthetic peptide 108–120, but not 69–81, partially neutralized authentic HPV11 virions in the more sensitive quantitative RT-PCR infectivity assay. The higher sensitivity of the quantitative (as compared to nested) RT-PCR assay may explain this discrepancy, as this assay can also measure partial neutralization. These results corroborate and extend previous findings where mAb to or antisera raised against aa 108–120 of HPV16 L2 cross-neutralized heterologous HPV11 pseudovirions, whereas neutralization of HPV6 by a mAb to aa 69–81 of HPV16 L2 was not reported [28,44].
The extent of cross-neutralization for heterologous HPV was further analyzed with pseudovirions of the genital types HPV6 and HPV18, none of which scored positive with the antisera. Of note, the L2 epitope 108–120 is identical for HPV6 and 11, consistent with the fact that antiserum to L1+108-120 did neither neutralize HPV11 (both by quantitative RT-PCR assay and nested PCR), nor HPV6 (by pseudovirion assay).
Our RT-PCR results demonstrate for the first time that HPV16 L2 aa 69–81, in the context of an assembled L1 pentamer, induce antisera that cross-neutralize native HPV11 virions, whereas antisera raised against the 69–81 peptide alone did not neutralize. The 10-fold higher titer to L2 induced by L1-L2 pentamers/VLP compared to synthetic L2 peptide vaccination might account for this difference, supporting the concept of boosting L2 immunogenicity by the L1 display system. Although L1+69-81 assembled into capsomers appears superior to L1+108-120 VLP in the capacity to induce neutralizing antisera, this difference may arise primarily from characteristics of the epitopes including their (cross)-neutralizing ability, rather than the multiplicity of the carrying particle. Further studies are required to compare the potency of chimeric pentamers with that of VLP to induce Ab to surface exposed heterologous peptides.
Serum titers against L2 obtained in this (and in previous) studies are generally several orders of magnitude lower than the anti-L1 titers obtained with VLP vaccines. The latter correlate with high vaccination efficiency in preventing HPV infection and associated disease, although an anti-L1 Ab threshold level as surrogate marker for protection has yet to be defined. Nevertheless, low Ab titers to L2 may be clinically (at least partially) protective, considering the slow internalization kinetics of papillomavirus with a t(1/2) time of 4 h following cell attachment, during which virions remain susceptible to neutralizing Ab present in various body fluids [59,60].
In conclusion, incorporation of cross-neutralizing L2 epitopes into a self-assembled L1 carrier may be a possible strategy to generate broad-spectrum vaccines to protect against multiple HPV types.
Acknowledgements
This work was supported by a grant from the Austrian Science Foundation (FWF, P18990-B13) to RK. Partial support from PHS NCI CA47622 (NDC) and SPORE in Cervical Cancer P50 CA098252 (RBSR abd NDC) and an American Cancer Society Research Scholar Grant RSG MBC-103744 (RBSR) is acknowledged.
References
- [1].de Villiers EM, Fauquet C, Broker TR, Bernard HU, zur Hausen H. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- [2].Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- [3].Pfister H. Chapter 8: human papillomavirus and skin cancer. J Natl Cancer Inst Monogr. 2003:52–6. doi: 10.1093/oxfordjournals.jncimonographs.a003483. [DOI] [PubMed] [Google Scholar]
- [4].Salzman NP, Howley PM, editors. The Papillomaviruses. Vol. 2. Plenum Press; New York: 1987. The Papovaviridae; p. 392. [Google Scholar]
- [5].zur Hausen H. Papillomavirus infection- a major cause of human cancers. Biochim Biophys Acta. 1996;1288:F55–78. doi: 10.1016/0304-419x(96)00020-0. [DOI] [PubMed] [Google Scholar]
- [6].Kirnbauer R. Papillomavirus-like particles for serology and vaccine development. Intervirology. 1996;39:54–61. doi: 10.1159/000150475. [DOI] [PubMed] [Google Scholar]
- [7].Baker TS, Newcomb WW, Olson NH, Cowsert LM, Olson C, Brown JC. Structures of bovine and human papillomaviruses. Analysis by cryoelectron microscopy and three-dimensional image reconstruction. Biophys J. 1991;60:1445–56. doi: 10.1016/S0006-3495(91)82181-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen XS, Garcea RL, Goldberg I, Casini G, Harrison SC. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol Cell. 2000;5:557–67. doi: 10.1016/s1097-2765(00)80449-9. [DOI] [PubMed] [Google Scholar]
