Abstract
Klotho deficiency is a characteristic feature of chronic kidney disease in which anemia and cardiovascular complications are prevalent. Disruption of the Klotho gene in mice results in hypervitaminosis D and a syndrome resembling accelerated aging that includes osteopenia and vascular calcifications. Given that the bone microenvironment and its cellular components considerably influence hematopoiesis, in the present study, we addressed the in vivo role of klotho in blood cell formation and differentiation. Herein, we report that genetic ablation of Klotho in mice results in a significant increase in erythropoiesis and a decrease in the hematopoietic stem cell pool size in the bone marrow, leading to impaired hematopoietic stem cell homing in vivo. Our data also suggest that high vitamin D levels are only partially responsible for these hematopoietic changes in Klotho−/− mice. Importantly, we found similar hematopoietic abnormalities in Klotho−/− fetal liver cells, suggesting that the effects of klotho in hematopoietic stem cell development are independent of the bone microenvironment. Finally, injection of klotho protein results in hematopoietic changes opposite to the ones observed in Klotho−/− mice. These observations unveil a novel role for the antiaging hormone klotho in the regulation of prenatal and postnatal hematopoiesis and provide new insights for the development of therapeutic strategies targeting klotho to treat hematopoietic disorders associated with aging.
Hematopoiesis is a complex and tightly regulated process of blood cell formation that is hierarchically coordinated. During normal hematopoiesis, diverse blood cell types are produced by the bone marrow (BM) in a manner related to physiologic requirement. Certain conditions may trigger additional production of blood cells. When the oxygen content of body tissues is low, the kidneys produce and release erythropoietin (Epo), a hormone that stimulates the BM to produce more red blood cells (RBCs). Aging is associated with disruption of normal hematopoiesis, resulting in an increase in the prevalence of anemia, the emergence of hematopoietic malignancies, and the development of leukemias.1,2 Deterioration of vital organ function, such as kidney and heart, is also associated with age-related changes, as seen in chronic kidney disease (CKD) and cardiovascular disease (CVD).
The antiaging hormone klotho, predominantly expressed in the kidneys, is emerging as a multifunctional protein regulating vital cellular functions.3–5 Klotho was serendipitously discovered by Kuro-o et al6 when they observed symptoms of accelerated aging associated with a mutation in a specific gene in mice. Klotho exists in a membrane-bound form expressed at high levels in the kidney and, to a lesser extent, in other tissues, whereas a soluble form of klotho is secreted into blood, urine, and cerebrospinal fluid after cleavage of the extracellular domain.7–10 Earlier studies convincingly demonstrate that membrane-bound klotho (α-klotho) is indispensable for signaling of the phosphatonin fibroblast growth factor 23 and that secreted klotho functions as an endocrine hormone responsible for the multiple organ defects observed in Klotho−/− mice.11–14
Maintaining mineral ion homeostasis is critical and involves a delicate and concerted action between bone- and kidney-derived endocrine factors that operate through a complex feedback mechanism(s). Patients with CKD often present with bone diseases, such as osteopenia, osteoporosis, or osteomalacia, as a result of significant derangement of mineral metabolism.15,16 In patients with CKD, failure of appropriate fibroblast growth factor 23/Klotho signaling results in hyperphosphatemia and vascular calcifications.17 Klotho expression is decreased progressively with loss of renal function,18 whereas blood levels of fibroblast growth factor 23 are elevated and are associated with increased CVD and mortality in these patients and in patients undergoing dialysis.19–22 Moreover, abnormal blood cell production leading to severe anemia is a common complication in CKD and CVD and is caused by insufficient renal production of Epo.23,24
Disruption of the Klotho gene in mice due to mutations or inactivation (Klotho−/− mice) results in growth retardation and early demise, osteopenia, extensive vascular calcifications, and skin atrophy, coupled with phosphate retention and hypervitaminosis D.6,13,25–27 Conversely, overexpression of Klotho has been shown to rescue the klotho-deficient phenotype and extend the life span in mice, suggesting that Klotho functions as an aging suppressor gene in mammals.6,28 Loss of klotho is further known to cause endothelial dysfunction by promoting oxidative stress.29 It has been well appreciated that aging and oxidative stress adversely affect hematopoiesis by altering the niche functions.30,31 An earlier report has also highlighted that klotho deficiency in mice results in reduced B lymphopoiesis, suggesting changes in immune regulatory functions by klotho.32 In addition, klotho expression at the mRNA level has been found to be significantly decreased in resting human CD4+ lymphocytes proportionally to advancing age.33
Signals emanating from the BM microenvironment and extrinsic soluble factors associated with the bone and marrow milieu are known to modulate hematopoietic stem cell (HSC) proliferation and differentiation.34,35 Identifying the contributing factors involved in the regulation of hematopoiesis is an area of active research. Several lines of evidence highlight the role of bone-forming cells, the osteoblasts, in the HSC niche; postnatal depletion of osteoblasts negatively regulates the HSC pool size in the BM, whereas an increase in osteoblast number is associated with an augmentation in HSC number.36–39 In addition, a series of advances indicate the importance of the bone-resorbing osteoclasts in regulation of the HSC microenvironment. Osteoclasts actively participate in HSC mobilization from the BM to the circulation and also promote formation of the HSC niche by controlling the maturation of osteoblasts.40–44 Not only do bone cells participate in the regulation of hematopoiesis but the mineral content of the niche may also have a key function in localization of adult hematopoiesis, as reported in studies showing involvement of the calcium-sensing receptor and vitamin D signaling in this process.45,46 Therefore, alterations in bone modeling and remodeling processes and/or mineralization seem to have a prominent effect on the modulation or formation of the hematopoietic niche. However, the regulation of mineral ion balance and hematopoiesis still remains largely a naive area.
Because the bone environment and its components and the process of aging are closely linked to the regulation of hematopoiesis, and klotho deficiency is associated with a marked defect in skeletal mineralization and premature aging-like features, we hypothesized that klotho is involved in the regulation of RBC production and differentiation. In the present study, we demonstrate that loss of klotho severely affects erythropoiesis and HSC number and function. More important, we show that klotho affects hematopoiesis independently of changes in the BM environment and that the absence of klotho results in aberrant hematopoiesis prenatally, providing evidence for a novel and direct role for klotho in hematopoietic development. Although the kidney is the adult hematopoietic organ in zebra fish equivalent to mammalian BM,47–49 the present data demonstrate for the first time, to our knowledge, a link between the kidney-bone-hematopoiesis axes in the mammalian system and attest that klotho is a key factor in the process of hematopoiesis.
Materials and Methods
Mice
Klotho heterozygous mice (Klotho+/−) were purchased from the Mutant Mouse Regional Resource Center (University of California, Davis, CA) and were interbred to obtain Klotho-null mice (Klotho−/−). 1α(OH)ase heterozygous mice were a gift from Dr. René St-Arnaud (Genetics Unit, Shriners Hospital, Montreal, QC, Canada). Klotho heterozygous and 1α(OH)ase heterozygous mice were bred to obtain Klotho−/−/1α(OH)ase−/− double mutants. B6.SJL-Ptprca/BoyAiTac (CD45.1; Ly5.1) mice were purchased from Taconic Farms Inc. (New York, NY). All mice were kept on a light/dark (12 hours/12 hours) cycle at 23°C and received standard laboratory chow and water ad libitum. Genomic DNA was obtained from tail snips, and routine PCR was performed to identify the genotypes. PCR conditions were as follows: klotho—initial denaturation at 94°C for 5 minutes, 35 cycles of denaturation at 94°C for 1 minute, annealing at 63°C for 1 minute and extension at 72°C for 30 seconds, and final extension at 72°C for 10 minutes; 1α(OH)ase—94°C for 5 minutes, annealing at 56°C for 1 minute and extension at 72°C for 1 minute, followed by final extension at 72°C for 10 minutes. The following primers were used: KL0787-12, 5′-GATGGGGTCGACGTCA-3′; KL0787-13, 5′-TAAAGGAGGAAAGCCATTGTC-3′; KL0787-20, 5′-ATGCTCCAGACATTCTCAGC-3′; Neo3a, 5′-GCAGCGCATCGCCTTCTATC-3′; and 1α(OH)ase (forward and reverse), 5′-GCACCTGGCTCAGGTAGCTCTTC-3′ and 5′-GTCCCAGACAGAGACATCCGT-3′. All the animals were maintained in the New York University (NYU) College of Dentistry Animal Facility in accordance with the general guidelines of the NYU School of Medicine Division of Laboratory Animal Resources. All the animal studies were approved by the NYU Institutional Animal Care and Use Committee.
