Abstract
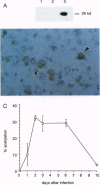
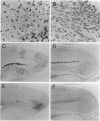
Virus-induced apoptosis has been well characterized in vitro, but the role of apoptosis in viral pathogenesis is not well understood. The suicide of a cell in response to viral infection is postulated to be an important host defense for the organism, leading to a reduction in its total viral burden. However, virus-induced death of nonregenerating cells in the central nervous system may be detrimental to the host. Therefore, to investigate the role of apoptosis in the pathogenesis of fatal encephalitis, we constructed a recombinant alphavirus chimera that expresses the antiapoptotic gene, bcl-2, in virally infected neural cells. Infection of neonatal mice with the alphavirus chimera expressing human bcl-2 [Sindbis virus (SIN)/bcl-2] resulted in a significantly lower mortality rate (7.5%) as compared with infection with control chimeric viruses containing a chloramphenicol acetyltransferase (CAT) reporter gene (SIN/CAT) (78.1%) or bcl-2 containing a premature stop codon (SIN/bcl-2stop) (72.1%) (P < 0.001). Viral titers were reduced 5-fold 1 day after infection and 10-fold 6 days after infection in the brains of SIN/bcl-2-infected mice as compared to SIN/CAT or SIN/bcl-2stop-infected mice. In situ end labeling to detect apoptotic nuclei demonstrated a reduction in the number of foci of apoptotic cells in the brains of mice infected with SIN/bcl-2 as compared with SIN/bcl-2stop. The reduction in apoptosis was associated with a reduction in the number of foci of cells expressing alphavirus RNA. Thus, the antiapoptotic gene, bcl-2, suppresses viral replication and protects against a lethal viral disease, suggesting an interaction between cellular genetic control of viral replication and cell death.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari B., Coates P. J., Greenstein B. D., Hall P. A. In situ end-labelling detects DNA strand breaks in apoptosis and other physiological and pathological states. J Pathol. 1993 May;170(1):1–8. doi: 10.1002/path.1711700102. [DOI] [PubMed] [Google Scholar]
- Antoni B. A., Sabbatini P., Rabson A. B., White E. Inhibition of apoptosis in human immunodeficiency virus-infected cells enhances virus production and facilitates persistent infection. J Virol. 1995 Apr;69(4):2384–2392. doi: 10.1128/jvi.69.4.2384-2392.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum M. J., Clem R. J., Miller L. K. An apoptosis-inhibiting gene from a nuclear polyhedrosis virus encoding a polypeptide with Cys/His sequence motifs. J Virol. 1994 Apr;68(4):2521–2528. doi: 10.1128/jvi.68.4.2521-2528.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clem R. J., Fechheimer M., Miller L. K. Prevention of apoptosis by a baculovirus gene during infection of insect cells. Science. 1991 Nov 29;254(5036):1388–1390. doi: 10.1126/science.1962198. [DOI] [PubMed] [Google Scholar]
- Clem R. J., Miller L. K. Apoptosis reduces both the in vitro replication and the in vivo infectivity of a baculovirus. J Virol. 1993 Jul;67(7):3730–3738. doi: 10.1128/jvi.67.7.3730-3738.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook N. E., Clem R. J., Miller L. K. An apoptosis-inhibiting baculovirus gene with a zinc finger-like motif. J Virol. 1993 Apr;67(4):2168–2174. doi: 10.1128/jvi.67.4.2168-2174.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis R. E., Yuan J. Y., Horvitz H. R. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Fairbairn D. W., Carnahan K. G., Thwaits R. N., Grigsby R. V., Holyoak G. R., O'Neill K. L. Detection of apoptosis induced DNA cleavage in scrapie-infected sheep brain. FEMS Microbiol Lett. 1994 Jan 15;115(2-3):341–346. doi: 10.1111/j.1574-6968.1994.tb06661.x. [DOI] [PubMed] [Google Scholar]
- Gagliardini V., Fernandez P. A., Lee R. K., Drexler H. C., Rotello R. J., Fishman M. C., Yuan J. Prevention of vertebrate neuronal death by the crmA gene. Science. 1994 Feb 11;263(5148):826–828. doi: 10.1126/science.8303301. [DOI] [PubMed] [Google Scholar]