- [9].Trus BL, Roden RB, Greenstone HL, Vrhel M, Schiller JT, Booy FP. Novel structural features of bovine papillomavirus capsid revealed by a three-dimensional reconstruction to 9Å resolution. Nat Struct Biol. 1997;4:413–20. doi: 10.1038/nsb0597-413. [DOI] [PubMed] [Google Scholar]
- [10].Breitburd F, Kirnbauer R, Hubbert NL, Nonnenmacher B, Trin-Dinh-Desmarquet C, Orth G, et al. Immunization with viruslike particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J Virol. 1995;69:3959–63. doi: 10.1128/jvi.69.6.3959-3963.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Christensen N, Reed C, Cladel N, Han R, Kreider J. Immunization with viruslike particles induces long-term protection of rabbits against challenge with cottontail rabbit papillomavirus. J Virol. 1996;70:960–5. doi: 10.1128/jvi.70.2.960-965.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jansen K, Rosolowsky M, Schultz L, Markus H, Cook J, Donnelly J, et al. Vaccination with yeast-expressed cottontail rabbit papillomavirus (CRPV) virus-like particles protects rabbits from CRPV-induced papilloma formation. Vaccine. 1995;13:1509–14. doi: 10.1016/0264-410x(95)00103-8. [DOI] [PubMed] [Google Scholar]
- [13].Kirnbauer R, Chandrachud L, O’Neil B, Wagner E, Grindlay G, Armstrong A, et al. Virus-like particles of bovine papillomavirus type-4 in prophylactic and therapeutic immunization. Virology. 1996;219:37–44. doi: 10.1006/viro.1996.0220. [DOI] [PubMed] [Google Scholar]
- [14].Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci USA. 1992;89:12180–4. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Suzich JA, Ghim S, Palmer-Hill FJ, White WI, Tamura JK, Bell J, et al. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomas. Proc Natl Acad Sci USA. 1995;92:11553–7. doi: 10.1073/pnas.92.25.11553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Harper DM, Franco EL, Wheeler CM, Moscicki AB, Romanowski B, Roteli-Martins CM, et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet. 2006;367:1247–55. doi: 10.1016/S0140-6736(06)68439-0. [DOI] [PubMed] [Google Scholar]
- [17].Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364:1757–65. doi: 10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- [18].Harro CD, Pang YY, Roden RB, Hildesheim A, Wang Z, Reynolds MJ, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst. 2001;93:284–92. doi: 10.1093/jnci/93.4.284. [DOI] [PubMed] [Google Scholar]
- [19].Koutsky LA, Ault KA, Wheeler CM, Brown DR, Barr E, Alvarez FB, et al. A controlled trial of a human papillomavirus type 16 vaccine. N Engl J Med. 2002;347:1645–51. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- [20].Villa LL, Costa RL, Petta CA, Andrade RP, Ault KA, Giuliano AR, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16 and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6:271–8. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- [21].Munoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–27. doi: 10.1056/NEJMoa021641. [DOI] [PubMed] [Google Scholar]
- [22].Roden RB, Weissinger EM, Henderson DW, Booy F, Kirnbauer R, Mushinski JF, et al. Neutralization of bovine papillomavirus by antibodies to L1 and L2 capsid proteins. J Virol. 1994;68:7570–4. doi: 10.1128/jvi.68.11.7570-7574.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Pastrana DV, Gambhira R, Buck CB, Pang YY, Thompson CD, Culp TD, et al. Cross-neutralization of cutaneous and mucosal papillomavirus types with anti-sera to the amino terminus of L2. Virology. 2005;337:365–72. doi: 10.1016/j.virol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- [24].Roden RBS, Yutzy WH, Fallon R, Inglis S, Lowy DR, Schiller JT. Minor capsid protein of human genital papillomaviruses contains sub-dominant, cross-neutralizing epitopes. Virology. 2000;270:254–7. doi: 10.1006/viro.2000.0272. [DOI] [PubMed] [Google Scholar]
- [25].Kawana K, Yasugi T, Kanda T, Kino N, Oda K, Okada S, et al. Safety and immunogenicity of a peptide containing the cross-neutralization epitope of HPV16 L2 administered nasally in healthy volunteers. Vaccine. 2003;21:4256–60. doi: 10.1016/s0264-410x(03)00454-7. [DOI] [PubMed] [Google Scholar]
- [26].Campo MS. Animal models of papillomavirus pathogenesis. Virus Res. 2002;89:249–61. doi: 10.1016/s0168-1702(02)00193-4. [DOI] [PubMed] [Google Scholar]