Blood Collection and Hematologic Analysis
Peripheral blood was collected after euthanasia from 6-week-old mice by cardiac puncture into EDTA-coated tubes (BD Biosciences, San Jose, CA) to prevent clotting. Blood samples were then shipped overnight to Cornell University Animal Health Diagnostic Center (Ithica, NY) for automated complete blood cell count.
Tissue Collection
BM was isolated from dissected tibiae and femora from 6-week-old mice by flushing in Iscove's modified Dulbecco's medium (IMDM) (Sigma-Aldrich, St. Louis, MO) supplemented with 20% fetal bovine serum (20% IMDM) (HyClone; Thermo Scientific, Wilmington, DE) through a 27-gauge needle (Becton Dickinson Co., Franklin Lakes, NJ). Marrow cells were dispersed by manual agitation and then were filtered to remove foreign particles. Spleens from 6-week-old mice were surgically removed and were homogenized into a cell suspension in 20% IMDM. Timed pregnant Klotho+/− female mice were sacrificed at 15.5 days postcoitum, and fetal livers were collected by caesarean section and were homogenized into a cell suspension in 20% IMDM. Genomic DNA was obtained from tail snips, and routine PCR as described previously herein was used to identify the genotypes of the embryos.
Flow Cytometry Analysis
Tissues were dissected from 6-week-old or E15.5 embryo mice, and flow cytometry analysis for peripheral blood, BM, spleen, and fetal liver cells was performed in a BD FACSort flow cytometer equipped with 488 argon lasers (BD Biosciences). For immunostaining, cells were washed and then resuspended in 1× PBS containing 0.1% bovine serum albumin. Mouse Fc receptor was blocked before staining using CD16/32 antibody to reduce nonspecific binding. After the addition of antibodies, cells were incubated for 40 minutes on ice; for peripheral blood, RBCs were further lysed using BD FACS lysing solution (BD Biosciences). Labeled cells were then washed with 1× PBS and were analyzed by flow cytometry. Appropriate isotype controls were kept for each set. Forward and side scatter patterns were gated to exclude debris. A total of 50,000 events were collected and analyzed using FlowJo software version 7.6.5 (Tree Star Inc., Ashland, OR). Erythroid lineage was assessed using Ter119 APC/CD71 phosphatidylethanolamine (PE) markers combined with the forward scatter (FSC) properties.50 CXCR4 expression was analyzed using PE-tagged CXCR4 antibody. Hematopoietic stem/progenitor cells were differentiated using SLAM markers (CD150 PE/CD48 APC), Sca1 fluorescein isothiocyanate (Ly6A-E), cKit Percp Cy5.5 (CD117), CD90 PE (Thy-1), and APC-tagged lineage cocktail composed of antibodies against CD3, B220 (CD45R), Ly6G and Ly6C (Gr1), CD11b (Mac1), and TER-119. CKit+Sca1+ cells were gated on the lineage-negative fraction to analyze LSK (lin−cKit+Sca1+). The LSK cells were then analyzed using a Thy-1low gate to obtain the KTLS population (LSK Thylow). CD45.1 PE and CD45.2 fluorescein isothiocyanate antibodies were used to differentiate donor and recipient populations after transplantation. All the antibodies except the SLAM markers were purchased from BD Pharmingen (San Jose, CA). SLAM markers CD150 and CD48 were purchased from eBioscience Inc. (San Diego, CA).
Gene Expression Analysis
Total RNA from bone, BM, spleen, kidney, adult liver, and fetal liver from 6-week-old or E15.5 wild-type (WT) and Klotho−/− mice was extracted using TRIzol reagent (Sigma-Aldrich) according to the manufacturer's protocol and was quantified using a NanoDrop 2000 spectrophotometer (Thermo Scientific). RNA (1 μg) from each sample was reverse transcribed to cDNA in 10 μL of final volume using a high-capacity cDNA reverse transcription kit with random hexamers (Applied Biosystems, Foster City, CA). Real-time PCR analysis was performed using an Eppendorf Mastercycler ep gradient S realplex2 machine (Eppendorf, Hamburg, Germany) in a final reaction volume of 25 μL containing 1 μL of the prepared cDNA of each gene, 12.5 μL of PerfeCta SYBR Green PCR SuperMix (Quanta BioSciences Inc., Gaithersburg, MD), and 1 μmol/L of primers amplifying the genes of interest. Thermal cycle conditions were as follows: 60°C for 2 minutes, 95°C for 10 minutes, and 40 cycles at 95°C for 15 seconds and 60°C for 1 minute. Analyses were performed in duplicate. Samples without reverse transcriptase were used as negative controls. The expression of klotho was measured in BM, spleen, kidney, and fetal liver; the expression of Epo, Hif-1α, and Hif-2α was analyzed in bone, BM, kidney, and liver; and the expression of transferrin, transferrin receptor, glucose transporter type 1, and phosphoglycerate kinase 1 was analyzed in BM and liver. All quantitative RT-PCR values were normalized to the housekeeping gene HPRT, and differences in gene expression between control (WT) mice and Klotho−/− mice were calculated based on the ΔCT method. The primer sequences are shown in Table 1.
Table 1.
Mouse Primer Sequences Used for Real-Time PCR Analysis
| Gene | Forward sequence | Reverse sequence |
|---|---|---|
| Klotho | 5′-ACTTGGCCTTTATTAGCCGGGTCT-3′ | 5′-AGATGGCCTCTTCCCTGTGTTCAA-3′ |
| Erythropoietin (Epo) | 5′-TCTACGTAGCCTCACTTCACT-3′ | 5′-ACCCGGAAGAGCTTGCAGAAA-3′ |
| Hypoxia-inducible factor-1α (HIF-1α) | 5′-TCTCGGCGAAGCAAAGAGTCT-3′ | 5′-TAGACCACCGGCATCCAGAAG-3′ |
| Hypoxia-inducible factor-2α (HIF-2α) | 5′-GGGAACACTACACCCAGTGC-3′ | 5′-TCTTCAAGGGATTCTCCAAGG-3′ |
| Transferrin | 5′-CCCTCTGTGACCTGTGTATTG-3′ | 5′-CTTTCTCAACGAGACACCTGAA-3′ |
| Transferrin receptor | 5′-TCCTGTCGCCCTATGTATCT-3′ | 5′-CGAAGCTTCAAGTTCTCCACTA-3′ |
| Glucose transporter-1 (Glut-1) | 5′-CCCAGGTGTTTGGCTTAGA-3′ | 5′-CAGAAGGGCAACAGGATACA-3′ |
| Phosphoglycerate kinase-1 (Pgk-1) | 5′-CACAGAAGGCTGGTGGATTT-3′ | 5′-CTTTAGCGCCTCCCAAGATAG-3′ |
| HPRT | 5′-AAGCCTAAGATGAGCGCAAG-3′ | 5′-TTACTAGGCAGATGGCCACA-3′ |
ELISA
Serum Epo and stromal-derived factor-1α (SDF-1α) levels were determined using Quantikine enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN) according to the manufacturer's instructions. Serum samples were collected from 6-week-old WT and Klotho−/− mice and were run in duplicate for each assay.
Colony-Forming Unit Assay
A colony-forming unit assay was performed for BM, spleen, and fetal liver cells from 6-week-old or E15.5 WT and Klotho−/− mice. After isolation, the cells were incubated for 1 hour at 37°C and 5% CO2 to allow for adherence to eliminate major stromal cell fractions. BM or fetal liver cells (1 × 104) and spleen cells (1 × 105) from the nonadherent fraction were plated in methyl cellulose medium (MethoCult M3231; STEMCELL Technologies Inc., Vancouver, BC, Canada). Murine recombinant growth factors, including IL-3 (4 ng/mL), stem cell factor (20 ng/mL), granulocyte macrophage colony-stimulating factor (2 ng/mL), and Epo (2 U/mL) (PeproTech, Rocky Hill, NJ), were added to the medium. The plates were incubated in humidified atmosphere at 37°C and 5% CO2. After incubation (8 to 12 days), the colonies were scored based on morphology as burst-forming unit–erythroid) or granulocyte-erythroid-megakaryocyte-macrophage under a Nikon TMS inverted phase contrast microscope (Nikon Instruments, Melville, NY).
Transwell Migration Assay
Chemotactic responses of the WT and Klotho−/− BM cells to SDF-1α were assessed in vitro by Transwell migration assay. Briefly, 105 BM cells from 6-week-old WT and Klotho−/− mice were seeded to the upper chamber of the 8-μm BD Falcon 24-well cell culture insert (BD Biosciences). One hundred nanograms of SDF-1α (PeproTech) was added to the lower wells, containing 600 μL of medium. For each experiment, some wells were kept in both sets without SDF-1α to detect spontaneous migration. The cells were allowed to migrate for 3 hours at 37°C and 5% CO2. The migrated cells were collected and manually counted. The percentage of migration was calculated as follows: (total number of input cells/number of cells migrated) × 100.
