Abstract
Although various lines of evidence suggest that oxidative stress plays a role in human prostate cancer initiation and progression, there is a paucity of direct evidence for its role in tumor initiation. To begin to address this issue, we developed a novel tumorigenesis model by reducing the expression of multiple selenoproteins (SPs) in mouse prostatic epithelium. This was accomplished via the prostate-specific deletion of Trsp, a gene that encodes a transfer RNA (Sec tRNA) required for the insertion of selenocysteine residues into SPs during their translation. By 6 weeks of age, Trsp-deficient mice exhibited widespread prostatic intraepithelial neoplasia lesions in all prostatic lobes, which then progressed to high-grade dysplasia and microinvasive carcinoma by 24 weeks. In contrast to other murine prostate cancer models, Trsp-deficient mice required neither the deletion of a tumor suppressor nor the transgenic introduction of an oncogene for prostatic intraepithelial neoplasia lesion development. In keeping with the antioxidant functions of several SPs, we found increases in lipid peroxidation markers in Trsp-deficient epithelial cells. This novel model of prostate neoplasia provides evidence for the existence of a selenoprotein or selenoproteins capable of acting as a tumor suppressor in the murine prostate.
Prostate cancer is the second most common cancer in men after skin cancer in the United States, and it has been estimated that approximately 242,000 men were diagnosed with this disease, and that approximately 28,000 men died of this malignancy in 2012 [Surveillance, Epidemiology, and End Results (SEER) Program; http://seer.cancer.gov/statfacts/html/prost.html]. Adequate selenium (Se) levels have long been proposed as one of the factors preventing the development of various types of malignancy (reviewed in Brigelius-Flohe1 and Davis et al2) including prostate cancer. Se is an essential dietary micronutrient that is found in association with various organic molecules, including the 21st amino acid, selenocysteine (Sec).3 The latter molecule is essential for the normal function of the selenoprotein (SP) family of proteins. Indeed, SP synthesis requires that Sec residues be inserted into the growing polypeptide chains opposite specific UGA codons that are present within the coding regions of SP mRNAs.4 Although in the eukaryotic nucleus, UGA ordinarily specifies a translational stop, in the case of the selenoprotein gene coding regions, the UGA codon is recognized by a specific transfer RNA, Sec tRNA (designated Sec tRNA[Ser]Sec), that is encoded by the Trsp gene. Recognition of the appropriate UGA codon(s) in SPs is enabled by a group of proteins that recognize a Sec insertion sequence that is located within the 3′ untranslated regions of SP mRNAs.5
There are 25 human and 24 murine SP genes,6 approximately one-third of which function as antioxidants. This family includes the important glutathione peroxidases (GPx) 1 through 47 that are responsible for protecting cells against reactive oxygen species (ROS)-mediated and reactive nitrogen species–mediated damage to cellular macromolecules.6 Other members of the SP family, such as the thioredoxin reductases, are critical regulators of intracellular redox potential, and thus regulate the activities of redox-sensitive signal transduction molecules and transcription factors.7,8
Although the function of many SPs is still unknown, other than that they may participate in antioxidant and anabolic processes, the SPs that are most likely to be relevant to an increased cancer risk are those of the GPx family, the 15-kDa selenoprotein (Sep15), and the thioredoxin reductases, previously implicated in various cancers, including prostate cancer (reviewed in Rayman9 and Davis2). It has been proposed that SPs may exert protective effects, not only against tumorigenesis, but also against cancer progression and metastasis.10,11 There is evidence of an association between Se and reductions in DNA damage and oxidative stress, together with data showing an effect of SP genotype on cancer risk, thus suggesting that alterations in SP activity may have a role in tumorigenesis (reviewed in Rayman9). To begin to study this possibility, SP levels were reduced via the transgenic expression of a mutant Sec tRNA (i6A−) to determine what effect this would have on prostate tumorigenesis in transgenic mice expressing the SV40 large-T and small-t oncogenes (C3(1)/Tag) in their prostates.12 Interestingly, the bigenic mice exhibited accelerated tumor development, suggesting that one or more SPs were involved in suppressing tumor evolution.
There has been considerable speculation and circumstantial evidence supporting a role for oxidative stress in human prostate cancer.13,14 Although much work implicating ROS has been performed on human prostate cancer cell lines in vitro, as well as in xenografted and transgenic mice,15,16 there is a paucity of direct evidence in either humans or mice demonstrating that oxidative stress plays a tumor-initiating role in this disease.
The targeted removal of Trsp in all tissues was embryonic lethal, indicating that there are one or more SPs that are essential for normal development.17 Hence, investigation of SP deficiency in vivo has required a conditional (Cre-LoxP) mutagenesis approach. Studying the consequences of tissue-specific Trsp gene loss allows insight into the role of the SP family in any given organ or cell type. Thus, to determine whether prostate-specific deletion of SP would have any effect on the normal murine prostate, we interbred mice carrying loxP-flanked (floxed) Trsp alleles (Trspfl/fl)17 with mice expressing a prostate-specific Cre transgene.18 Our results demonstrate that Trsp deletion in the prostatic epithelium leads to the rapid development of prostatic intraepithelial neoplasia (PIN) lesions.
It should be noted that our findings neither contradict nor are made irrelevant by the negative results of the SELECT trial (Selenium and Vitamin E Cancer Prevention Trial).19 This trial, which enrolled men with normal to high Se blood levels at entry, was intended to evaluate whether dietary Se (with and without vitamin E) supplementation would decrease the risk of prostate cancer development. Its purpose was not to demonstrate whether SP levels were relevant to human prostate tumorigenesis. Although generalized SP deficiency leads to the rapid development of PIN lesions in mice, in humans, isolated SP deficiencies would likely exert more subtle effects. It is conceivable nonetheless, and in view of the evidence that oxidative stress may be important to the pathogenesis of the human disease,13,14,20 that our Trsp model represents an abbreviated scenario of a process that might ordinarily be played out over many decades in humans.
