Abstract
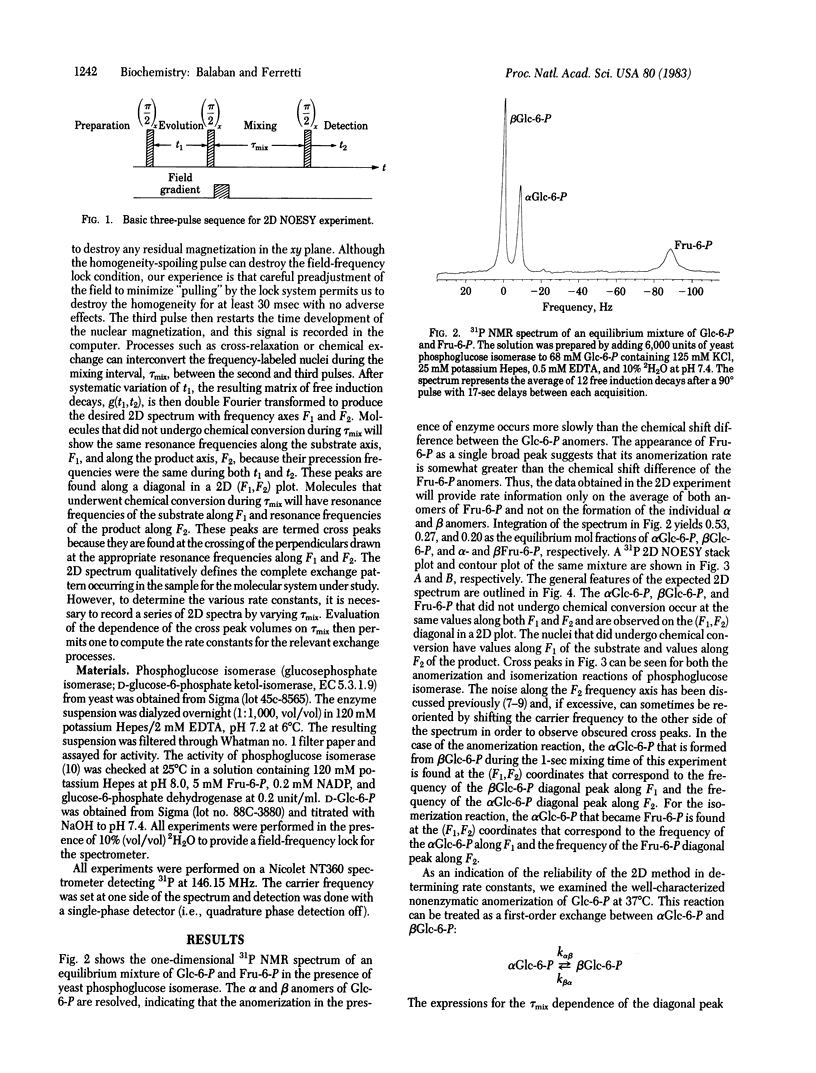
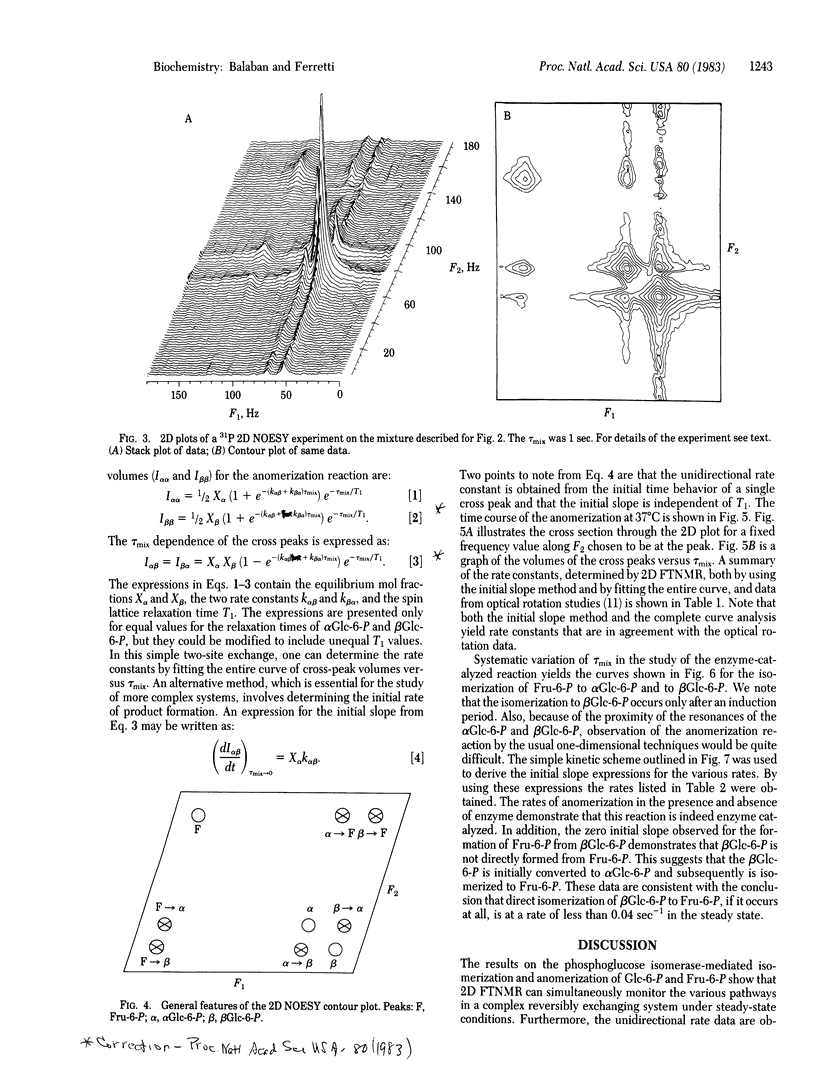
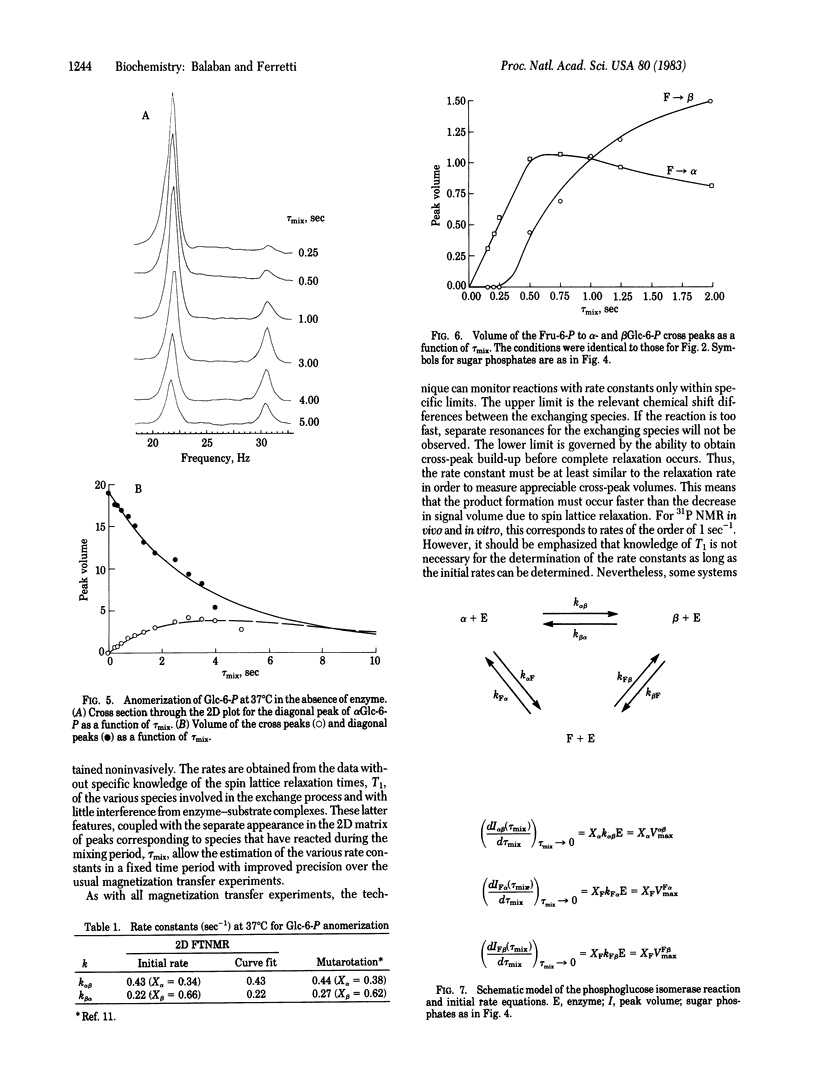
The application of two-dimensional (2D) Fourier-transform NMR to the determination of rate constants of complex enzyme-catalyzed reactions in the steady state is described. The yeast phosphoglucose isomerase (EC 5.3.1.9)-catalyzed anomerization of glucose 6-phosphate (Glc-6-P) as well as its isomerization to fructose 6-phosphate (Fru-6-P) was chosen as an example. The 2D technique permitted the simultaneous monitoring of the time course of the anomerization and isomerization steps, from which the various reaction rates were determined. The results obtained in the steady state demonstrate the usefulness of the 2D technique by confirming that the anomerization of Glc-6-P is enzyme catalyzed and that the isomerization of the alpha anomer of Glc-6-P to Fru-6-P is at