- Griebel P. J., Ohmann H. B., Lawman M. J., Babiuk L. A. The interaction between bovine herpesvirus type 1 and activated bovine T lymphocytes. J Gen Virol. 1990 Feb;71(Pt 2):369–377. doi: 10.1099/0022-1317-71-2-369. [DOI] [PubMed] [Google Scholar]
- Henderson S., Huen D., Rowe M., Dawson C., Johnson G., Rickinson A. Epstein-Barr virus-coded BHRF1 protein, a viral homologue of Bcl-2, protects human B cells from programmed cell death. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8479–8483. doi: 10.1073/pnas.90.18.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz J. M., Huang H. V. Utilization of heterologous alphavirus junction sequences as promoters by Sindbis virus. J Virol. 1992 Feb;66(2):857–864. doi: 10.1128/jvi.66.2.857-864.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw V. S., Olsen C. W., Dybdahl-Sissoko N., Evans D. Apoptosis: a mechanism of cell killing by influenza A and B viruses. J Virol. 1994 Jun;68(6):3667–3673. doi: 10.1128/jvi.68.6.3667-3673.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. C., Moench T. R., Griffin D. E., Johnson R. T. The pathogenesis of spinal cord involvement in the encephalomyelitis of mice caused by neuroadapted Sindbis virus infection. Lab Invest. 1987 Apr;56(4):418–423. [PubMed] [Google Scholar]
- Jackson A. C., Moench T. R., Griffin D. E., Johnson R. T. The pathogenesis of spinal cord involvement in the encephalomyelitis of mice caused by neuroadapted Sindbis virus infection. Lab Invest. 1987 Apr;56(4):418–423. [PubMed] [Google Scholar]
- Jeurissen S. H., Wagenaar F., Pol J. M., van der Eb A. J., Noteborn M. H. Chicken anemia virus causes apoptosis of thymocytes after in vivo infection and of cell lines after in vitro infection. J Virol. 1992 Dec;66(12):7383–7388. doi: 10.1128/jvi.66.12.7383-7388.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamita S. G., Majima K., Maeda S. Identification and characterization of the p35 gene of Bombyx mori nuclear polyhedrosis virus that prevents virus-induced apoptosis. J Virol. 1993 Jan;67(1):455–463. doi: 10.1128/jvi.67.1.455-463.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanishi M. Epstein-Barr virus induces fragmentation of chromosomal DNA during lytic infection. J Virol. 1993 Dec;67(12):7654–7658. doi: 10.1128/jvi.67.12.7654-7658.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent-Crawford A. G., Krust B., Muller S., Rivière Y., Rey-Cuillé M. A., Béchet J. M., Montagnier L., Hovanessian A. G. The cytopathic effect of HIV is associated with apoptosis. Virology. 1991 Dec;185(2):829–839. doi: 10.1016/0042-6822(91)90554-o. [DOI] [PubMed] [Google Scholar]
- Levine B., Huang Q., Isaacs J. T., Reed J. C., Griffin D. E., Hardwick J. M. Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature. 1993 Feb 25;361(6414):739–742. doi: 10.1038/361739a0. [DOI] [PubMed] [Google Scholar]
- Lewis J., Wesselingh S. L., Griffin D. E., Hardwick J. M. Alphavirus-induced apoptosis in mouse brains correlates with neurovirulence. J Virol. 1996 Mar;70(3):1828–1835. doi: 10.1128/jvi.70.3.1828-1835.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X. H., Mungal S., Ayscue A., Meissner J. D., Wodnicki P., Hockenbery D., Lockett S., Herman B. Bcl-2 protooncogene expression in cervical carcinoma cell lines containing inactive p53. J Cell Biochem. 1995 Mar;57(3):509–521. doi: 10.1002/jcb.240570316. [DOI] [PubMed] [Google Scholar]
- Lu Y. Y., Koga Y., Tanaka K., Sasaki M., Kimura G., Nomoto K. Apoptosis induced in CD4+ cells expressing gp160 of human immunodeficiency virus type 1. J Virol. 1994 Jan;68(1):390–399. doi: 10.1128/jvi.68.1.390-399.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lustig S., Jackson A. C., Hahn C. S., Griffin D. E., Strauss E. G., Strauss J. H. Molecular basis of Sindbis virus neurovirulence in mice. J Virol. 1988 Jul;62(7):2329–2336. doi: 10.1128/jvi.62.7.2329-2336.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyaard L., Otto S. A., Jonker R. R., Mijnster M. J., Keet R. P., Miedema F. Programmed death of T cells in HIV-1 infection. Science. 1992 Jul 10;257(5067):217–219. doi: 10.1126/science.1352911. [DOI] [PubMed] [Google Scholar]