Materials and Methods
Generation of Mice
Trspfl/fl mice17 were interbred with a PB*Cre4 mouse line expressing Cre recombinase under the control of a composite promoter, ARR2PB, a derivative of the rat prostate-specific probasin (PB) promoter that is fused to two androgen response elements (ARR2).18 Cre expression in the PB*Cre4 line occurs postnatally, under androgen control, and hence allows excisions of floxed genes in the prostatic epithelium. Each line was backcrossed for n = 10 generations onto the C57BL/6J background. Mice were housed in a viral antibody–free barrier facility in accordance with Canadian Council on Animal Care guidelines, and with ethical approval by the University of Calgary Animal Care Committee.
Genotyping
Tail-tip clippings from pups were taken immediately postweaning and processed for genotyping for both the Trspfl/fl and the Cre transgene as previously described.17,18
Histology and Immunohistochemistry
The dissected lower urogenital tracts of the mice were fixed in 10% formaldehyde, embedded in paraffin, and sectioned at 5 μm. Sections were deparaffinized with xylene and graded alcohols, and sections were stained with H&E for microscopy. For immunohistochemistry, sections were deparaffinized as above, and antigen retrieval was performed using 10 mmol/L sodium citrate (pH 6.0). For cryosections, animals were perfused with 4% paraformaldehyde after a lethal dose of ketamine–xylazine, and dissected lower urogenital tracts were embedded in optimal cutting temperature compound and cryosectioned at 10 μm. Antibodies used were against Sep15, GPx4, dTR1 (Epitomics, Burlingame, CA), Ki-67 (Novocastra, Newcastle Upon Tyne, UK) pAKT, phospho-p42/44 mitogen-activated protein kinase (MAPK), phospho-p38 MAPK, and pS6 (Cell Signaling Technology, Danvers, MA), nitrotyrosine (Millipore, Billerica, MA), malondialdehyde (MDA; Abcam, Cambridge, UK), and 4-hydroxynonenal (4-HNE; Millipore); and either the mouse IgG Vectastain Elite ABC kit or the rabbit IgG ABC kit from Vector Laboratories (Burlingame, CA) were used as secondary reagents. Color development was performed with DAB substrate (Sigma-Aldrich, St. Louis, MO), and sections were counterstained with hematoxylin. PAS staining was performed at the Calgary Laboratory Services pathology laboratory according to standard protocols.
Quantification of Proliferation
The proliferation index was determined as the percentage of Ki67-positive cells over total cells counted. At least 1000 cells per animal were counted over five different fields of view, and four mice were included per group. Student's t-test (two-tailed, unequal variance) was used for statistical analyses.
Apoptosis Assay
The dissected lower urogenital tracts of the mice were fixed in 10% formaldehyde, embedded in paraffin, and sectioned at 5 μm. Sections were deparaffinized with xylene and graded alcohols before the TUNEL assay, which was performed with the ApopTag Peroxidase in Situ Apoptosis Detection kit according to the manufacturer's instructions (Chemicon International, Temecula, CA).
Results
Deleting the Trsp Gene Leads to Loss of SPs in the Prostatic Epithelium
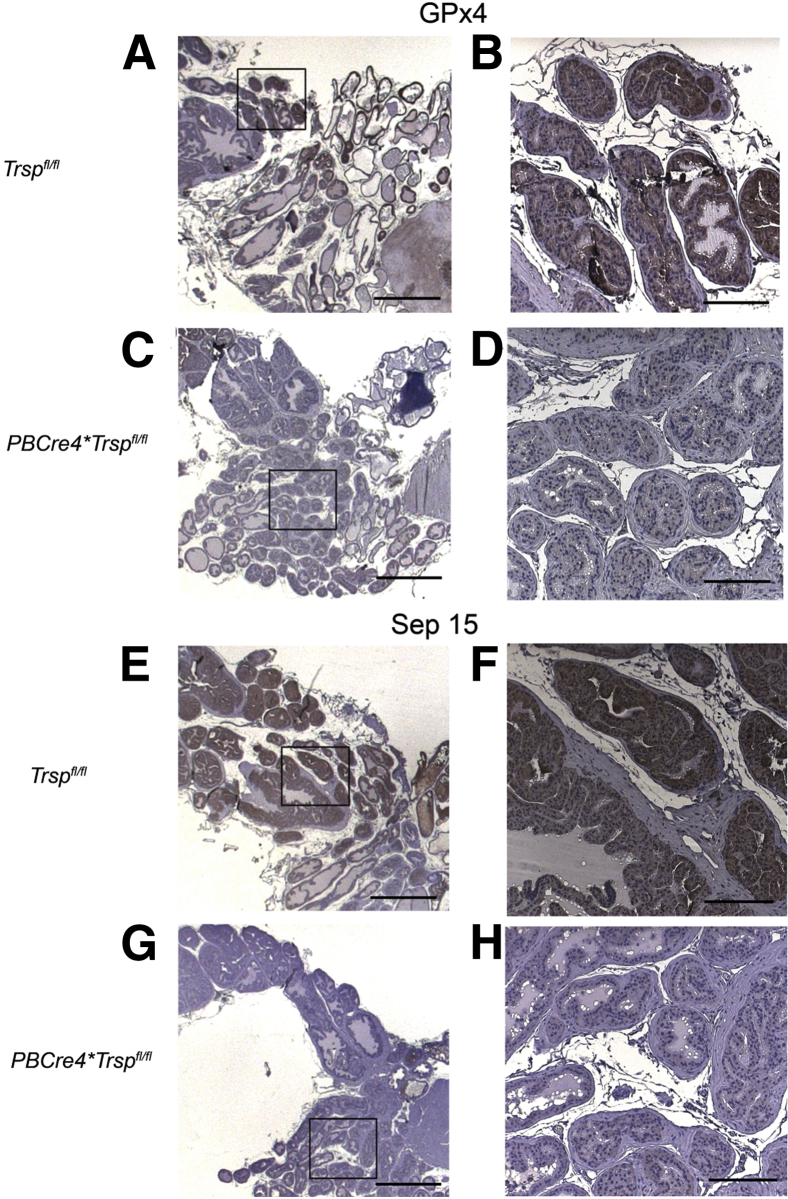
It has been previously shown that Trsp knockout mice are embryonic lethal21; therefore, to get prostate-specific deletion of Trsp, we crossed mice with floxed Trsp alleles to a mouse line (PBCre4) expressing Cre recombinase under the control of a composite promoter, ARR2PB.18 Using immunohistochemistry, we observed that prostate-specific excision of the Trsp gene led to a loss of GPx4 (Figure 1, C and D) and Sep15 (Figure 1, G and H) in the prostatic epithelium (controls are shown in Figure 1, A, B, E, and F). The reduced selenoprotein expression was detectable as early as 6 weeks of age (Figure 1). Loss of GPx4 was less dramatic than that of Sep15, perhaps owing to some residual protein being recognized by the anti-GPx4 antibody despite the premature translational termination that is predicted to occur in the absence of Sec tRNA, or alternatively, to a cross-reactive epitope present in the prostatic epithelium. Similarly, reduction of GPx1 was more dramatic than that of the TR1 selenoprotein (Table 1). Taken together, the results thus showed that Cre recombinase–driven excision of the Trsp gene inhibited the expression of various SPs in the prostatic epithelium.