- [27].Christensen ND, Kreider JW, Kan NC, DiAngelo SL. The open reading frame L2 of cottontail rabbit papillomavirus contains antibody-inducing neutralizing epitopes. Virology. 1991;181:572–9. doi: 10.1016/0042-6822(91)90890-n. [DOI] [PubMed] [Google Scholar]
- [28].Kawana K, Yoshikawa H, Taketani Y, Yoshiike K, Kanda T. Common neutralization epitope in minor capsid protein L2 of human papillomavirus types 16 and 6. J Virol. 1999;73:6188–90. doi: 10.1128/jvi.73.7.6188-6190.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Bachmann MF, Zinkernagel RM, Oxenius A. Immune responses in the absence of costimulation: viruses know the trick. J Immunol. 1998;161:5791–4. [PubMed] [Google Scholar]
- [30].Yuan H, Estes PA, Chen Y, Newsome J, Olcese VA, Garcea RL, et al. Immunization with a pentameric L1 fusion protein protects against papillomavirus infection. J Virol. 2001;75:7848–53. doi: 10.1128/JVI.75.17.7848-7853.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Rose R, White W, Li M, Suzich J, Lane C, Garcea R. Human papillomavirus type 11 recombinant L1 capsomeres induce virus-neutralizing antibodies. J Virol. 1998;72:6151–4. doi: 10.1128/jvi.72.7.6151-6154.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Fligge C, Giroglou T, Streeck RE, Sapp M. Induction of type-specific neutralizing antibodies by capsomeres of human papillomavirus type 33. Virology. 2001;283:353–7. doi: 10.1006/viro.2000.0875. [DOI] [PubMed] [Google Scholar]
- [33].Varsani A, Williamson AL, de Villiers D, Becker I, Christensen ND, Rybicki EP. Chimeric human papillomavirus type 16 (HPV-16) L1 particles presenting the common neutralizing epitope for the L2 minor capsid protein of HPV-6 and HPV-16. J Virol. 2003;77:8386–93. doi: 10.1128/JVI.77.15.8386-8393.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Slupetzky K, Shafti-Keramat S, Lenz P, Brandt S, Grassauer A, Sara M, et al. Chimeric papillomavirus-like particles expressing a foreign epitope on capsid surface loops. J Gen Virol. 2001;82:2799–804. doi: 10.1099/0022-1317-82-11-2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Chackerian B, Lowy DR, Schiller JT. Induction of autoantibodies to mouse CCR5 with recombinant papillomavirus particles. Proc Natl Acad Sci USA. 1999;96:2373–8. doi: 10.1073/pnas.96.5.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ailenberg M, Silverman M. Controlled hot start and improved specificity in carrying out PCR utilizing touch-up and loop incorporated primers (TULIPS) Biotechniques. 2000;29(5):1018–24. doi: 10.2144/00295st03. [DOI] [PubMed] [Google Scholar]
- [37].Sambrook J, Fritsch EF, Maniatis T. Molecular cloning. A laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1989. [Google Scholar]
- [38].Shafti-Keramat S, Handisurya A, Kriehuber E, Meneguzzi G, Slupetzky K, Kirnbauer R. Different heparan sulfate proteoglycans serve as cellular receptors for human papillomaviruses. J Virol. 2003;77:13125–35. doi: 10.1128/JVI.77.24.13125-13135.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Smith LH, Foster C, Hitchcock ME, Leiserowitz GS, Hall K, Isseroff R, et al. Titration of HPV-11 infectivity and antibody neutralization can be measured in vitro. J Invest Dermatol. 1995;105:438–44. doi: 10.1111/1523-1747.ep12321173. [DOI] [PubMed] [Google Scholar]
- [40].Culp TD, Christensen ND. Quantitative. RT-PCR assay for HPV infection in cultured cells. J Virol Methods. 2003;111:135–44. doi: 10.1016/s0166-0934(03)00170-8. [DOI] [PubMed] [Google Scholar]
- [41].Buck CB, Pastrana DV, Lowy DR, Schiller JT. Efficient intracellular assembly of papillomaviral vectors. J Virol. 2004;78:751–7. doi: 10.1128/JVI.78.2.751-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pastrana DV, Buck CB, Pang YY, Thompson CD, Castle PE, FitzGerald PC, et al. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004;321:205–16. doi: 10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- [43].Kawana K, Matsumoto K, Yoshikawa H, Taketani Y, Kawana T, Yoshiike K, et al. A surface immunodeterminant of human papillomavirus type 16 minor capsid protein L2. Virology. 1998;245:353–9. doi: 10.1006/viro.1998.9168. [DOI] [PubMed] [Google Scholar]
