Abstract
Background
Monoclonal antibodies directed at IgE demonstrate clinical efficacy in subjects with peanut allergy, but previous studies have not addressed the kinetics of the clinical response or the role of mast cells and basophils in the food-induced allergic response.
Objective
We sought to determine the kinetics of the clinical response to omalizumab and whether clinical improvement is associated with either mast cell or basophil suppression.
Methods
Subjects with peanut allergy were treated with omalizumab for 6 months and assessed for clinical and cellular responses. At baseline, subjects had a double-blind, placebo-controlled oral food challenge (OFC), skin prick test titration (SPTT), and basophil histamine release (BHR) to peanut. BHR was repeated at week 2 and then weekly until it decreased to less than 20% of baseline values. The OFCs and SPTTs were repeated after the BHR reduction (or at week 8 if BHR did not decrease) and again at 6 months.
Results
Fourteen subjects enrolled in the study. At the second food challenge, there was a significant increase in the threshold dose of peanut inducing allergic symptoms (80 to 6500 mg, P < .01). Peanut-induced BHR was either completely suppressed (n = 5) or 10-fold more allergen was required to induce maximal BHR (n = 9), and SPTT responses were not significantly changed from baseline. After 6 months of omalizumab, further changes in the OFC threshold dose or BHR were not observed, but a significant suppression in SPTTs was identified.
Conclusions
The clinical response to omalizumab occurs early in treatment when the basophil, but not the mast cell, is suppressed, supporting a role for the basophil in acute food reactions.
Keywords: Omalizumab, peanut allergy, oral food challenge, basophil, mast cell
Omalizumab is an mAb directed at IgE that is currently approved for the treatment of allergic asthma. In the presence of omalizumab, basophil FcεRI expression is markedly suppressed after just a week of treatment, whereas mast cell expression FcεRI is suppressed after 10 weeks.1 Because of the different kinetics of basophil and mast cell suppression of IgE receptor expression, we hypothesized that we could differentiate the relative contributions of the basophil and mast cell in the acute allergic response by performing allergen challenges both early and late in omalizumab therapy. Indeed, in a recent study of patients with cat allergy, at an early time point after beginning omalizumab, both the basophil response to allergen and the clinical response to nasal allergen challenge were suppressed. At the same time, the nasal mast cell response was unaffected,2 demonstrating a temporal association between basophil suppression and clinical improvement.
In the current study we extend our previous findings from a nasal allergy model and explore the role of the basophil and mast cell in the systemic allergic response using food allergy as a model of systemic anaphylaxis. Previously, a different mAb to IgE (HU-901) was evaluated in patients with peanut allergy and demonstrated efficacy for the drug at the highest dose.3 Omalizumab has also been studied in patients with peanut allergy.4 After 24 weeks, there was a nearly statistically significant increase in the relative change in the amount of peanut inducing allergic symptoms at food challenge in the treated group compared with the placebo group. Neither of these past studies included significant mechanistic assessments. The aims of the current study were therefore to determine (1) whether patients with peanut allergy could tolerate a greater amount of peanut after treatment, (2) the kinetics of the clinical response, and (3) whether the clinical response was correlated with a change in either the mast cell or basophil response to allergen.
METHODS
Study subjects
Adult subjects between 18 and 50 years of age were recruited by advertisement and referral. Subjects met the following inclusion criteria for enrollment: clinical history of early-onset peanut allergy, serum total IgE level of 30 to 700 IU/mL, weight within the dosing guidelines for omalizumab, peanut-specific IgE level of greater than 0.35 IU/mL, and peanut skin prick test wheal size of 3 mm greater than that elicited by the saline control. Subjects needed to have peanut allergen–induced basophil histamine release (Pn-BHR) of greater than 20% of total leukocyte content (or 10% to 19% of total leukocyte content and >50% of optimal anti-IgE–induced basophil histamine release [BHR]), which was based on previous studies in cat allergy2 and pilot studies in peanut allergy (unpublished data). A robust baseline Pn-BHR value was necessary to test the hypothesis that suppression of the basophil allergen response was associated with clinical response. All subjects had to fail a double-blind peanut oral food challenge (OFC) at a cumulative dose of 1000 mg or less (OFC1).