In Vivo Homing Experiments
BM cells (2 × 106 cells suspended in 1× PBS) from 6-week-old WT and Klotho−/− mice (CD45.2; Ly5.2) were isolated and injected into the tail vein of nonmyeloablated or myeloablated B6.SJL (CD45.1; Ly5.1) recipient mice (n = 5 per set for each experiment). Myeloablation was achieved by a lethal dose of irradiation (900 rads). Twenty hours after transplantation, peripheral blood, spleen, and BM were collected from recipient mice and were analyzed for the presence of CD45.2+ donor cells by flow cytometry. A minimum of 50,000 events were acquired using a BD FACSort flow cytometer equipped with 488 argon lasers (BD Biosciences).
Klotho Protein Injections
Mouse recombinant klotho protein (10 μg/kg) (R&D Systems) was injected i.p. into 8-week-old C57/Bl/6 mice every other day for 3 days. Saline (1× PBS) was injected as vehicle. Peripheral blood, spleen, and BM cells were immunostained for erythroid and hematopoietic stem/progenitor markers and were analyzed by flow cytometry as described previously herein.
Statistical Analysis
Statistical differences between groups were analyzed by unpaired Student's t-test. More than six mice were analyzed for each set per experiment, and all the values are expressed as means ± SEM. Graphs were plotted using GraphPad Prism software version 5.0 (GraphPad Software Inc., San Diego, CA). A P ≤ 0.05 was considered statistically significant.
Results
Klotho Expression in Hematopoietic Tissues
Klotho is predominantly expressed in the renal distal tubular epithelial cells and, to a lesser extent, in a variety of other tissues, such as the parathyroid gland, choroid plexus in the brain, placenta, and small intestine.6,51–53 To dissect the role of klotho in hematopoiesis, we examined whether klotho transcripts are expressed in hematopoietic tissues. We analyzed klotho expression in total BM cells and splenocytes from 6-week-old WT mice and in fetal liver cells from E15.5 WT embryos by real-time PCR. Adult mouse kidney cells were used as a positive control. As expected, klotho was highly expressed in kidney, which is the primary source of klotho production. These results showed that klotho mRNA is also expressed in BM, spleen, and fetal liver cells, albeit at much lower levels than in kidney, suggesting a possible role for klotho signaling in hematopoiesis. The real-time data are expressed as fold change with respect to the housekeeping gene HPRT (Supplemental Figure S1A).
Hematologic Phenotype of Klotho−/− Mice
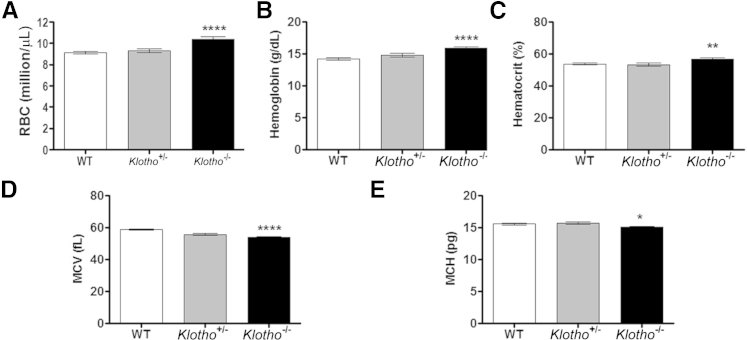
To assess the effect of klotho in steady-state hematopoiesis, we compared the hematopoietic output of 6-week-old Klotho−/− mice with that of their WT and Klotho+/− (heterozygous) littermates. Complete blood cell count analysis revealed a significant increase in RBC numbers, hemoglobin levels, and hematocrit values in Klotho−/− mice (Figure 1, A–C). Conversely, a significant decrease in RBC indices, such as mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH), was detected in peripheral blood of Klotho−/− mice (Figure 1, D and E). No differences were found in any of the examined hematologic parameters between WT and Klotho+/− (heterozygous) mice.
Figure 1.
Complete blood cell count analysis of 6-week-old WT, heterozygous (Klotho+/−), and KlothoKlotho-null (Klotho−/−) mice. A: RBC counts (WT, n = 23; +/−, n = 8; −/−, n = 18). B: Hemoglobin levels (WT, n = 19; +/−, n = 9; −/−, n = 16). C: Hematocrit values (WT, n = 19; +/−, n = 10; −/−, n = 16). D: MCV (WT, n = 21; +/−, n = 10; −/−, n = 20). E: MCH (WT, n = 16; +/−, n = 10; −/−, n = 16). Data represent the means ± SEM. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗∗P < 0.0001.
Loss of Klotho Results in Increased BM Erythropoiesis without Extramedullary Erythropoiesis
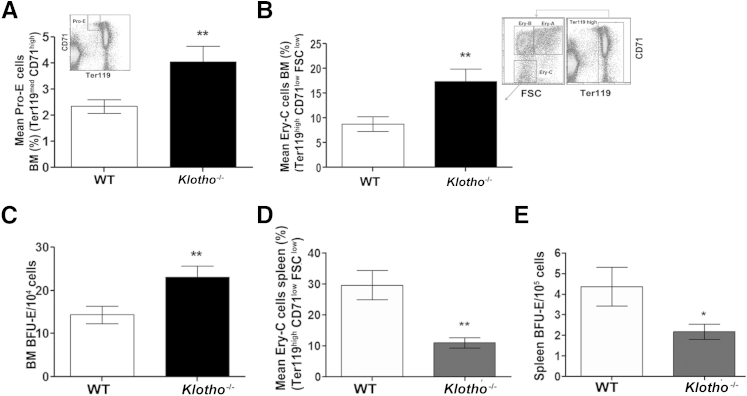
We further examined the effect of klotho deficiency in erythroid cell production and differentiation in BM as the origin of hematopoiesis in adult mice. Total BM cellularity was modestly decreased in Klotho−/− mice compared with WT littermates (data not shown). Successive stages of erythroid commitment were determined based on cell size (FSC) coupled with expression of Ter119 and the transferrin receptor CD71. We observed a significant increase in immature pro-erythroblasts (Pro-E) (Ter119med CD71high) in the BM of Klotho−/− mice (Figure 2A). Further analysis based on cell size showed that the absence of klotho resulted in a marked increase in the relatively mature erythroid C (Ery-C) fraction (Ter119highCD71lowFSClow) of erythroblasts in comparison to WT littermates (Figure 2B). However, there were no discernible changes in the intermediate Ery-A (Ter119highCD71high FSChigh) and Ery-B (Ter119 highCD71medFSClow) populations (Supplemental Figure S1, B and C). In addition, an in vitro colony-forming unit assay showed that Klotho−/− BM cells generated more erythroid colonies (burst-forming unit–erythroid) than WT cells, indicating the presence of increased functional erythroid progenitors in Klotho−/− BM (Figure 2C).
Figure 2.
BM and splenic erythropoiesis in 6-week-old WT and Klotho−/− mice. A: Flow cytometry analysis shows an increase in primitive Pro-Es (Ter119med CD71high) in Klotho−/− mice (Pro-E: WT, n = 12; Klotho−/−, n = 9); inset shows flow cytometry gating. B: Erythroid lineage commitment was analyzed using Ter119 and CD71 markers coupled with FSC measurements. A mature Ery-C (Ter119high CD71low FSClow) population was observed in Klotho−/− compared with WT littermates (Ery-C: WT, n = 9; Klotho−/−, n = 7); inset shows flow cytometry gating on the Ter119high population. C: Colony-forming unit assay showing burst-forming unit–erythroid (BFU-E) colonies produced by Klotho−/− BM cells (WT, n = 13; Klotho−/−, n = 11). Cells from each mouse were plated in triplicate, and the number of colonies was scored based on morphologic features. D: Graphic representation of flow cytometry analysis of the mature Ery-C population in Klotho−/− splenocytes (WT, n = 9; Klotho−/−, n = 8). E: Colony-forming unit assay showing splenic BFU-E colonies in Klotho−/− mice (WT, n = 4; Klotho−/−, n = 4). Cells from WT and Klotho−/− spleens were plated in triplicate. Data represent the means ± SEM. ∗P < 0.05 and ∗∗P < 0.01.