Figure 1.
Reduced expression of selenoproteins in prostates of PBCre4;Trspfl/fl mice. Tissue sections of prostates from 6- to 10-week-old Trspfl/fl and PBCre4Trspfl/fl mice were immunostained with antibodies against GPx4 and Sep15. PBCre4;Trspfl/fl prostates show lack of detectable expression of these selenoproteins in the luminal epithelium (C, D, G, and H), as a consequence of Trsp excision. Staining results were replicated on prostates from three to six experimental and control mice. Boxed areas in A, C, E, and G correspond to higher-magnification images in B, D, F, and H, respectively. Scale bars: 200 μm (A, C, E, and G); 20 μm (B, D, F, and H).
Table 1.
Summary of Immunostaining of PBCre4;Trspfl/fl Prostates
| Target | Trspfl/fl | PBCRe4;Trspfl/fl |
|---|---|---|
| Sep15 | + | − |
| GPx1 | + | − |
| GPx4 | + | Decreased |
| TR1 | + | Decreased |
| Ki-67 | Minimal | + |
| TUNEL staining | Minimal | + |
| p42/p44 MAPK | − | + |
| pAKT | − | + |
| pS6 | − | + |
| p38MAPK | − | + |
| NF-κB | − | − |
| HIF1α | − | − |
| Nitrotyrosine | + | + |
| MDA | Minimal | + |
| 4-HNE | Minimal | + |
Prostates of 6- to 29-week-old PBCre4;Trspfl/fl and Trspfl/fl controls were immunostained. Minimal denotes a modest level of positive staining but at levels lower than in the other groups.
+, positive staining; −, lack of staining.
Lack of SP Expression in Prostatic Epithelium Leads to PIN and Microinvasive Adenocarcinoma
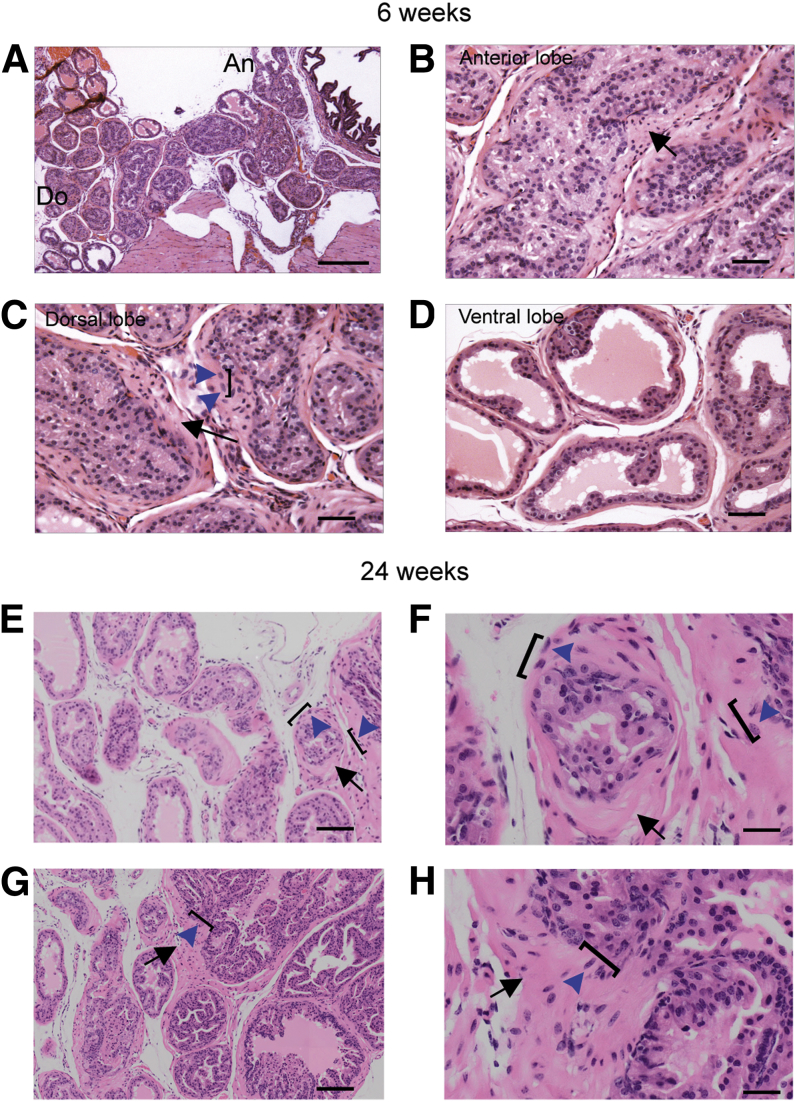
Decreased levels of SPs induced by the transgenic expression of a mutant Sec tRNA (i6A−) in the prostate were previously linked to accelerated development of prostate cancer in C3(1)/Tag mice.12 Herein, we investigated whether Trsp excision in the prostatic epithelium would lead to abnormal morphology indicative of early-stage prostate cancer. Indeed, we found that PBCre4;Trspfl/fl mice demonstrated abnormal morphology and PIN lesions in all three lobes of the prostate as early as 6 weeks of age (Figure 2, A–D). Abnormal histopathology was observed with cellular disorganization, cellular and nuclear enlargement and atypia, and basement membrane thickening with cellular microinvasion into the stroma. Dramatic hyperchromasia and mitosis, and cell stratification were observed (Supplemental Figure S1A). As the mice aged, the phenotype became more pronounced such that high-grade PIN lesions and more extreme examples of the above phenotypes were observed by 24 weeks of age (Figure 2, E–H, and Supplemental Figure S1B). Control animals at this stage exhibited no evidence of any abnormal histopathology (Supplemental Figure S1, C–F). Thus, PBCre4;Trspfl/fl mice between 6 and 29 weeks displayed all of the characteristic histopathological features of prostate cancer with 100% penetrance, including high-grade PIN lesions and microinvasive adenocarcinoma, in contrast to control Trspfl/fl animals, which exhibited neither of these features. There was no additional progression past high-grade lesions and invasive adenocarcinoma into metastasis when animals were aged to 30 weeks. In summary, these results indicated that Trsp excision, and hence, lack of SPs in the prostatic epithelium, was sufficient to lead to high-grade PIN lesions and microinvasive carcinoma.