Key exclusion criteria included severe persistent asthma, FEV1 of less than 80% of predicted value or oral corticosteroid use for asthma in last 6 months, history of severe allergic reaction to peanut requiring intensive care unit admission, late-onset peanut allergy (defined as patients who had previously tolerated peanut on a regular basis before their initial reaction), and eosinophilic enteropathy. This study was approved by the Johns Hopkins Institutional Review Board and the National Institute of Allergy and Infectious Diseases’ Data Safety Monitoring Board.
Study design
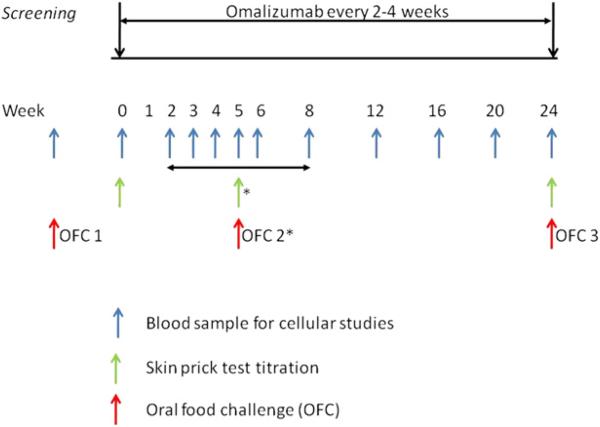
The study was a 6-month open-label study of 14 subjects enrolled from July 2009 to October 2010. The overall study design is depicted in Fig 1. Omalizumab was dosed according to the package insert. Procedures performed at the baseline visit included blood sampling for basophil and serum studies and skin prick test titration (SPTT) to peanut before initiating omalizumab treatment. Subjects returned for blood sampling at week 2 and then weekly until week 6 to monitor Pn-BHR. When a subject's Pn-BHR decreased to less than 20% of baseline values (assessed based on the area under the dose-response curve to peanut allergen [Pn-BHR AUC]), a second OFC (OFC2) and SPTT were performed. If a subject's Pn-BHR AUC value had not decreased to less than 20% of baseline values by week 6, OFC2 was performed at week 8. The BHR values and timing were chosen based on our previous study with cat allergy.2 Pn-BHR was followed monthly after OFC2 in those subjects whose Pn-BHR AUC values had not decreased to less than 20% of baseline values. After 6 months of omalizumab, a final OFC (OFC3) and SPTT to peanut were completed.
FIG 1.
Overall study design. *OFC2 and SPTT at this time point occurred when Pn-BHR AUC values were less than 20% of baseline values or at week 8.
Total and peanut-specific IgE levels
Total and peanut-specific IgE levels were measured with the ImmunoCAP 250 (Phadia, Uppsala, Sweden).
BHR
Venous blood was drawn into syringes containing PBS-EDTA, and basophils were isolated by using a single Percoll-based density gradient (Pharmacia, Piscataway, NJ) with Accuspin separation tubes (Sigma-Aldrich, St Louis, Mo). Enriched basophils were enumerated and stimulated for BHR with anti-IgE (0.03-1 mg/mL; HP6061; Hybridoma Reagent Laboratories, Baltimore, Md) and peanut allergen (0.001-10,000 ng/mL; lot 120814; Greer Laboratories, Lenoir, NC) in duplicate for 45 minutes at 37°C by using calcium-containing buffers.5 Automated fluorometry was used to measure BHR in cell-free supernatants.6 Results for each stimulus are reported as a percentage of the total histamine content found in an aliquot of perchloric acid–lysed leukocytes after subtraction of spontaneous BHR from cells in buffer alone as follows:
The results are reported as the mean ± SEM BHR at each concentration of allergen tested.
SPTTs
SPTTs were performed in duplicate on the dorsum of the forearms with 8 concentrations of peanut allergen by using a Greer pick. Commercial peanut extract (lot 120814, approximately 910 μg of crude protein per milliliter, Greer Laboratories) was used (“stock” concentration), as well as a 1:10, 1:50, 1:100, 1:500, 1:1000, 1:5000, and 1:10,000 dilution of the stock solution generated, with albumin saline and phenol as a diluent. The average of each duplicate at each dilution was taken as that patient's response. The results are reported as the average of all 8 dilution wheal responses.