Because the spleen is an important organ that supports erythropoiesis in stress and inflammation, we next analyzed splenic erythropoiesis. Klotho mutant mice developed postnatal growth retardation and early mortality, with most Klotho−/− mice dying by 9 weeks after birth.6 Klotho−/− mice were significantly smaller than their control littermates, and their spleen size was also found to be markedly decreased. However, even when the spleen size was corrected to body weight, the ratio of Klotho−/− spleen weight to body weight was one-third that of their WT littermates (Supplemental Figure S1D). In addition, total spleen cellularity was also decreased in Klotho−/− mice compared with their WT littermates (Supplemental Figure S1E). Although there were no remarkable changes in primitive erythroid lineage cells in the spleens of Klotho−/− mice (data not shown), we observed a significant decrease in the mature Ery-C (Ter119highCD71lowFSClow) fraction of the erythroid population (Figure 2D) accompanied by a decrease in the number of burst-forming unit–erythroid colonies generated by Klotho−/− splenocytes (Figure 2E). These data suggested a decline in splenic erythropoiesis and rules out the possibility of extramedullary hematopoiesis in the absence of klotho.
Enhanced Erythropoiesis in Klotho−/− Mice Is Mediated through Epo and the HIF Signaling Pathway
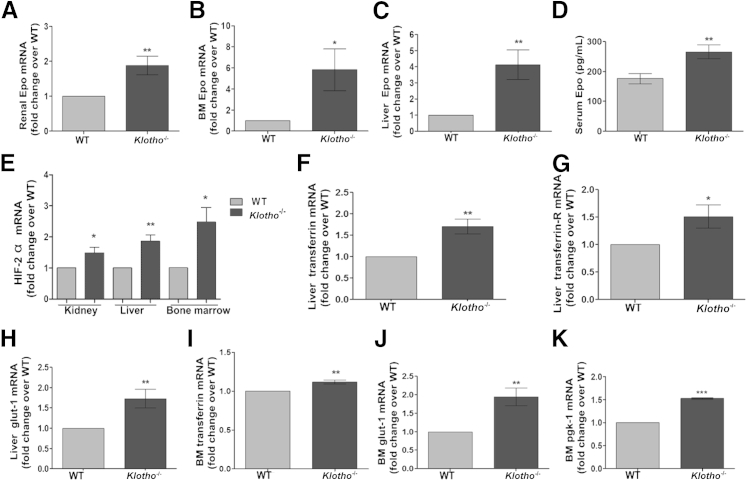
BM erythropoiesis is under the tight control of Epo secreted from the kidneys. Therefore, we examined whether enhanced erythropoiesis in Klotho−/− mice is Epo mediated. Epo mRNA expression was significantly up-regulated in Klotho−/− kidney, BM, and liver cells (Figure 3, A–C). Similarly, serum Epo levels were significantly elevated in klotho−/− mice compared with their WT littermates, indicating enhanced Epo secretion in the absence of klotho (Figure 3D). Previous studies have demonstrated that the aging kidney is exposed to hypoxia, to which it responds by activation of HIF signaling and up-regulation of HIF-regulated genes.54 Because Klotho−/− mice present with aging-like features and Epo is an HIF target gene, we next examined HIF-1α and HIF-2α expression in WT and Klotho−/− kidneys. Renal mRNA expression of HIF-1α (Supplemental Figure S1F) and HIF-2α (Figure 3E) was similarly up-regulated in Klotho−/− mice, consistent with the notion that activation of HIF by hypoxia results in increased Epo production. Although the kidney was the major Epo-producing organ, it has been reported that in some pathologic conditions, hepatic Epo production is increased.55 Therefore, we studied the expression of important HIF target genes in the liver and BM of WT and Klotho−/− littermates. The expression of glucose transporter type 1, phosphoglycerate kinase 1, transferrin, and transferrin receptor was significantly up-regulated in the liver and BM in Klotho−/− mice (Figure 3, F–K), confirming activation of the HIF pathway and erythropoiesis. These findings suggest that enhanced erythropoiesis in Klotho−/− mice is Epo induced and is driven by augmented HIF signaling and may serve as a mechanism to respond to stress conditions, such as anemia and inflammation.
Figure 3.
Increased erythropoiesis by activation of HIF signaling and up-regulation of HIF target genes in 6-week-old klotho−/− mice. Real-time quantitative PCR of renal Epo mRNA (WT, n = 5; Klotho−/−, n = 5) (A), BM Epo mRNA (WT, n = 14; Klotho−/−, n = 6) (B), and liver Epo mRNA (WT, n = 9; Klotho−/−, n = 7) (C). D: Serum Epo levels (WT, n = 14; Klotho−/−, n = 14). E: Real-time quantitative PCR of HIF-2α in kidney (WT, n = 6; Klotho−/−, n = 5), liver (WT, n = 5; Klotho−/−, n = 4), and BM (WT, n = 6; Klotho−/−, n = 4). F: Liver transferrin (WT, n = 5; Klotho−/−, n = 6). G: Liver transferrin receptor (WT, n = 6; Klotho−/−, n = 6). H: Liver glucose transporter type 1 (glut-1) (WT, n = 6; Klotho−/−, n = 6). I: BM transferrin (WT, n = 6; Klotho−/−, n = 4). J: BM glut-1 (WT, n = 6; Klotho−/−, n = 4). K: BM phosphoglycerate kinase 1 (pgk-1) (WT, n = 6; Klotho−/−, n = 4). Data represent the means ± SEM, normalized to HPRT and plotted as fold change over WT (where WT = 1). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
Absence of Klotho Results in Altered Localization of Hematopoietic Stem/Progenitor Cells
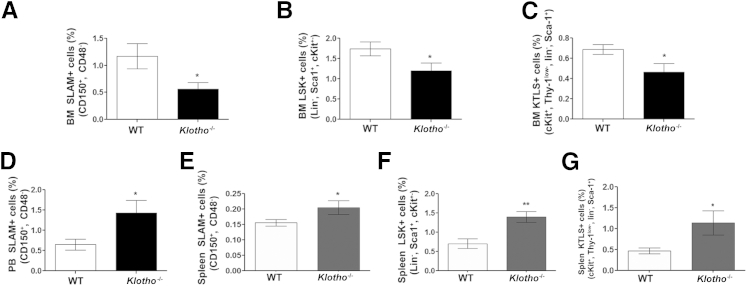
To investigate whether increased erythropoiesis in Klotho−/− mice is due to expansion of the HSC compartment and/or increased differentiation of progenitor cells, we analyzed by flow cytometry the frequency of HSCs in BM, peripheral blood, and spleen. These data show a significant decrease in the CD150+CD48− (SLAM) population highly enriched in HSCs and in the frequency of the Lin−cKit+Sca-1+ (LSK) and the more primitive cKit+ Thy-1low Lin− Sca1+ (KTLS) fraction in the BM of Klotho−/− mice compared with WT littermates (Figure 4, A–C). These findings suggest that loss of klotho results in an overall reduction in the HSC pool size in the BM possibly due to an increase in differentiation of HSCs to the erythroid lineage. We observed an increase in the circulating progenitors marked by SLAM (CD150+CD48−) markers in Klotho−/− mice (Figure 4D). Moreover, we observed an increase in the phenotypic HSCs in Klotho−/− spleens as detected by elevated SLAM (CD150+CD48−) (Figure 4E), LSK (Lin−Sca-1+cKit+) (Figure 4F), and the most primitive KTLS (cKit+ Thy-1low Lin− Sca-1+) (Figure 4G) hematopoietic stem/progenitor cells. The increase in circulating and splenic HSCs in Klotho−/− mice suggested a possible homing defect during development. Collectively, these observations indicate that the hematopoietic milieu of mice lacking Klotho is severely disturbed compared with control WT littermates.
Figure 4.
Altered localization of hematopoietic stem/progenitor cells in Klotho−/− mice. Frequency of the HSC population stained for SLAM (CD150+ CD48−) (WT, n = 14; Klotho−/−, n = 14) (A), the Lin− Sca-1+ (C) Kit+ (LSK) HSC population (WT, n = 9; Klotho−/−, n = 7) (B), and the primitive c-Kit+Thy1+Lin−Sca 1+ (KTLS) HSC population (WT, n = 7; Klotho−/−, n = 7) (C) in the Klotho−/− BM compartment. D: Circulating SLAM (CD150+ CD48−) population in peripheral blood (PB) (WT, n = 7; Klotho−/−, n = 6). Retention of phenotypic SLAM (WT, n = 11; Klotho−/−, n = 8) (E), Lin− Sca-1+ c-Kit+ (LSK) (WT, n = 9; Klotho−/−, n = 9) (F), and primitive c-Kit+Thy1+Lin−Sca 1+ (KTLS) population (WT, n = 10; Klotho−/−, n = 9) (G) in splenocytes from Klotho−/− mice compared with their WT littermates. Data represent the means ± SEM. ∗P < 0.05 and ∗∗P < 0.01.