Figure 2.
Characteristic features of tumorigenesis in prostates of PBCre4;Trspfl/fl mice. Abnormal morphology and PIN lesions observed in all three lobes of the prostate at 6 weeks (A–D). Abnormal histopathology including cellular disorganization, cellular and nuclear enlargement and atypia (blue arrowheads and square brackets), and basement membrane thickening with cellular microinvasion into the stroma (black arrows). The phenotype was more pronounced in older mice (shown at 24 weeks), with high-grade PIN lesions and more extreme examples of the above phenotypes (E–H). F and H: Regions showing cellular and nuclear disorganization, enlargement and atypia, microinvasion (blue arrows and square brackets), and basement membrane thickening (black arrows). Penetrance was 100% with animals in all age groups displaying PIN lesions. Representative images of prostates from five to eight mice from each group and time point are shown. F and H are higher magnifications of E and G, respectively. Scale bars: 200 μm (A); 50 μm (B–D); 100 μm (E and G); 20 μm (F and H). An, anterior lobe; Do, dorsal lobe.
Increased Proliferation and Apoptosis in the Prostates of PBCre4;Trspfl/fl Mice
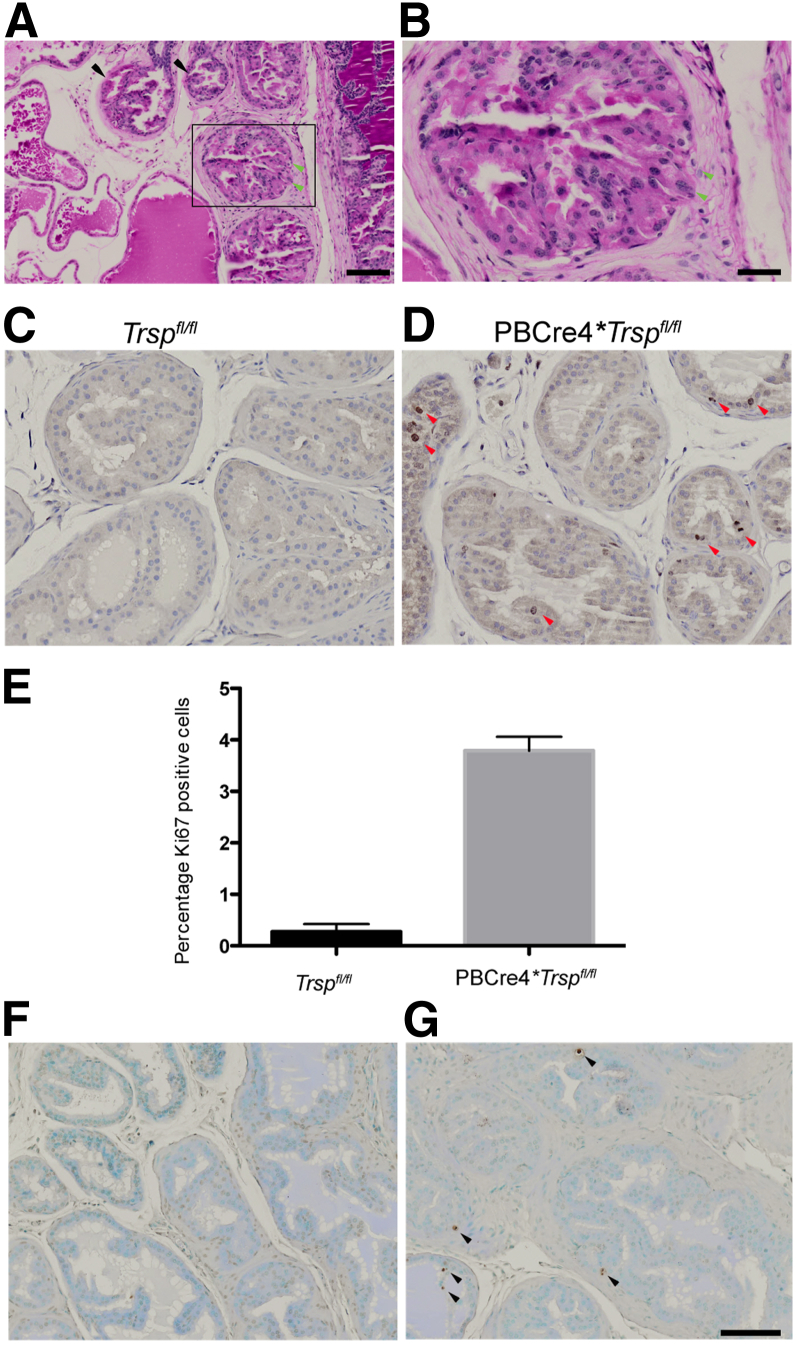
Further signs of tumorigenesis were confirmed by PAS staining of PBCre4;Trspfl/fl prostates, which showed thinning and disruption of the basement membrane in the prostate tubules and microinvasion of luminal epithelial cells into the stroma (Figure 3, A and B). The Ki-67 marker was used to assess proliferation in the prostatic epithelium of PBCre4;Trspfl/fl mice. Luminal epithelial cells were found to be positive for Ki67 in the prostates of the Cre-positive animals (Figure 3D). This was observed in prostates of young adult mice at 6 weeks of age as well as in the luminal epithelium and PIN lesions of older mice, at a time when the prostate is fully developed and cell division is minimal, as seen in the prostates of control Trspfl/fl mice (Figure 3C). Quantification of the Ki-67 in the prostates of 6-week-old PBCre4;Trspfl/fl demonstrated a striking increase in the numbers of Ki67-positive cells (Figure 3E).