Food challenge
All food challenges were double-blind and placebo-controlled by using defatted peanut flour or oat flour as placebo.7 The cumulative dose for the screening food challenge was 1,000 mg of peanut protein, which was administered over 7 doses, whereas it was 10,000 mg of peanut protein at OFC2 and OFC3, which was administered over 13 doses. The challenge was terminated at the first signs of a convincing allergic reaction (termed the threshold dose), and appropriate rescue medications were administered. Symptoms were recorded as oral, skin, gastrointestinal, upper respiratory, lower respiratory, and cardiovascular.
Statistical analysis
For continuous variables that were normally distributed, we used the paired student t test. For nonnormally distributed data, we used the Wilcoxon matched-pairs signed-ranks test. Figures show medians or means ± SEMs, as specified in the figure legends.
For ease of presentation, 3 time points are discussed: OFC1, OFC2, and OFC3. OFC1 refers to the screening food challenge and SPTT and Pn-BHR results obtained at the baseline visit. OFC2 refers to the second food challenge, Pn-BHR measurement immediately proximal to the second food challenge (which determined the time of OFC2), and an SPTT obtained at the time of OFC2. OFC3 refers to the third food challenge, Pn-BHR measurement immediately proximal to OFC3, and SPTTs obtained at OFC3.
RESULTS
Subjects’ characteristics
Fifty-one subjects underwent screening history and laboratory evaluation. Of those, 14 underwent a screening food challenge (OFC1) and enrolled in the study. Reasons for nonenrollment included qualified but chose not to enroll (n = 6), total IgE/weight ratio out of range with acceptable BHR and peanut IgE levels (n = 8), low FEV1 (n = 1), history of late-onset peanut allergy (n = 1), inadequate BHR with acceptable peanut IgE levels (n = 7), and inadequate peanut IgE levels or skin prick test results (n = 14).
Subjects were predominantly young female adults with a median skin prick test wheal size of 7.8 mm (range, 3-22 mm) and a peanut IgE level of 14 kUA/L (range, 1-184 kUA/L). Baseline patients’ characteristics are displayed in Table I.
TABLE I.
Baseline patients’ characteristics (n = 14)
| Characteristic | Value |
|---|---|
| Age (y [range]) | 23 (18-44) |
| Female sex (%) | 79 |
| Skin prick test–induced peanut wheal (mm [range]) | 7.8 (3-22) |
| Total IgE (kU/L [range]) | 155 (36-527) |
| Peanut-specific IgE (kUA/L [range]) | 14.3 (1-184) |
| Peanut-specific/total IgE ratio (range) | 0.071 (0.005-0.41) |
| Monthly dose of omalizumab (mg [range]) | 300 (150-750) |
All values are medians.
Of the 14 subjects enrolled, 1 was unable to complete OFC2 and OFC3 because of low FEV1 but continued treatment and participated in cellular studies. Two additional subjects were unable to complete OFC3 and provide Pn-BHR/SPTT data after OFC2 because of compliance issues. One subject was not able to participate in the active portion of OFC3 because of scheduling issues but was able to provide SPTT data. See Table E1 in this article's Online Repository at www.jacionline.org for numbers of evaluable subjects at each time point.
Five subjects (group A) had a decrease in Pn-BHR AUC values to less than 20% of baseline values before week 8 and therefore met the criteria to undergo OFC2 before the 8-week time point (median day 31/approximately week 4). Nine subjects (group B) did not have such a decrease in peanut-induced BHR by this time point, and OFC2 was performed around week 8. As expected, subjects in group A had significantly lower peanut-specific IgE levels at baseline compared with those in group B (median, 2.1 vs 30.5 kUA/L, respectively; P = .01; Table II).2 Group A also had a lower peanut/total IgE ratio than group B (2% vs 32%, P = .004).
TABLE II.