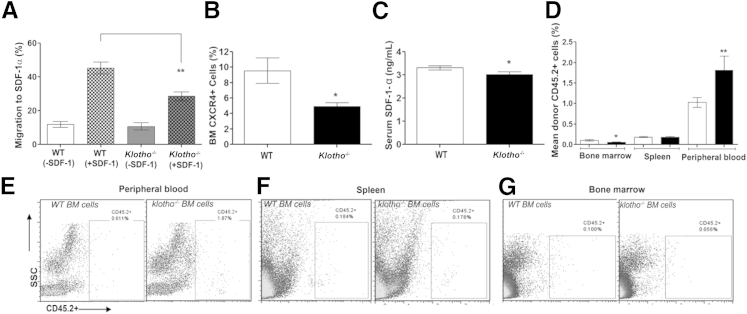
Migratory Properties of BM Cells from Klotho−/− Mice Are Severely Impaired, Resulting in Disrupted Homing in Vivo
The present peripheral blood, BM, and spleen data demonstrated that loss of klotho results in highly perturbed erythropoiesis and suggest that this may be related to altered homing or retention of hematopoietic stem/progenitor cells in the Klotho-deficient BM. The homing mechanism is dependent on expression of the chemokine SDF-1α/CXCL-12 and other cell adhesion molecules and extracellular matrix proteins that guide the hematopoietic stem/progenitor cells to the niche. To address the hypothesis, we performed an in vitro Transwell migration assay that compared the migratory function of BM cells from WT and Klotho−/− mice toward SDF-1α. The findings showed that BM cells from Klotho-deficient mice exhibited a twofold decrease in chemotaxis toward an SDF-1α gradient compared with WT mice (Figure 5A). Spontaneous migration in the absence of SDF-1α was also assessed simultaneously and was found to be low, with no difference between WT and Klotho−/− mice. Furthermore, we analyzed the expression of CXCR4, the putative receptor for SDF-1α, and found that the reduced chemotactic response of Klotho−/− BM cells was associated with a decrease in overall CXCR4+ population in Klotho−/− BM (Figure 5B). These findings were confirmed by quantifying serum levels of SDF-1α in WT and Klotho−/− mice. SDF-1α levels were found to be significantly reduced in mice lacking Klotho, indicating that trafficking of stem/progenitor cells between peripheral blood and the BM compartment is impaired (Figure 5C). These results were further confirmed by an in vivo assay in which we compared the direct and competitive steady-state homing potential of BM cells from WT and Klotho−/− mice. Studies have shown that lethal irradiation creates a noncompetitive host HSC pool that can be easily replaced by donor HSCs, whereas a nonmyeloablated environment may better portray natural trafficking patterns of HSCs in vivo and allow greater recovery and better survival of donor cells.56–58 Therefore, we transplanted Klotho−/− or WT BM cells into nonmyeloablated and myeloablated B6.SJL WT recipient mice and assessed their direct homing efficiency 20 hours after transplantation. We found a marked reduction in the ability of Klotho−/− cells to lodge in a normal BM microenvironment (Figure 5, D and G, and Supplemental Figure S2, C and D) compared with transplanted WT BM cells. Furthermore, these experiments showed that most transplanted Klotho−/− BM cells were localized in the peripheral blood (Figure 5, D and E, and Supplemental Figure S2, A and D). However, localization of Klotho−/− and WT transplanted BM cells was similar in the spleens of nonmyeloablated and myeloablated recipient mice (Figure 5, D and F, and Supplemental Figure S2, B and D). Together, these results confirm a defect in the homing ability of Klotho−/− BM cells and clearly demonstrate that lack of klotho results in impaired lodgment and retention of BM cells in a normal microenvironment owing to a defect in their migratory function.
Figure 5.
The perturbed in vitro and in vivo homing ability of Klotho−/− BM cells. A:In vitro migration experiment of the migratory capacity of Klotho−/− BM cells toward an SDF-1α gradient (WT, n = 5; Klotho−/−, n = 5). B: Flow cytometry of CXCR4 in Klotho−/− BM compared with WT mice (WT, n = 11; Klotho−/−, n = 8). C: SDF-1α concentration measured by ELISA in the serum of WT (n = 10) and Klotho−/− (n = 10) mice. Samples were measured in duplicate. D: Graphic representation of flow cytometry analysis of the BM, spleen, and peripheral blood after transplantation of WT (n = 8) or Klotho−/− (n = 8) BM cells in myeloablated B6.SJL recipient mice. E–G: Flow cytometry profiles of the peripheral blood, spleen, and BM after transplantation. SSC, side scatter. Data represent the means ± SEM. ∗P < 0.05 and ∗∗P < 0.01.
Increased Vitamin D Levels Are Only Partially Responsible for the Hematopoietic Changes in Klotho−/− Mice
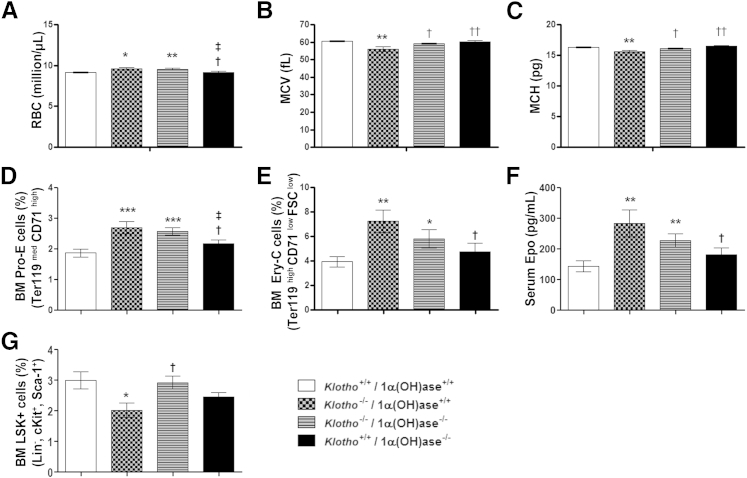
Because Klotho−/− mice exhibit highly elevated vitamin D levels and vitamin D is known to regulate physiologic functions of klotho,59 we investigated whether elimination of vitamin D activity can rescue the hematopoietic changes observed in Klotho−/− mice. We generated compound mutant mice lacking both Klotho and 1α(OH)ase, the enzyme responsible for converting vitamin D to its active form. A complete blood cell count analysis was performed in WT, Klotho−/−/1α(OH)ase+/+, Klotho−/−/1α(OH)ase−/−, and Klotho+/+/1α(OH)ase−/− littermates. As shown in Figure 6A, although Klotho−/−/1α(OH)ase−/− double-mutant mice still displayed an increased number of circulating RBCs, genetic ablation of 1α(OH)ase from Klotho−/− mice restored the MCV and MCH to normal levels in the double mutants (Figure 6, B and C).
Figure 6.
Genetic ablation of 1α(OH)ase partially rescued hematopoietic changes in Klotho−/− mice. A–C: Complete blood cell count analysis of WT, Klotho−/−/1α(OH)ase+/+, Klotho−/−/1α(OH)ase−/−, and Klotho+/+/1α(OH)ase−/− mice. RBC counts [WT, n = 14; Klotho−/−/1α(OH)ase+/+, n = 11; Klotho−/−/1α(OH)ase−/−, n = 16; Klotho+/+/1α(OH)ase−/−, n = 11] (A), MCV [WT, n = 12; Klotho−/−/1α(OH)ase+/+, n = 13; Klotho−/−/1α(OH)ase−/−, n = 15; Klotho+/+/1α(OH)ase−/−, n = 15] (B), and MCH [WT, n = 17, klotho−/−/1α(OH)ase+/+, n = 12; Klotho−/−/1α(OH)ase−/−, n = 17; Klotho+/+/1α(OH)ase−/−, n = 17] (C). D–F: Flow cytometry analysis of BM cells from all four genotypes. Percentage of the Pro-E population [WT, n = 16; Klotho−/−/1α(OH)ase+/+, n = 14; Klotho−/−/1α(OH)ase−/−, n = 12; Klotho+/+/1α(OH)ase−/−, n = 13] (D) and the Ery-C population [WT, n = 13; Klotho−/−/1α(OH)ase+/+, n = 10; Klotho−/−/1α(OH)ase−/−, n = 9; Klotho+/+/1α(OH)ase−/−, n = 12] (E) and serum Epo levels [WT, n = 9, Klotho−/−/1α(OH)ase+/+, n = 6; Klotho−/−/1α(OH)ase−/−, n = 9, Klotho+/+/1α(OH)ase−/−, n = 7] (F). G: Percentage of LSK progenitors [WT, n = 7; Klotho−/−/1α(OH)ase+/+, n = 7; Klotho−/−/1α(OH)ase−/−, n = 7; Klotho+/+/1α(OH)ase−/−, n = 7]. Data represent the means ± SEM. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 versus WT; †P < 0.05, ††P < 0.01 versus Klotho−/−/1α(OH)ase+/+; ‡P < 0.05 versus Klotho−/−/1α(OH)ase−/−.