- Morey A. L., Ferguson D. J., Fleming K. A. Ultrastructural features of fetal erythroid precursors infected with parvovirus B19 in vitro: evidence of cell death by apoptosis. J Pathol. 1993 Feb;169(2):213–220. doi: 10.1002/path.1711690207. [DOI] [PubMed] [Google Scholar]
- Noteborn M. H., Todd D., Verschueren C. A., de Gauw H. W., Curran W. L., Veldkamp S., Douglas A. J., McNulty M. S., van der EB A. J., Koch G. A single chicken anemia virus protein induces apoptosis. J Virol. 1994 Jan;68(1):346–351. doi: 10.1128/jvi.68.1.346-351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Social controls on cell survival and cell death. Nature. 1992 Apr 2;356(6368):397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- Rao L., Debbas M., Sabbatini P., Hockenbery D., Korsmeyer S., White E. The adenovirus E1A proteins induce apoptosis, which is inhibited by the E1B 19-kDa and Bcl-2 proteins. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7742–7746. doi: 10.1073/pnas.89.16.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C. Bcl-2 and the regulation of programmed cell death. J Cell Biol. 1994 Jan;124(1-2):1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed J. C., Cuddy M., Slabiak T., Croce C. M., Nowell P. C. Oncogenic potential of bcl-2 demonstrated by gene transfer. Nature. 1988 Nov 17;336(6196):259–261. doi: 10.1038/336259a0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S. Alphaviruses--vectors for the expression of heterologous genes. Trends Biotechnol. 1993 Jan;11(1):18–22. doi: 10.1016/0167-7799(93)90070-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaljohn A. L., Johnson E. D., Dalrymple J. M., Cole G. A. Non-neutralizing monoclonal antibodies can prevent lethal alphavirus encephalitis. Nature. 1982 May 6;297(5861):70–72. doi: 10.1038/297070a0. [DOI] [PubMed] [Google Scholar]
- Sherman L. A., Griffin D. E. Pathogenesis of encephalitis induced in newborn mice by virulent and avirulent strains of Sindbis virus. J Virol. 1990 May;64(5):2041–2046. doi: 10.1128/jvi.64.5.2041-2046.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAYLOR R. M., HURLBUT H. S. The isolation of Coxsackie-like viruses from mosquitoes. J Egypt Med Assoc. 1953;36(9):489–494. [PubMed] [Google Scholar]
- Takizawa T., Matsukawa S., Higuchi Y., Nakamura S., Nakanishi Y., Fukuda R. Induction of programmed cell death (apoptosis) by influenza virus infection in tissue culture cells. J Gen Virol. 1993 Nov;74(Pt 11):2347–2355. doi: 10.1099/0022-1317-74-11-2347. [DOI] [PubMed] [Google Scholar]
- Terai C., Kornbluth R. S., Pauza C. D., Richman D. D., Carson D. A. Apoptosis as a mechanism of cell death in cultured T lymphoblasts acutely infected with HIV-1. J Clin Invest. 1991 May;87(5):1710–1715. doi: 10.1172/JCI115188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimoto Y., Croce C. M. Analysis of the structure, transcripts, and protein products of bcl-2, the gene involved in human follicular lymphoma. Proc Natl Acad Sci U S A. 1986 Jul;83(14):5214–5218. doi: 10.1073/pnas.83.14.5214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubol S., Tucker P. C., Griffin D. E., Hardwick J. M. Neurovirulent strains of Alphavirus induce apoptosis in bcl-2-expressing cells: role of a single amino acid change in the E2 glycoprotein. Proc Natl Acad Sci U S A. 1994 May 24;91(11):5202–5206. doi: 10.1073/pnas.91.11.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Sabbatini P., Debbas M., Wold W. S., Kusher D. I., Gooding L. R. The 19-kilodalton adenovirus E1B transforming protein inhibits programmed cell death and prevents cytolysis by tumor necrosis factor alpha. Mol Cell Biol. 1992 Jun;12(6):2570–2580. doi: 10.1128/mcb.12.6.2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Yamaoka S., Goto T., Nakai M., Tsujimoto Y., Hatanaka M. The human T-cell leukemia virus type I Tax protein induces apoptosis which is blocked by the Bcl-2 protein. J Virol. 1994 May;68(5):3374–3379. doi: 10.1128/jvi.68.5.3374-3379.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]