Participants’ characteristics according to peanut-induced BHR response before week 8
| Characteristic | Group A | Group B | P value* |
|---|---|---|---|
| Decrease in Pn-BHR AUC of >80% compared with baseline values before week 8 | Yes | No | NA |
| No. of subjects | 5 | 9 | NA |
| Time of OFC2 (d [range]) | 31 (20-35) | 55 (41-77) | .003 |
| Peanut-specific IgE (% [range]) | 1.9 (0.5-3.3) | 32 (3.3-41) | .004 |
| Peanut-specific IgE (kUA/L [range]) | 2.1 (1.1-13) | 30.5 (8.3-184) | .01 |
| Total IgE (kU/L [range]) | 201 (110-523) | 129 (36-527) | .64 |
| Dose of peanut protein inducing allergic symptoms at OFCs for all subjects (mg [range]) | OFC1: 80 (30-380); n = 5 | OFC1: 80 (10-700); n = 9 | .84 |
| OFC2: 6,500 (3,080-10,000); n = 5 | OFC2: 6,790 (180-10,000); n = 8 | .76 | |
| OFC3: 8,540 (1,830-10,000); n = 4 | OFC3: 2,455 (1,830-10,000); n = 6 | .43 | |
| Dose of peanut protein inducing allergic symptoms at OFCs for subjects who completed all OFCs (mg [range]) | OFC1: 80 (55-380); n = 4 | OFC1: 55 (10-700); n = 6 | .51 |
| OFC2: 8,250 (3,080-10,000); n = 4 | OFC2: 2,705 (180-10,000); n = 6 | .27 | |
| OFC3: 8,540 (1,830-10,000); n = 4 | OFC3: 2,455 (1,830-10,000); n = 6 | .43 | |
| Omalizumab received before OFC 2 | |||
| No. of doses | 1 (1-3) | 3 (2-5) | .03 |
| Total mg | 300 (300-675) | 900 (300-1,875) | .03 |
All values are medians.
Wilcoxon rank sum test.
Food challenge response
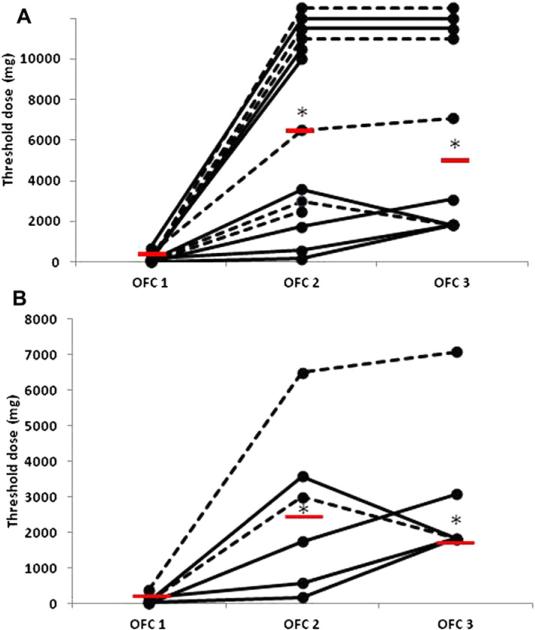
There was no significant difference in food challenge threshold responses between groups A and B (Table II and Fig 2, A). Among all subjects, the median threshold dose of peanut protein at OFC1 was 80 mg (n = 14; range, 10-700 mg). The median threshold dose increased to 6,500 mg at OFC2 (P = .002 compared with OFC1, Wilcoxon matched-pairs signed-ranks test; n = 13) and 5,080 mg at OFC3 (P = .005 compared with OFC1, Wilcoxon matched-pairs signed-ranks test; n = 10; Fig 2, A). The threshold dose at OFC2 was highly variable between subjects, with 4 subjects tolerating the full 10,000-mg challenge without symptoms and some reacting to the challenge at less than 1,000 mg. Fig E1 in this article's Online Repository at www.jacionline.org shows the distribution of the fold increase in threshold dose for the 13 subjects who completed OFC2, with a median 56-fold increase (range, 3.2-1,000).
FIG 2.
OFC results. A, Results for all subjects. B, Results for 6 subjects who completed all 3 OFCs and did not reach 10,000 mg. The median threshold dose for each time point is indicated by a red bar. *P < .05, see text for details. Dashed lines indicate group A subjects; solid lines indicate group B subjects.
To most accurately represent the kinetics of the OFC threshold changes, Fig 2, B, shows the threshold dose for the 6 subjects who were represented at all 3 OFCs and did not reach the OFC stopping dose of 10,000 mg and demonstrates that there was no significant difference between OFC2 and OFC3. The median threshold dose was 2,455 mg and 1,830 mg for this subset at OFC2 and OFC3, respectively (P = .75, Wilcoxon matched-pairs signed-ranks test; n = 6).