least 10 times faster than the isomerization of the beta anomer of Glc-6-P. These results are compared with reaction rates obtained by rapid-quench methods and the mechanistic implications are discussed. Extrapolation of these results suggests that the 2D Fourier-transform NMR method should be applicable in intact biological tissues.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey J. M., Fishman P. H., Pentchev P. G. Studies on mutarotases. II. Investigations of possible rate-limiting anomerizations in glucose metabolism. J Biol Chem. 1968 Sep 25;243(18):4827–4831. [PubMed] [Google Scholar]
- Brown T. R., Ogawa S. 31P nuclear magnetic resonance kinetic measurements on adenylatekinase. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3627–3631. doi: 10.1073/pnas.74.9.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. K., Redfield A. G. Double nuclear magnetic resonance observation of electron exchange between ferri- and ferrocytochrome c. Science. 1970 Sep 18;169(3951):1204–1206. doi: 10.1126/science.169.3951.1204. [DOI] [PubMed] [Google Scholar]
- Plesser T., Wurster B., Hess B. Determination of the kinetic constants of glucosephosphate isomerase by non-linear optimization. Isomerization and anomerization function. Eur J Biochem. 1979 Jul;98(1):93–98. doi: 10.1111/j.1432-1033.1979.tb13165.x. [DOI] [PubMed] [Google Scholar]
- SALAS M., VINUELA E., SOLS A. SPONTANEOUS AND ENZYMATICALLY CATALYZED ANOMERIZATION OF GLUCOSE 6-PHOSPHATE AND ANOMERIC SPECIFICITY OF RELATED ENZYMES. J Biol Chem. 1965 Feb;240:561–568. [PubMed] [Google Scholar]
- Schray K. J., Benkovic S. J., Benkovic P. A., Rose I. A. Catalytic reactions of phosphoglucose isomerase with cyclic forms of glucose 6-phosphate and fructose 6-phosphate. J Biol Chem. 1973 Mar 25;248(6):2219–2224. [PubMed] [Google Scholar]