However, flow cytometry analysis of erythroid populations in the BM showed that the frequency of immature erythroid population (Pro-E; pro-erythroid) and the more mature Ery-C fraction was similarly increased in Klotho−/− and Klotho−/−/1α(OH)ase−/− double-mutant mice but not in 1α(OH)ase−/− mice (Figure 6, D and E). Similarly, circulating Epo levels were highly elevated in Klotho−/− and Klotho−/−/1α(OH)ase−/− mutant mice compared with WT and 1α(OH)ase−/− mice (Figure 6F). Erythropoiesis seemed to be unaffected in 1α(OH)ase−/− mice, as indicated by the examined parameters. Therefore, these data suggest that the increase in erythropoiesis in the absence of klotho is independent of vitamin D levels and is most likely associated with loss of klotho function. There was no difference in the frequency of hematopoietic progenitor cells (LSK; Lin−Sca-1+cKit+) among WT, Klotho−/−/1α(OH)ase−/− double mutants, and 1α(OH)ase−/− mice, in contrast to decreased LSK frequency in Klotho−/− mice (Figure 6G), suggesting that excess vitamin D negatively affects hematopoietic progenitor numbers. Taken together, these findings suggest that elimination of vitamin D could not completely rescue the hematopoietic abnormalities observed in Klotho−/− mice, signifying the importance of klotho in the regulation of hematopoiesis.
Klotho Protein Administration Influences Steady-State Hematopoiesis in Vivo
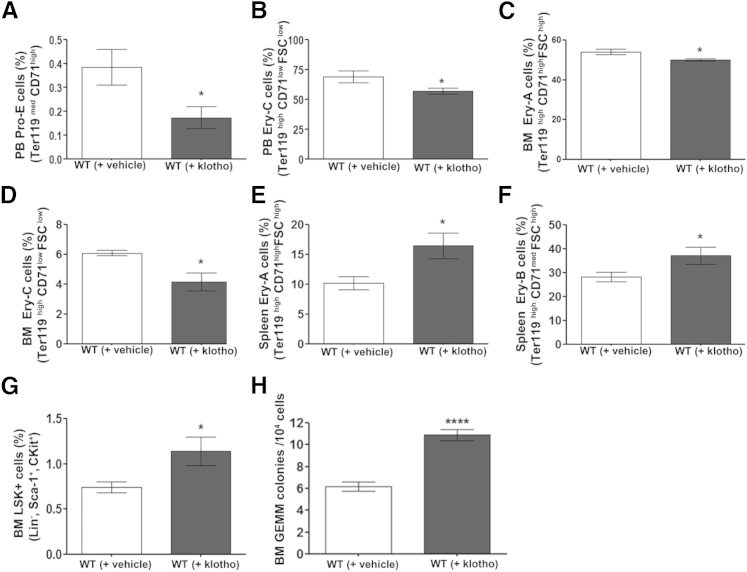
These findings so far clearly reveal that lack of klotho adversely affects hematopoiesis. To determine whether the effect of klotho is direct, we investigated whether exogenous administration of klotho protein can modulate steady-state hematopoiesis. Intraperitoneal injection of klotho protein in 8-week-old WT mice resulted in differential regulation of HSCs in various tissues analyzed. Flow cytometry analysis showed a decrease in erythroid progenitors in peripheral blood and BM (Figure 7, A–D), followed by augmented splenic erythropoiesis (Figure 7, E and F) in klotho-injected mice compared with vehicle-injected mice. Moreover, administration of klotho protein resulted in higher LSK (Lin−Sca-1+cKit+) hematopoietic stem/progenitor cell levels (Figure 7G) in BM and more progenitor cell (granulocyte-erythroid-megakaryocyte–macrophage) colonies in vitro (Figure 7H). These data show that klotho administration results in hematopoietic changes opposite to the ones observed in klotho-deficient mice. Taken together, these results clearly demonstrate a novel role for klotho in the regulation of steady-state hematopoiesis. However, the exact mechanism of the differential modulation of hematopoiesis requires further investigation.
Figure 7.
Klotho protein injection influenced hematopoiesis in WT mice. A–G: Flow cytometry analysis of erythroid populations in peripheral blood (PB) and BM and of erythroid cells in spleen. Peripheral blood: Percentage of the Pro-E population [WT (+ vehicle), n = 3; WT (+ Klotho), n = 10] (A) and the Ery-C population [WT (+ vehicle), n = 6; WT (+ klotho), n = 7] (B). BM: Percentage of the Ery-A population [WT (+ vehicle), n = 5; WT (+ klotho), n = 9] (C) and the Ery-C population [WT (+ vehicle), n = 5; WT (+ klotho), n = 8] (D). Spleen: Percentage of the Ery-A population [WT (+ vehicle), n = 7; WT (+ klotho), n = 8] (E) and the Ery-B population [WT (+ vehicle), n = 7; WT (+ klotho), n = 7] (F). G: Percentage of LSK (lin− Sca-1+ c-Kit+) progenitors in the BM of injected mice [WT (+ vehicle), n = 8; WT (+ klotho), n = 6]. H: Colony-forming unit assay of BM granulocyte-erythroid-megakaryocyte-macrophage (GEMM) mix colonies in klotho-injected mice [WT (+ vehicle), n = 8; WT (+ klotho), n = 10]. Data represent the means ± SEM. ∗P < 0.05, ∗∗∗∗P < 0.0001.
Klotho Deficiency Results in Disrupted Hematopoiesis Independent of the BM Microenvironment
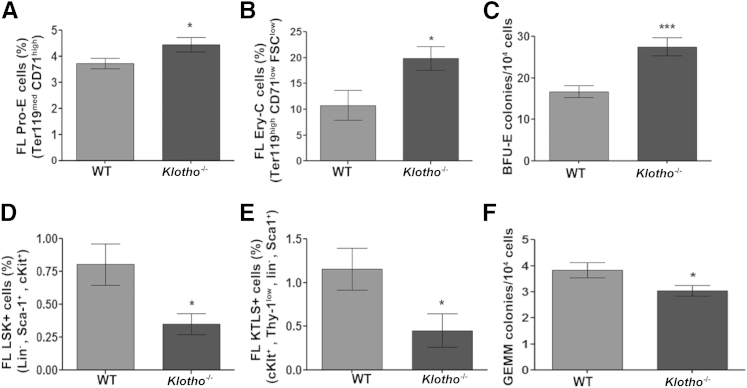
The BM hematopoietic changes observed postnatally in Klotho-deficient mice could be associated with the dramatic reduction in osteoblast and osteoclast numbers and bone mineral density in Klotho−/− bones. However, the decrease in HSCs in Klotho−/− BM and their retention in the spleen along with the defect in their homing ability (as seen in vitro and in vivo) suggest that klotho deficiency impairs hematopoiesis regardless of the BM microenvironment. To address this hypothesis, we studied prenatal hematopoiesis in Klotho−/− mice. Because embryonic hematopoiesis in fetal liver declines after E15.5, we isolated fetal liver cells from time pregnant female mice on E15.5 and studied changes in fetal liver hematopoiesis. The yield of fetal liver cells from Klotho−/− mice was significantly lower compared with WT littermates (Supplemental Figure S2E). We found that fetal liver hematopoiesis in Klotho−/− mice was similarly affected, with changes similar to those detected in the BM compartment of adult Klotho−/− mice. Analysis of the erythroid population showed an increase in early erythroid cells (Pro-E; Ter119medCD71high) (Figure 8A) and in the more mature Ery-C fraction (Ter119highCD71lowFSClow) (Figure 8B) of erythroblasts in Klotho−/− fetal liver cells, a finding supported by enhanced erythroid colony-forming ability in vitro (Figure 8C). More importantly, we observed a marked reduction in Lin−Sca1+cKit+ (LSK) and cKit+ Thy-1low Lin− Sca-1+ (KTLS) HSCs in Klotho−/− fetal liver cells compared with WT fetal livers (Figure 8, D and E). This was concomitant with a significant decrease in the ability of Klotho−/− fetal liver cells to form granulocyte-erythroid-megakaryocyte–macrophage colonies in vitro (Figure 8F). These data indicate a global defect in the HSC turnover in the absence of klotho. This novel observation, that klotho deficiency affects prenatal hematopoiesis, clearly demonstrates a potential role for the antiaging hormone klotho in hematopoietic development.
Figure 8.