At OFC2, on average, subjects achieved 80% of the maximum threshold dose reached at either OFC2 or OFC3 (range, 10% to 100%). At OFC3, on average, subjects achieved 91% of the maximum threshold dose reached at either OFC2 or OFC3 (range, 51% to 100%).
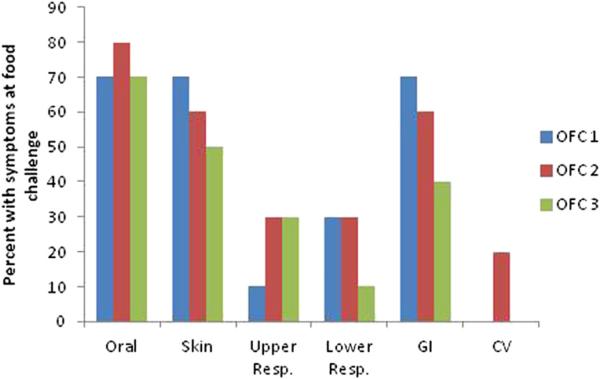
Among the 10 subjects who completed all OFCs, the frequency of organ-specific allergic symptoms at OFCs was similar between the food challenges (Fig 3). There was a decrease in both skin and gastrointestinal symptoms, but these changes did not meet statistical significance. At OFC1, all 10 subjects who completed the 3 OFCs were treated with antihistamines, whereas 8 of the 10 and 7 of the 10 subjects were treated with antihistamines at OFC2 and OFC3, respectively (P = not significant). Of the 10 subjects who completed all OFCs, 3 were treated with epinephrine at all 3 OFCs, whereas an additional subject received epinephrine at OFC3 (P = not significant for comparisons among OFCs).
FIG 3.
Occurrence of allergic symptoms during OFCs for the 10 subjects who completed all 3 food challenges. Values are the percentages of subjects who reported symptoms in each organ system. There were no significant differences between OFC1, OFC2, and OFC3. CV, Cardiovascular; GI, gastrointestinal; Resp., respiratory.
Peanut allergen SPTTs
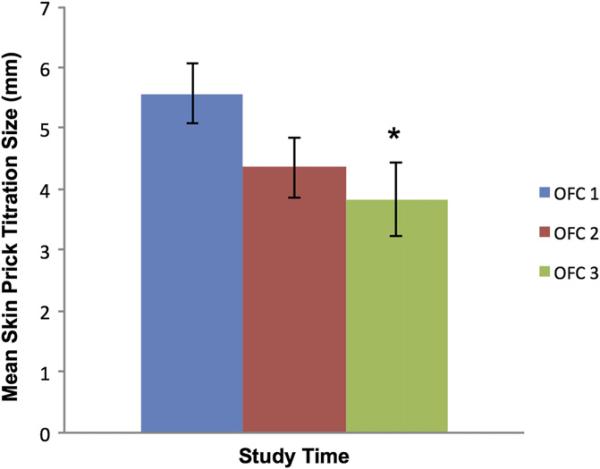
Skin prick test responses were similar between groups A and B, and therefore results are not stratified by group. The mean SPTT wheal size at the time of OFC1 was 5.6 mm. By the time of OFC2, the mean wheal size had decreased, but this did not meet statistical significance (4.4 mm; P = .11 compared with OFC 1, paired t test; Fig 4). By the time of OFC3, the mean wheal size was significantly suppressed compared with that at OFC1 (3.8 mm; P = .03 compared with OFC1, paired t test). Significant differences were not seen with wheal size at the maximum concentration used when comparing OFC1 values with those obtained at either OFC2 or OFC3.
FIG 4.
Mean skin prick test results ± SEMs. *P < .05 compared with OFC1.
BHR
Seven (50%) of 14 subjects displayed greater than 10% spontaneous histamine release before initiation of omalizumab (median for group of 7, 14%; range, 11% to 63%). By OFC2, the median spontaneous histamine release was significantly suppressed relative to that at OFC1 (median for group at OFC1, 8.1; median at OFC2, 4; P = .02, n = 14).