Figure 3.
Microinvasive carcinoma, increased proliferation, and apoptosis in prostates of PBCre4;Trspfl/fl mice. Positive PAS staining in PBCre4;Trspfl/fl prostates (A). B is a higher magnification of the boxed region in A. Thinning and disruption of basement membrane (A, black arrows) and microinvasion of luminal epithelium cells in stroma (A and B, green arrowheads) were observed. Compared to prostates of control mice (C), increased numbers of luminal epithelial cells positive for Ki-67 were observed in PBCre4;Trspfl/fl (D, red arrowheads) and confirmed through quantification (E). P < 0.0002 versus control Trspfl/fl mice. Compared to Trspfl/fl mice (F), increased TUNEL-positive cells (G, black arrowheads) were also observed in the prostates of PBCre4;Trspfl/fl mice. PAS staining was performed on 25-week-old mice; Ki-67 and TUNEL were performed on 6-week-old mice. A minimum of three to five animals were used per condition. Scale bars: 100 μm (A, C, D, F, and G); 20 μm (B).
The TUNEL assay, to identify the presence of apoptotic cells in the prostatic epithelium of PBCre4;Trspfl/fl mice, revealed increased numbers of apoptotic luminal epithelial cells (Figure 3G) compared to control prostates (Figure 3F).
SP Loss in the Prostate Leads to Activation of Pro-Oncogenic Pathways and Oxidative Stress
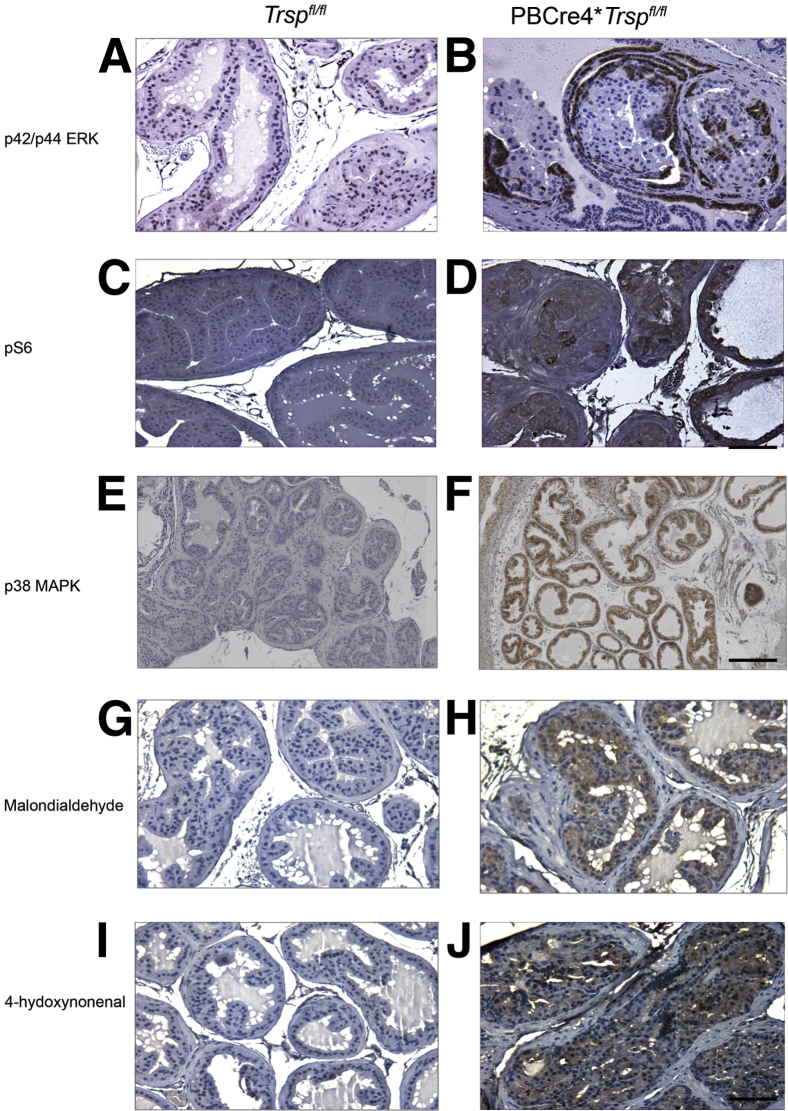
To further investigate the mechanisms through which SP loss resulted in PIN development, we performed immunohistochemistry on the prostate tissue of mice using a series of antibodies against various effectors of pro-oncogenic pathways. We found up-regulation of p42/p44 MAPK (Figure 4, A and B) and phospho-S6 (Figure 4, C and D), consistent with activation of both the MAPK and PI3K pathways (Table 1). Interestingly, activated p42/p44 MAPK seemed to be present primarily in cells on the outermost regions of the tubules with PIN lesions (Figure 4B), whereas pS6 levels tended to be increased in cells within the tubule lumens (Figure 4D).
Figure 4.
Activation of pro-oncogenic and oxidative stress signals. Up-regulation of phospho-p42/p44 MAPK (p42/p44 ERK) (B), and phospho-S6 (pS6) (D) signals were seen in prostates of PBCre4;Trspfl/fl mice, but not in controls (A and C). Increases in the levels of phospho-p38MAPK (F) and of the lipid peroxidation markers malondialdehyde (H) and 4-hydroxynonenal (J) were also observed in the PBCre4;Trspfl/fl prostatic epithelium compared to controls (E, G, and I). Prostates from 6- to 29-week-old mice were used; representative immunostaining is shown for 10-week-old mice (p38MAPK) and 24-week-old mice (MDA and 4-HNE) with three to five animals used per condition. Scale bars: 100 μm (A–D and G–J); 200 μm (E and F).