Klotho deficiency affected fetal liver (FL) hematopoietic milieu. Flow cytometry analysis of E15.5 FL cells. Immature erythroid cells (Pro-E; Ter119medCD71high) (WT, n = 9; Klotho−/−, n = 10) (A) and Ery-C fraction (Ter119high CD71low FSClow) (WT, n = 8; Klotho−/−, n = 6) (B) in Klotho−/− FLs. C: Colony-forming unit assay of burst-forming unit–erythroid (BFU-E) colonies produced by Klotho−/− FL cells (WT, n = 7; Klotho−/−, n = 9). Cells from each mouse were plated in triplicate, and the number of colonies in each plate was counted. Frequency of Lin− Sca-1+ c-Kit+ (LSK) (WT, n = 10; Klotho−/−, n = 8) (D) and c-Kit+Thy1+Lin−Sca-1+ (KTLS) (WT, n = 7; Klotho−/−, n = 8) (E) HSC populations in Klotho−/− FLs. F: Colony-forming unit assay of primitive granulocyte-erythroid-megakaryocyte-macrophage (GEMM) colonies in Klotho−/− FL cells (WT, n = 7; Klotho−/−, n = 9). Cells from each mouse were plated in triplicate, and the number of colonies in each plate was counted. Data represent the means ± SEM. ∗P < 0.05, ∗∗∗P < 0.001.
Discussion
Several lines of evidence suggest that aging has a major effect on HSC function and localization.60–66 Klotho has previously been identified as an aging-suppressor gene that has an active role in the renal control of calcium, phosphate, and vitamin D metabolism, and Klotho gene deficiency is associated with renal and skeletal abnormalities, including low-bone-turnover osteopenia characterized by a decreased bone formation rate, reduced osteoblast and osteoclast numbers, and severely impaired bone mineral density.26,67–69 Defects in bone ossification and altered mineral ion metabolism have adverse effects in the BM microenvironment that houses the hematopoietic stem and progenitor cells.38,70 Several studies have reported that decreased osteoblast and osteoclast numbers and bone mineral content result in reduced B-lymphocyte and HSC numbers.37–39,44,45,71–74 In addition, defective endochondral ossification causes aberrant B lymphopoiesis in mice.75,76 The present study is the first to demonstrate that normal function of klotho is required for balanced erythropoiesis and to establish a novel role for klotho in the regulation of RBC formation. We show that mice deficient in Klotho exhibit perturbed prenatal and postnatal hematopoiesis and, hence, are incapable of sustaining a balanced hematopoietic milieu.
Klotho deficiency is a characteristic feature of CKD in which anemia and vascular calcifications are prevalent. Moreover, kidney disease is associated with inflammation and aging. Direct involvement of klotho in vascular calcifications was demonstrated in recent studies.17,18 Therefore, understanding the extrarenal functions of klotho is of great clinical significance for therapeutic intervention in pathologic conditions associated with CKD and CVD. This study shows that loss of klotho in mice results in reduced MCV and MCH in circulating RBCs. Erythropoiesis is tightly regulated between the kidney and BM microenvironment by the production of Epo, a hormone secreted by the kidney and acting on the erythroid progenitors in the BM. BM erythropoiesis is a complex and dynamic process that involves proliferation of immature erythroblasts and maturation through successive stages, leading to enucleation of mature erythrocytes. As a response to inherited or acquired anemia, the kidneys produce Epo to increase RBC production and oxygenation. Hypoxia acts as a physiologic stimulus to induce Epo transcription.77,78 Studies have shown that administration of recombinant Epo can induce klotho expression in a rat nephropathy model, suggesting that these kidney-derived proteins can interact in vivo.79 Herein we demonstrate that despite the low MCV and MCH, klotho deficiency in mice results in increased erythropoiesis through activation of the HIF signaling pathway and subsequent increased Epo secretion and up-regulation of renal Epo mRNA. Although the kidney is the primary site for Epo production in adult animals, other tissues also produce Epo. It has been recently reported that osteoblasts can also produce Epo through activation of the HIF pathway under physiologic and pathophysiologic conditions.80 In the present studies, we found that the expression of HIF-1α and HIF-2α was significantly attenuated in Klotho−/− bone, resulting in local suppression of Epo (unpublished data, S.V.M., L.M.C., C.C., D.S.). These findings suggest that reduced osteoblast numbers and osteopenia in Klotho−/− mice may be responsible for the inhibition of HIF and Epo in bone and further support the hypothesis for tissue-specific hypoxic induction of Epo, leading to increased erythrocyte production.
We found that klotho deficiency results in decreased frequency of Lin−cKit+Sca1+ (LSK) and cKit+ Thy-1low Lin− Sca-1+ (KTLS) HSCs in the BM and fetal liver. However, HSC numbers were increased in the blood and spleen of Klotho−/− mice, suggesting that in the absence of klotho, primitive HSC populations reside in sites other than the BM. These observations raised the possibility that the decrease in BM HSC numbers may be due to altered homing or retention of the cells in the correct microenvironment. Retention and homing of HSCs to the BM depends on the presence of chemokines, extracellular matrix proteins, and osteoblasts. The present data show that Klotho deletion results in a decreased response of BM cells to a gradient of SDF-1α, low serum SDF-1α, and reduced CXCR4+ cells in the BM, suggesting that the Klotho−/− BM stroma does not support HSC retention, and that the migratory function of Klotho−/− BM cells is impaired. Furthermore, in vivo homing assays confirmed these observations and demonstrated that transplanted Klotho−/− BM cells were unable to competently lodge in a normal BM microenvironment regardless of whether there was competition (nonmyeloablation) or not (myeloablation). Also, trafficking from the marrow to the periphery was increased in klotho deficiency. The present findings, together with the current understanding that loss of osteoblasts negatively regulates the size of the HSC niche and results in defective HSC localization,37–39,45,81 suggest that i) impaired bone mineralization and loss of osteoblasts and osteoclasts in Klotho−/− mice may alter the BM microenvironment, rendering it nonconducive to supporting normal hematopoiesis, ii) the decrease in HSC numbers in Klotho−/− mice may be due to an accelerated differentiation of these cells toward the erythroid lineage, and/or iii) the abnormalities found in Klotho−/− HSCs may be due to a cell-autonomous defect in their ability to engage the niche in vivo. Preliminary data from the Despina Sitara Laboratory (Department of Basic Science and Craniofacial Biology New York University College of Dentistry, and Department of Medicine New York University School of Medicine, NY) show that after long-term competitive repopulation transplantations, Klotho−/− BM cells do not engraft as competently as WT BM cells to the BM environment of lethally irradiated WT mice (unpublished data, S.V.M., L.M.C., C.C., D.S.). These findings suggest that Klotho deletion results in an intrinsic abnormality of the HSCs to efficiently engraft to a normal BM niche and that HSCs do not depend on the Klotho−/− host BM environment. More transplantation studies are currently under way to confirm these observations.
Previously published data show that Klotho-null mice exhibit B lymphopenia and low levels of IL-7, which is essential for early B-cell development in the BM, and they attribute these changes to aging.32 Klotho mRNA expression has also been found to be significantly decreased in resting human CD4+ lymphocytes proportionally to advancing age.33 Additionally, a shift toward myeloid lineage commitment and reduced B lymphopoiesis are well-documented phenomena in both murine and human aging.65,82,83 Taken together, the effect of klotho deficiency in lymphopoiesis and myelopoiesis and the mechanisms mediating these effects require further investigation.
Lack of klotho disturbs mineral ion homeostasis and vitamin D balance, leading to hyperphosphatemia, hypercalcemia, and hypervitaminosis D. Previous studies convincingly demonstrated that genetic ablation of vitamin D can reverse phosphate and calcium excess and rescue aging and growth retardation in Klotho−/− mice.59,84 In addition, the vitamin D receptor is known to play an important role in hematopoiesis.46 Therefore, we investigated whether the mechanism of altered hematopoiesis in Klotho−/− mice is vitamin D mediated. We observed that the altered erythropoiesis in Klotho−/− mice could not be completely rescued in Klotho−/−/1α(OH)ase−/− double-mutant mice, suggesting that the effect of klotho on erythroid cell production is independent of vitamin D levels. However, the low mean cell hemoglobin content and microcytosis were normalized in the double-mutant mice, indicating a possible involvement of vitamin D in maintaining normal RBC size. To our knowledge, there are no data available directly linking vitamin D to RBC mass, and the role of 1,25-dihydroxyvitamin D3 in the proliferation of erythroid precursors has been controversial.85,86 Moreover, studies have shown that increased intracellular calcium levels in circulating RBCs result in a decrease in RBC volume.87 This is caused by activation of calcium channels present in RBCs, leading to RBC membrane deformation and a subsequent increase in Ca2+ permeability, inducing significant cell dehydration and cell shrinkage.87 Therefore, it is possible that the effect on MCV in Klotho−/− mice is mediated by the hypercalcemia that these mice exhibit. Further studies are needed to determine whether calcium plays a role in the effects of klotho on erythropoiesis. In addition, genetic ablation of 1α(OH)ase resulted in normal numbers of BM hematopoietic progenitor cells (LSK; Lin−Sca-1+cKit+) compared with Klotho−/− mice, suggesting that high vitamin D levels associated with klotho deficiency may be responsible for the decline in HSC numbers.