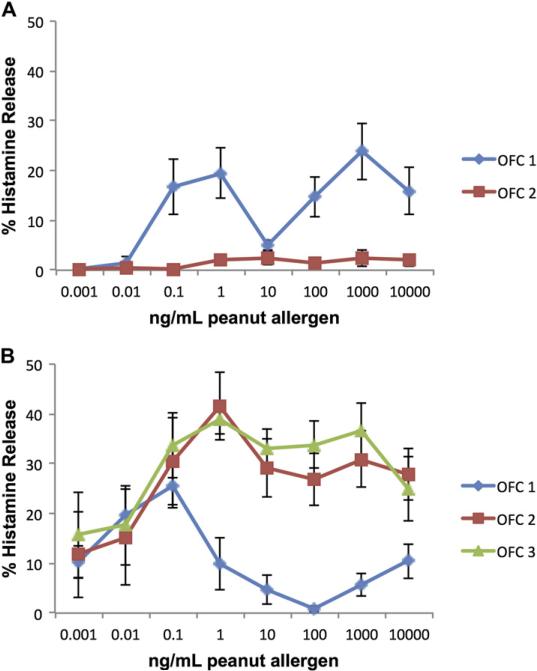
As a group, there was no change in the Pn-BHR AUC value between OFC1 and OFC2 (median Pn-BHR AUC at OFC 1, 77 [range, 27-142]; median Pn-BHR AUC at OFC2, 59 [range, 6-542]; P = .36). However, by definition, there was a significant decrease in the Pn-BHR AUC values in group A (n = 5; median Pn-BHR AUC at baseline, 72 [range, 52-133]; median Pn-BHR AUC at OFC2, 11 [range, 6-14]; P = .04).
Omalizumab treatment led to near suppression of Pn-BHR in group A (Fig 5, A) and an increase in the amount of allergen required to achieve maximal histamine release in group B (Fig 5, B). At OFC1 in group B, maximal histamine release was observed at 0.1 ng/mL peanut allergen. At OFC2 and OFC3, 10 times more allergen was required to achieve maximal release, which was seen at 1 ng/mL allergen. In addition to this decrease in basophil sensitivity in group B, there was also an increase in BHR at OFC2 and OFC3 at greater than 0.1 ng/mL peanut allergen.
FIG 5.
Peanut-induced BHR across 8 concentrations of allergen at OFCs. A, Group A. B, Group B. Values are means ± SEMs.
DISCUSSION
This study of omalizumab treatment in adults with peanut allergy is unique in that we completed food challenges and cellular studies both early and later in treatment. Our food challenge outcomes demonstrate a 56-fold increase in the threshold challenge dose, and in most subjects this change was apparent remarkably early in therapy. Assuming approximately 300 mg of protein per peanut, this represents an increase in the threshold dose from less than 1 peanut to approximately 21 peanuts. However, the change in food challenge response was highly variable between subjects, which is consistent with previously published studies of anti-IgE in peanut allergy.3,4 Notably, there was little individual or group improvement in the challenge threshold seen between OFC2 and OFC3.
Interestingly, the threshold doses identified at food challenge in the current study were markedly higher than those in previously published studies of anti-IgE antibodies in patients with peanut allergy. Reasons for this might be related to enrollment of children with off-label omalizumab dosing in the previous omalizumab/peanut study and possible under-dosing in the HU-901 study. Our study was not powered to identify factors that might predict response to omalizumab, and larger studies will be needed to address predictors of therapeutic response.
Although earlier studies of asthma suggested that 16 weeks might be necessary to demonstrate a clinical benefit with omalizumab,8 a recent study of inner-city children with asthma also demonstrated a clinical effect early in treatment.9 Furthermore, our previous study in a nasal model of cat allergy demonstrated a 50% reduction in nasal allergen response early in therapy,2 suggesting that allergen-mediated clinical responses are inhibited early in omalizumab therapy. Although US Food and Drug Administration approval of omalizumab is unlikely for food allergy in general, the rapid increase in threshold dose observed here suggest that temporary treatment with omalizumab might have a role when combined with oral immunotherapy for food allergy. A pilot study has already shown promising results in patients with milk allergy.10
The symptoms recorded during OFCs did not appear to change during treatment, although the threshold dose of peanut required to induce these symptoms was increased. Thus it took a higher oral dose of allergen to elicit gastrointestinal (local) and non-gastrointestinal (systemic) symptoms during OFC2. In the case of cutaneous symptoms at OFC2, we have the insight offered by direct skin testing with peanut allergen. Although the skin test reactivity measured by using SPTTs was not significantly suppressed at OFC2, a substantially higher ingested dose of peanut allergen was required to elicit skin symptoms. This discordance in skin test results and symptoms suggests 2 possible explanations for the systemic symptoms during allergic reactions. If systemic symptoms during OFCs result from circulating antigen, then the discordance suggests that treatment with omalizumab reduces the transport of allergen to the circulation and thus to the skin. An alternative interpretation is that allergen does not circulate and the systemic effects of OFCs result from mediators released locally but transported systemically. In this scenario the allergen skin test would not necessarily be a marker for responses to circulating mediators. However, early serum transfer studies11 provide strong support for the first interpretation.