Because decreased Se and SP levels have been associated with increased oxidative stress,22,23 we investigated whether Trsp excision would be accompanied by markers of oxidative stress. Indeed, we found that PBCre4;Trspfl/fl prostates displayed increased p38 MAPK staining (Figure 4, E and F). Although we were not able to discern differences in NF-κB, hypoxia-inducible factor-1α (HIF-1α), and nitrotyrosine, we did find striking increases in the levels of the lipid peroxidation markers MDA (Figure 4, G and H) and 4-HNE (Figure 4, I and J). The latter results were consistent with Trsp loss being accompanied by oxidative stress–induced lipid peroxidation.
Discussion
Our study demonstrates that interbreeding Trspfl/fl and PB*Cre4 mice, not only effectively reduced prostatic epithelial expression of SPs, but also led to development of PIN lesions and early carcinoma in mice within the first 2 months of age. We speculate that increased oxidative stress, for example, due to reduced levels of SPs such as GPx-1, -4, and Sep15, might play a role in this process, perhaps via the triggering of pro-oncogenic pathways. Indeed, dramatically lowered expression of specific SPs in the prostates of the PBCre4;Trspfl/fl mice was associated with up-regulation of the cell proliferation indicator, Ki-67, and markers of pro-oncogenic pathway activation, including pS6 and pMAPK. Furthermore, there was increased apoptosis in the prostates of the experimental animals consistent with an increased rate of cell turnover, likely exacerbated by the combination of ROS-mediated cell damage and nutrient deprivation affecting cells that were accumulating within the avascular intratubular compartment. Although we found increased staining in phosphorylated p38 MAPK, a further indication of oxidative stress in the PBCre4;Trspfl/fl prostates, we were unable to detect differences in the levels or localization of other protein sensors commonly associated with oxidative stress such as NF-κB and HIF-1α. This may be due to these proteins, and especially HIF-1α, being labile and hence undetectable in our processed specimens.
In support of oxidative stress being present in the prostates of Trsp-deficient animals, we found an up-regulation of the lipid peroxidation markers, MDA and 4-HNE, indicative of oxidative stress within the prostatic epithelium. GPx4 acts, not only on H2O2, but also on soluble fatty acid hydroperoxides (reviewed in Brigelius-Flohe24), and is the primary antioxidant enzyme capable of directly reducing lipid hydroperoxides within all cellular membranes (reviewed in Brigelius-Flohe24). The ability of GPx4 to act on lipid hydroperoxides plays a major role in cytoprotection against ROS. In keeping with this idea, GPx4-overexpressing mice were found to be more resistant to oxidative insults (reviewed in Brigelius-Flohe24 and Liang et al25). We found decreased levels of GPx4 in the prostates of PBCre4;Trspfl/fl mice, suggesting that deficiency of this SP accounted for the increased 4-HNE and MDA immunostaining we observed in the PIN lesions.
The widespread nature of the lesions throughout the lobes of the prostate and their occurrence in all Trsp-deficient mice argue against a stochastic process such as might be expected if oxidative stress were randomly inducing DNA mutations in key growth control genes. Instead, the histopathology was consistent with a field effect, whereby large numbers of progenitors were being stimulated to turn over. This would be in keeping with the ability of ROS to stimulate various pro-growth effectors,26,27 such as the increased levels of phospho-p42/44 MAPK, phospho-p38 MAPK p42/p44 MAPK, p38 MAPK, and phospho-S6 that we observed in the PIN lesions. It is feasible, once a generalized hyperplasia is established, that ROS-induced mutations, cytogenetic aberrations, or epigenetic alteration could act to promote the selection of clones with increased malignancy. This could have accounted for the increased levels of dysplasia and microinvasion we observed in the Trsp-deficient prostates of older mice.
Normal cellular SP activity is thought to be protective against tumorigenesis.2 Although the function of some SPs is still unknown, SPs whose deficiency may predispose to an increased cancer risk include members of the GPx family, Sep15, and the thioredoxin reductases.28 The loss of Trsp would lead to deficiencies in all SPs expressed in mouse prostate. Thus, in addition to GPx4, there could be other SPs whose losses act to promote PIN development, and it is conceivable that PIN lesions result from a specific combination of SP deficiencies. However, in view of the unique properties of the GPx4 isoforms that provide a defense against lipid peroxidation events, we hypothesize that reduced levels of GPx4 render it a candidate, accounting for PIN lesions developing in Trsp-deficient mice. Furthermore, deletion of Gpx4 has been shown to phenocopy the effects of Trsp deletion in other cell types (eg, neuronal cells).29 It could thus be speculated that GPx4 might be one of the SPs whose deficiency promotes PIN development in Trsp-deficient prostates.
It is intriguing that many lines of evidence have pointed to a role for oxidative stress in the pathogenesis of human prostate cancer13,14 as well as in the progression to androgen independence.30 Our results raise the possibility that oxidative stress may have a direct oncogenic role in the pathogenesis of PINs, and suggest that severe Se deficiency, or gene polymorphisms that decrease the activity of SPs, might predispose humans to prostate cancer. Indeed, gene polymorphisms or decreased expression of specific SPs have already been implicated as potential etiological factors in human prostate cancer.31–36
Acknowledgment
We thank Carolina Salazar for animal care and technical help.
Footnotes
Supported by a grant from the Alberta Cancer Foundation, Alberta Innovates-Health Solutions (F.R.J.). F.R.J. was the recipient of a Canada Research Chairs award.
Disclosures: None declared.
Supplemental Data
Histology of PBCre4;Trspfl/fl and control Trspfl/fl mice. PIN lesions in 6-week (A) and 24-week (B) PBCre4;Trspfl/fl mice are accompanied by a dramatic increase in mitosis, cellular stratification (A and B), and noticeable basement membrane thickening (B). By contrast, none of these features were seen in the prostates of control animals. Prostate histology in control animals at 24 weeks is shown (C–F). Scale bars: 50 μm (A–B) and (D–F); and 100 μm (C).