- [44].Kawana Y, Kawana K, Yoshikawa H, Taketani Y, Yoshiike K, Kanda T. Human papillomavirus type 16 minor capsid protein L2 N-terminal region containing a common neutralization epitope binds to the cell surface and enters the cytoplasm. J Virol. 2001;75:2331–6. doi: 10.1128/JVI.75.5.2331-2336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Rose RC, Bonnez W, Da Rin C, McCance DJ, Reichman RC. Sero-logical differentiation of human papillomavirus types 11, 16 and 18 using recombinant virus-like particles. J Gen Virol. 1994;75:2445–9. doi: 10.1099/0022-1317-75-9-2445. [DOI] [PubMed] [Google Scholar]
- [46].Buck CB, Pastrana DV, Lowy DR, Schiller JT. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol Med. 2005;119:445–62. doi: 10.1385/1-59259-982-6:445. [DOI] [PubMed] [Google Scholar]
- [47].Zamora E, Handisurya A, Shafti-Keramat S, Borchelt D, Rudow G, Conant K, et al. Papillomavirus-like particles are an effective platform for amyloid-β immunization in rabbits and transgenic mice. J Immunol. 2006;177:2662–70. doi: 10.4049/jimmunol.177.4.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Chandrachud LM, Grindlay GJ, McGarvie GM, O’Neil BW, Wagner ER, Jarrett WF, et al. Vaccination of cattle with the N-terminus of L2 is necessary and sufficient for preventing infection by bovine papillomavirus-4. Virology. 1995;211:204–8. doi: 10.1006/viro.1995.1392. [DOI] [PubMed] [Google Scholar]
- [49].Bachmann MF, Zinkernagel RM. Neutralizing antiviral B cell responses. Annu Rev Immunol. 1997;15:235–70. doi: 10.1146/annurev.immunol.15.1.235. [DOI] [PubMed] [Google Scholar]
- [50].Lenz P, Day PM, Pang YY, Frye SA, Jensen PN, Lowy DR, et al. Papillomavirus-like particles induce acute activation of dendritic cells. J Immunol. 2001;166:5346–55. doi: 10.4049/jimmunol.166.9.5346. [DOI] [PubMed] [Google Scholar]
- [51].Rudolf MP, Fausch SC, Da Silva DM, Kast WM. Human dendritic cells are activated by chimeric human papillomavirus type-16 virus-like particles and induce epitope-specific human T cell responses in vitro. J Immunol. 2001;166:5917–24. doi: 10.4049/jimmunol.166.10.5917. [DOI] [PubMed] [Google Scholar]
- [52].Yang R, Murillo FM, Delannoy MJ, Blosser RL, Yutzy WHt, Uematsu S, et al. B lymphocyte activation by human papillomavirus-like particles directly induces Ig class switch recombination via TLR4-MyD88. J Immunol. 2005;174:7912–9. doi: 10.4049/jimmunol.174.12.7912. [DOI] [PubMed] [Google Scholar]
- [53].Pinto LA, Castle PE, Roden RB, Harro CD, Lowy DR, Schiller JT, et al. HPV-16 L1 VLP vaccine elicits a broad-spectrum of cytokine responses in whole blood. Vaccine. 2005;23:3555–64. doi: 10.1016/j.vaccine.2005.01.146. [DOI] [PubMed] [Google Scholar]
- [54].Pinto LA, Edwards J, Castle PE, Harro CD, Lowy DR, Schiller JT, et al. Cellular immune responses to human papillomavirus (HPV)-16 L1 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. J Infect Dis. 2003;188:327–38. doi: 10.1086/376505. [DOI] [PubMed] [Google Scholar]
- [55].Yang R, Murillo FM, Lin KY, Yutzy WHt, Uematsu S, Takeda K, et al. Human papillomavirus type-16 virus-like particles activate complementary defense responses in key dendritic cell subpopulations. J Immunol. 2004;173:2624–31. doi: 10.4049/jimmunol.173.4.2624. [DOI] [PubMed] [Google Scholar]
- [56].Chackerian B, Lowy DR, Schiller JT. Conjugation of a self-antigen to papillomavirus-like particles allows for efficient induction of protective autoantibodies. J Clin Invest. 2001;108:415–23. doi: 10.1172/JCI11849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Chackerian B, Lenz P, Lowy DR, Schiller JT. Determinants of autoantibody induction by conjugated papillomavirus virus-like particles. J Immunol. 2002;169:6120–6. doi: 10.4049/jimmunol.169.11.6120. [DOI] [PubMed] [Google Scholar]
- [58].Embers ME, Budgeon LR, Pickel M, Christensen ND. Protective immunity to rabbit oral and cutaneous papillomaviruses by immunization with short peptides of L2, the minor capsid protein. J Virol. 2002;76:9798–805. doi: 10.1128/JVI.76.19.9798-9805.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Culp TD, Christensen ND. Kinetics of in vitro adsorption and entry of papillomavirus virions. Virology. 2004;319:152–61. doi: 10.1016/j.virol.2003.11.004. [DOI] [PubMed] [Google Scholar]
- [60].Day PM, Lowy DR, Schiller JT. Papillomaviruses infect cells via a clathrin-dependent pathway. Virology. 2003;307:1–11. doi: 10.1016/s0042-6822(02)00143-5. [DOI] [PubMed] [Google Scholar]