By doing both skin test and basophil assessments at the times of the OFCs, we showed that mast cell responses were not significantly suppressed by the time of OFC2, when a dramatic clinical response was observed in some subjects. At the same time, we observed complete suppression of Pn-BHR in group A. In group B the Pn-BHR AUC was unchanged, but there was a 10-fold shift in the concentration of peanut allergen required to induce the maximal BHR response at OFC2 and OFC3. Although this is not as great a shift as the approximately 50-fold increase in allergen required to elicit clinical allergic symptoms observed in the OFCs, this temporal association between clinical response and basophil dose-response shift might suggest involvement of the basophil in the food allergic response. Rather than considering the full dose-response curve, it might be more appropriate to consider the change in the suboptimal region of the curve as an indicator of the changing responsiveness. Because we did not identify an overall suppression in Pn-BHR AUC in group B, further studies will be needed to confirm whether a shift in the basophil allergen-induced dose-response curve is clinically relevant. We suspect the marked increase in Pn-BHR seen in group B at higher allergen concentrations is due to decreasing IgE receptor density to optimize allergen cross-linking at higher concentrations of allergen, and this interpretation and others are further explored in the companion article.12
Our study of omalizumab in subjects with peanut allergy is similar to our previous study of omalizumab in a nasal model of cat allergy.2 In both studies a relationship between the rate of decrease in the in vitro basophil response and the specific/total IgE ratio was observed. In our previous study ratios of less than 4% predicted a fast loss in the basophil response. Similarly, in this study the rapid decreasewas observed with ratios of less than approximately 3.3%.
Spontaneous BHR is commonly reported in children with food allergy,13 but its significance remains poorly understood. Omalizumab significantly reduced unstimulated BHR, suggesting that it might be IgE mediated in vivo. To our knowledge, this is the first reported decrease in unstimulated BHR with anti-IgE therapy in persons with peanut allergy, although the clinical implications remain elusive.
Our study has several limitations. First, our study population included only subjects with greater than 20% BHR to peanut. This was necessary to ensure we had a basophil signal to monitor. Large studies have not been done to determine the sensitivity and specificity of BHR in diagnosing peanut allergy, and therefore our subjects might not be representative of the general population with peanut allergy. However, we are confident that all subjects were truly allergic because we performed baseline food challenges.
Second, by choosing to perform a food challenge at the time of basophil suppression, we introduced bias in the association between BHR and food challenge response. However, in group B, in which subjects were challenged at 8 weeks independently of basophil suppression, this bias was not introduced, and we did see an association between clinical response and basophil sensitivity.
Finally, we used the skin prick test as a biomarker for the allergen-induced mast cell response. Although it is possible that resident intestinal mucosal mast cells are downregulated more quickly, more significantly, or both than skin mast cells by omalizumab treatment, it is clear that mast cell reactivity, as evidenced by skin prick test responses, is not a reliable biomarker for clinical responses to omalizumab.
In summary, although all subjects increased the clinical threshold dose of peanut protein inducing allergic symptoms, there was wide variability in the response to omalizumab, and the degree of response was similar early and late in therapy. Basophil hyporesponsiveness to allergen assessed either as complete suppression of reactivity or as a shift in allergen sensitivity might be an indicator of the changing threshold in the OFCs.
Supplementary Material
Clinical implications.
In adults with peanut allergy receiving omalizumab, the threshold dose of peanut inducing allergic reactions at food challenge significantly increased from 80 to 6500 mg by 8 weeks of treatment.
Acknowledgments
We thank Alison Pack and Nga Brereton of the General Clinical Research Center for assistance with food challenges.
Supported by National Institutes of Health grants AI070345 and T32AI007056-31.