References
- 1.Brigelius-Flohe R. Selenium compounds and selenoproteins in cancer. Chem Biodivers. 2008;5:389–395. doi: 10.1002/cbdv.200890039. [DOI] [PubMed] [Google Scholar]
- 2.Davis C.D., Tsuji P.A., Milner J.A. Selenoproteins and cancer prevention. Annu Rev Nutr. 2012;32:73–95. doi: 10.1146/annurev-nutr-071811-150740. [DOI] [PubMed] [Google Scholar]
- 3.Hatfield D.L., Gladyshev V.N. How selenium has altered our understanding of the genetic code. Mol Cell Biol. 2002;22:3565–3576. doi: 10.1128/MCB.22.11.3565-3576.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hatfield D.L., Carlson B.A., Xu X.M., Mix H., Gladyshev V.N. Selenocysteine incorporation machinery and the role of selenoproteins in development and health. Prog Nucleic Acid Res Mol Biol. 2006;81:97–142. doi: 10.1016/S0079-6603(06)81003-2. [DOI] [PubMed] [Google Scholar]
- 5.Small-Howard A., Morozova N., Stoytcheva Z., Forry E.P., Mansell J.B., Harney J.W., Carlson B.A., Xu X.M., Hatfield D.L., Berry M.J. Supramolecular complexes mediate selenocysteine incorporation in vivo. Mol Cell Biol. 2006;26:2337–2346. doi: 10.1128/MCB.26.6.2337-2346.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kryukov G.V., Castellano S., Novoselov S.V., Lobanov A.V., Zehtab O., Guigo R., Gladyshev V.N. Characterization of mammalian selenoproteomes. Science. 2003;300:1439–1443. doi: 10.1126/science.1083516. [DOI] [PubMed] [Google Scholar]
- 7.Papp L.V., Lu J., Holmgren A., Khanna K.K. From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal. 2007;9:775–806. doi: 10.1089/ars.2007.1528. [DOI] [PubMed] [Google Scholar]
- 8.Patenaude A., Murthy M.R., Mirault M.E. Emerging roles of thioredoxin cycle enzymes in the central nervous system. Cell Mol Life Sci. 2005;62:1063–1080. doi: 10.1007/s00018-005-4541-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rayman M.P. Selenium in cancer prevention: a review of the evidence and mechanism of action. Proc Nutr Soc. 2005;64:527–542. doi: 10.1079/pns2005467. [DOI] [PubMed] [Google Scholar]
- 10.Jiang C., Kim K.H., Wang Z., Lu J. Methyl selenium-induced vascular endothelial apoptosis is executed by caspases and principally mediated by p38 MAPK pathway. Nutr Cancer. 2004;49:174–183. doi: 10.1207/s15327914nc4902_9. [DOI] [PubMed] [Google Scholar]
- 11.Ip C., Dong Y. Methylselenocysteine modulates proliferation and apoptosis biomarkers in premalignant lesions of the rat mammary gland. Anticancer Res. 2001;21:863–867. [PubMed] [Google Scholar]
- 12.Diwadkar-Navsariwala V., Prins G.S., Swanson S.M., Birch L.A., Ray V.H., Hedayat S., Lantvit D.L., Diamond A.M. Selenoprotein deficiency accelerates prostate carcinogenesis in a transgenic model. Proc Natl Acad Sci U S A. 2006;103:8179–8184. doi: 10.1073/pnas.0508218103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oberley T.D., Zhong W., Szweda L.I., Oberley L.W. Localization of antioxidant enzymes and oxidative damage products in normal and malignant prostate epithelium. Prostate. 2000;44:144–155. doi: 10.1002/1097-0045(20000701)44:2<144::aid-pros7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 14.Gupta-Elera G., Garrett A.R., Robison R.A., O'Neill K.L. The role of oxidative stress in prostate cancer. Eur J Cancer Prev. 2012;21:155–162. doi: 10.1097/CEJ.0b013e32834a8002. [DOI] [PubMed] [Google Scholar]
- 15.Ouyang X., DeWeese T.L., Nelson W.G., Abate-Shen C. Loss-of-function of Nkx3.1 promotes increased oxidative damage in prostate carcinogenesis. Cancer Res. 2005;65:6773–6779. doi: 10.1158/0008-5472.CAN-05-1948. [DOI] [PubMed] [Google Scholar]
- 16.Kumar B., Koul S., Khandrika L., Meacham R.B., Koul H.K. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008;68:1777–1785. doi: 10.1158/0008-5472.CAN-07-5259. [DOI] [PubMed] [Google Scholar]
- 17.Kumaraswamy E., Carlson B.A., Morgan F., Miyoshi K., Robinson G.W., Su D., Wang S., Southon E., Tessarollo L., Lee B.J., Gladyshev V.N., Hennighausen L., Hatfield D.L. Selective removal of the selenocysteine tRNA [Ser]Sec gene (Trsp) in mouse mammary epithelium. Mol Cell Biol. 2003;23:1477–1488. doi: 10.1128/MCB.23.5.1477-1488.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu X., Wu J., Huang J., Powell W.C., Zhang J., Matusik R.J., Sangiorgi F.O., Maxson R.E., Sucov H.M., Roy-Burman P. Generation of a prostate epithelial cell-specific Cre transgenic mouse model for tissue-specific gene ablation. Mech Dev. 2001;101:61–69. doi: 10.1016/s0925-4773(00)00551-7. [DOI] [PubMed] [Google Scholar]
- 19.Dunn B.K., Richmond E.S., Minasian L.M., Ryan A.M., Ford L.G. A nutrient approach to prostate cancer prevention: the Selenium and Vitamin E Cancer Prevention Trial (SELECT) Nutr Cancer. 2010;62:896–918. doi: 10.1080/01635581.2010.509833. [DOI] [PubMed] [Google Scholar]