D. W. MacGlashan has received research support from the National Institutes of Health (NIH). S. S. Saini has consultant arrangements with Genentech, Novartis, Pharmacyclics, and Medimmune; has received research support from the National Institute of Allergic and Infectious Diseases and Genentech; received the drug for this study from Genentech; and receives royalties from UpToDate. R. A. Wood has consulted for the Asthma and Allergy Foundation of America and has provided expert testimony for the NIH.
Abbreviations used
- BHR
Basophil histamine release
- OFC
Oral food challenge
- Pn-BHR
Peanut allergen–induced basophil histamine release
- Pn-BHR AUC
Area under the dose-response curve to peanut allergen
- SPTT
Skin prick test titration
Footnotes
Disclosure of potential conflict of interest: The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.Beck LA, Marcotte GV, MacGlashan JD, Togias A, Saini S. Omalizumab-induced reductions in mast cell FcεRI expression and function. J Allergy Clin Immunol. 2004;114:527–30. doi: 10.1016/j.jaci.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 2.Eckman JA, Sterba PM, Kelly DAV, Liu MC, Bochner BS, MacGlashan DW, et al. Effects of omalizumab on basophil and mast cell responses using an intranasal cat allergen challenge. J Allergy Clin Immunol. 2010;125:889–95. doi: 10.1016/j.jaci.2009.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leung DY, Sampson HA, Yunginger JW, Burks AW, Jr, Schneider LC, Wortel CH, et al. Effect of anti-IgE therapy in patients with peanut allergy. N Engl J Med. 2003;348:986–93. doi: 10.1056/NEJMoa022613. [DOI] [PubMed] [Google Scholar]
- 4.Sampson HA, Leung DY, Burks AW, Lack G, Bahna SL, Jones SM, et al. A phase II, randomized, double blind, parallel group, placebo controlled oral food challenge trial of Xolair (omalizumab) in peanut allergy. J Allergy Clin Immunol. 2011;127:1309–10. doi: 10.1016/j.jaci.2011.01.051. [DOI] [PubMed] [Google Scholar]
- 5.Vonakis BM, Vasagar K, Gibbons JSP, Gober L, Sterba PM, Chang H, et al. Basophil FcεRI histamine release parallels expression of Src-homology 2-containing inositol phosphatases in chronic idiopathic urticaria. JAllergy Clin Immunol. 2007;119:441–8. doi: 10.1016/j.jaci.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 6.Saini SS, Richardson JJ, Wofsy C, Lavens-Phillips S, Bochner BS, MacGlashan DW. Expression and modulation of FcεRIa and FcεRIb in human blood basophils. J Allergy Clin Immunol. 2001;107:832–41. doi: 10.1067/mai.2001.114653. [DOI] [PubMed] [Google Scholar]
- 7.Niggemann B, Wahn U, Sampson HA. Proposals for standardization of oral food challenge tests in infants and children. Pediatr Allergy Immunol. 1994;5:11–3. doi: 10.1111/j.1399-3038.1994.tb00212.x. [DOI] [PubMed] [Google Scholar]
- 8.Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. 2004;125:1378–86. doi: 10.1378/chest.125.4.1378. [DOI] [PubMed] [Google Scholar]
- 9.Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011;364:1005–15. doi: 10.1056/NEJMoa1009705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nadeau KC, Schneider LC, Hoyte L, Borras I, Umetsu DT. Rapid oral desensitization in combination with omalizumab therapy in patients with cow's milk allergy. J Allergy Clin Immunol. 2011;127:1622–4. doi: 10.1016/j.jaci.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sussman H, Davidson A, Walzer M. Absorption of undigested proteins in human beings. Arch Intern Med. 1928;42:409–14. [Google Scholar]
- 12.MacGlashan DW, Jr, Savage J, Wood R, Saini S. Suppression of the basophil response to allergen during treatment with omalizumab is dependent on 2 competing factors. J Allergy Clin Immunol. 2012;130:1130–5. doi: 10.1016/j.jaci.2012.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sampson HA, Broadbent KR, Bernhisel-Broadbent J. Spontaneous release of histamine from basophils and histamine-releasing factor in patients with atopic dermatitis and food hypersensitivity. N Engl J Med. 1989;321:228–32. doi: 10.1056/NEJM198907273210405. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.