- 20.Khandrika L., Kumar B., Koul S., Maroni P., Koul H.K. Oxidative stress in prostate cancer. Cancer Lett. 2009;282:125–136. doi: 10.1016/j.canlet.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bosl M.R., Takaku K., Oshima M., Nishimura S., Taketo M.M. Early embryonic lethality caused by targeted disruption of the mouse selenocysteine tRNA gene (Trsp) Proc Natl Acad Sci U S A. 1997;94:5531–5534. doi: 10.1073/pnas.94.11.5531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui J., Zhong R., Chu E., Zhang X.F., Zhang W.G., Fang C.F., Dong Q., Li F.L., Li H. Correlation between oxidative stress and L-type calcium channel expression in the ventricular myocardia of selenium-deficient mice. J Int Med Res. 2012;40:1677–1687. doi: 10.1177/030006051204000507. [DOI] [PubMed] [Google Scholar]
- 23.Demirci S., Kutluhan S., Naziroglu M., Uguz A.C., Yurekli V.A., Demirci K. Effects of selenium and topiramate on cytosolic Ca(2+) influx and oxidative stress in neuronal PC12 cells. Neurochem Res. 2013;38:90–97. doi: 10.1007/s11064-012-0893-z. [DOI] [PubMed] [Google Scholar]
- 24.Brigelius-Flohe R. Vitamin E: the shrew waiting to be tamed. Free Radic Biol Med. 2009;46:543–554. doi: 10.1016/j.freeradbiomed.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 25.Ran Q., Liang H., Gu M., Qi W., Walter C.A., Roberts L.J., 2nd, Herman B., Richardson A., Van Remmen H. Transgenic mice overexpressing glutathione peroxidase 4 are protected against oxidative stress-induced apoptosis. J Biol Chem. 2004;279:55137–55146. doi: 10.1074/jbc.M410387200. [DOI] [PubMed] [Google Scholar]
- 26.Abe J., Berk B.C. Reactive oxygen species as mediators of signal transduction in cardiovascular disease. Trends Cardiovasc Med. 1998;8:59–64. doi: 10.1016/S1050-1738(97)00133-3. [DOI] [PubMed] [Google Scholar]
- 27.Runchel C., Matsuzawa A., Ichijo H. Mitogen-activated protein kinases in mammalian oxidative stress responses. Antioxid Redox Signal. 2011;15:205–218. doi: 10.1089/ars.2010.3733. [DOI] [PubMed] [Google Scholar]
- 28.Rayman M. Selenium in cancer prevention: a review of the evidence and mechanism of action. Proc Nutr Soc. 2005;64:527–542. doi: 10.1079/pns2005467. [DOI] [PubMed] [Google Scholar]
- 29.Wirth E.K., Conrad M., Winterer J., Wozny C., Carlson B.A., Roth S., Schmitz D., Bornkamm G.W., Coppola V., Tessarollo L., Schomburg L., Kohrle J., Hatfield D.L., Schweizer U. Neuronal selenoprotein expression is required for interneuron development and prevents seizures and neurodegeneration. FASEB J. 2010;24:844–852. doi: 10.1096/fj.09-143974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiota M., Takeuchi A., Yokomizo A., Kashiwagi E., Tatsugami K., Kuroiwa K., Naito S. Androgen receptor signaling regulates cell growth and vulnerability to doxorubicin in bladder cancer. J Urol. 2012;188:276–286. doi: 10.1016/j.juro.2012.02.2554. [DOI] [PubMed] [Google Scholar]
- 31.Meplan C., Crosley L.K., Nicol F., Beckett G.J., Howie A.F., Hill K.E., Horgan G., Mathers J.C., Arthur J.R., Hesketh J.E. Genetic polymorphisms in the human selenoprotein P gene determine the response of selenoprotein markers to selenium supplementation in a gender-specific manner (the SELGEN study) FASEB J. 2007;21:3063–3074. doi: 10.1096/fj.07-8166com. [DOI] [PubMed] [Google Scholar]
- 32.Steinbrecher A., Meplan C., Hesketh J., Schomburg L., Endermann T., Jansen E., Akesson B., Rohrmann S., Linseisen J. Effects of selenium status and polymorphisms in selenoprotein genes on prostate cancer risk in a prospective study of European men. Cancer Epidemiol Biomarkers Prev. 2010;19:2958–2968. doi: 10.1158/1055-9965.EPI-10-0364. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Moreno O., Boque N., Redrado M., Milagro F., Campion J., Endermann T., Takahashi K., Saito Y., Catena R., Schomburg L., Calvo A. Selenoprotein-P is down-regulated in prostate cancer, which results in lack of protection against oxidative damage. Prostate. 2011;71:824–834. doi: 10.1002/pros.21298. [DOI] [PubMed] [Google Scholar]
- 34.Penney K.L., Schumacher F.R., Li H., Kraft P., Morris J.S., Kurth T., Mucci L.A., Hunter D.J., Kantoff P.W., Stampfer M.J., Ma J. A large prospective study of SEP15 genetic variation, interaction with plasma selenium levels, and prostate cancer risk and survival. Cancer Prev Res. 2010;3:604–610. doi: 10.1158/1940-6207.CAPR-09-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cooper M.L., Adami H.O., Gronberg H., Wiklund F., Green F.R., Rayman M.P. Interaction between single nucleotide polymorphisms in selenoprotein P and mitochondrial superoxide dismutase determines prostate cancer risk. Cancer Res. 2008;68:10171–10177. doi: 10.1158/0008-5472.CAN-08-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu Y.P., Yu G., Tseng G., Cieply K., Nelson J., Defrances M., Zarnegar R., Michalopoulos G., Luo J.H. Glutathione peroxidase 3, deleted or methylated in prostate cancer, suppresses prostate cancer growth and metastasis. Cancer Res. 2007;67:8043–8050. doi: 10.1158/0008-5472.CAN-07-0648. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Histology of PBCre4;Trspfl/fl and control Trspfl/fl mice. PIN lesions in 6-week (A) and 24-week (B) PBCre4;Trspfl/fl mice are accompanied by a dramatic increase in mitosis, cellular stratification (A and B), and noticeable basement membrane thickening (B). By contrast, none of these features were seen in the prostates of control animals. Prostate histology in control animals at 24 weeks is shown (C–F). Scale bars: 50 μm (A–B) and (D–F); and 100 μm (C).