The prevalence of anemia in patients with CKD is mainly attributed to reduced renal Epo production. Klotho expression is also reduced with kidney disease progression.18 However, we found that klotho deficiency in mice is associated with up-regulation in renal Epo production. It has been shown that Epo levels are increased with aging, as a compensation for increased erythrocyte turnover.88,89 With advanced aging, however, this compensatory mechanism eventually becomes inadequate, leading to the development of anemia.89 Moreover, previous studies have demonstrated that in the aging kidney, HIF signaling is activated by hypoxia, resulting in the coordinated induction of HIF-regulated genes, such as Epo and Glut-1.54 Therefore, the aging-related changes in Klotho-null mice may be partially responsible for the elevated Epo production and increased erythropoiesis in these mice. Furthermore, patients with CKD exhibit low serum levels of vitamin D,90 whereas Klotho-null mice have significantly elevated vitamin D levels.59 It has been shown that 1,25-dihydroxyvitamin D3 induces up-regulation of Epo-R expression followed by stimulation of the growth and proliferation of erythroid precursor cells.91 In addition, it has been previously reported that the hematon fraction of normal BM, a low-density floating layer that constitutes the most productive erythropoietic compartment, contains significantly high active vitamin D content.92 Therefore, it would be tempting to speculate that excess vitamin D in Klotho−/− mice may have also induced erythroid activity. However, the present finding that increased erythropoiesis in Klotho−/− mice was not affected by genetic ablation of 1α(OH)ase suggests that it is most likely a vitamin D–independent process. Further studies are required to address the exact mechanism of enhanced erythropoiesis associated with klotho deficiency.
In addition, exogenous administration of klotho resulted in hematopoietic changes opposite to the ones observed in Klotho-deficient mice. Based on these observations, the present study demonstrates for the first time that klotho directly regulates HSC differentiation and erythroid cell generation and maturation. Moreover, we observed that klotho deficiency affected the prenatal fetal liver erythroid compartment in a similar manner, clearly suggesting a novel function for klotho in the regulation of hematopoiesis beyond the skeletal abnormalities and senescence features ascribed to its deficiency. Embryonic hematopoiesis begins in the yolk sac and moves to the aorta-gonad-mesonephros region, followed by the fetal liver, which becomes the primary site of fetal hematopoiesis.93,94 The present findings that klotho expression is detected in fetal liver cells from WT mice and that loss of klotho results in hematopoietic changes in fetal liver similar to the ones observed in adult mice are crucial. These data strongly suggest that aberrant production or function of HSCs in Klotho-null mice occurs before translocation of HSCs from fetal liver to BM, linking klotho directly to the development of hematopoiesis. Given that fetal liver does not generate HSCs but rather serves as a remarkable organ for HSC expansion, the changes we confront may be due to events upstream of the fetal liver, suggesting an exciting possibility of klotho having a function in embryonic hematopoietic development. Additional studies are required to determine the role of klotho in migration and colonization of the fetal liver and the mechanisms of action of klotho in prenatal hematopoiesis.
In summary, the present observations show that klotho deficiency not only plays a role in aging and aging-related conditions, such as CKD, CVD, and osteopenia, but also severely affects hematopoietic development, suggesting that the therapeutic manipulation of klotho has considerable clinical implications. Taken as a whole, this study identifies klotho as a potent regulator of prenatal and postnatal hematopoiesis and provides clear evidence that it plays an essential role in hematopoietic development. The findings herein extend our knowledge of the processes participating in establishment of the HSC niche and provide a better understanding of the connections among renal function, bone development, hematopoiesis, and aging. Moreover, therapeutic targeting of klotho and its associated signaling components may serve as a mechanism to boost immune functions and treat blood cell disorders associated with aging or kidney disorders.
Acknowledgments
We thank Dr. David Levy and Eric Ohlson (New York University College of Dentistry, New York, NY) for assistance with use of the flow cytometer, Rick DeFrancisco and Dr. Tracy Stokol (Cornell University College of Veterinary Medicine, Ithaca, NY) for advice on blood sample analysis, and Manoj Kumar (Polytechnic Institute of New York University, Brooklyn, NY) for help with the experiments.
Footnotes
Supported in part by NIH grant P30DE020754 (to Dr. Nicola Partridge; a portion was used to support D.S.).
Disclosures: None declared.
Supplemental Data
A: Klotho mRNA expression in WT mouse tissues. RNA (1 μg) was reverse transcribed and amplified by real-time quantitative RT-PCR using specific primers for klotho and HPRT (as an internal control). Klotho mRNA expression in the kidney (n = 3), BM (n = 3), spleen (n = 3), and fetal liver (n = 3) is represented as the fold change relative to HPRT. B and C: Graphic representation of flow cytometry analysis of the intermediate erythroid populations Ery-A (WT, n = 10; klotho−/−, n = 9) (B) and Ery-B (WT, n = 10; klotho−/−, n = 7) (C) in the BM of 6-week-old WT and klotho−/− mice. D: Spleen weight to body weight ratio of 6-week-old WT (n = 7) and klotho−/− (n = 7) mice. E: Number of cells counted in homogenized spleens from 6-week-old WT (n = 7) and klotho−/− (n = 6) mice. F: Quantitative PCR analysis of HIF-1α gene expression in kidney (WT, n = 6; klotho−/−, n = 5), liver (WT, n = 5; klotho−/−, n = 4), and BM (WT, n = 7; klotho−/−, n = 6) from 6-week-old WT and klotho−/− mice. Data represent the means ± SEM. ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001.
A–C: Flow cytometry profiles of peripheral blood (PB) (A), spleen (B), and BM (C) 20 hours after transplantation of WT or klotho−/− BM cells (CD45.2; Ly5.2) into nonirradiated B6.SJL (CD45.1; Ly5.1) recipient mice. SSC, side scatter. D: Graphic representation of flow cytometry (n = 5 for each set). E: Number of cells counted in homogenized fetal livers (FL) from E15.5 WT (n = 6) and klotho−/− (n = 7) mice. Data represent the means ± SEM. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗∗P < 0.0001.
References
- 1.Henry C.J., Marusyk A., DeGregori J. Aging-associated changes in hematopoiesis and leukemogenesis: what's the connection? Aging (Albany NY) 2011;3:643–656. doi: 10.18632/aging.100351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothstein G. Disordered hematopoiesis and myelodysplasia in the elderly. J Am Geriatr Soc. 2003;51:S22–S26. doi: 10.1046/j.1532-5415.51.3s.3.x. [DOI] [PubMed] [Google Scholar]
- 3.Chang B., Kim J., Jeong D., Jeong Y., Jeon S., Jung S.I., Yang Y., Kim K.I., Lim J.S., Kim C., Lee M.S. Klotho inhibits the capacity of cell migration and invasion in cervical cancer. Oncol Rep. 2012;28:1022–1028. doi: 10.3892/or.2012.1865. [DOI] [PubMed] [Google Scholar]
- 4.Maekawa Y., Ohishi M., Ikushima M., Yamamoto K., Yasuda O., Oguro R., Yamamoto-Hanasaki H., Tatara Y., Takeya Y., Rakugi H. Klotho protein diminishes endothelial apoptosis and senescence via a mitogen-activated kinase pathway. Geriatr Gerontol Int. 2011;11:510–516. doi: 10.1111/j.1447-0594.2011.00699.x. [DOI] [PubMed] [Google Scholar]
- 5.Olauson H., Lindberg K., Amin R., Jia T., Wernerson A., Andersson G., Larsson T.E. Targeted deletion of Klotho in kidney distal tubule disrupts mineral metabolism. J Am Soc Nephrol. 2012;23:1641–1651. doi: 10.1681/ASN.2012010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuro-o M., Matsumura Y., Aizawa H., Kawaguchi H., Suga T., Utsugi T., Ohyama Y., Kurabayashi M., Kaname T., Kume E., Iwasaki H., Iida A., Shiraki-Iida T., Nishikawa S., Nagai R., Nabeshima Y.I. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 7.Bloch L., Sineshchekova O., Reichenbach D., Reiss K., Saftig P., Kuro-o M., Kaether C. Klotho is a substrate for alpha-, beta- and gamma-secretase. FEBS Lett. 2009;583:3221–3224. doi: 10.1016/j.febslet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C.D., Podvin S., Gillespie E., Leeman S.E., Abraham C.R. Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A. 2007;104:19796–19801. doi: 10.1073/pnas.0709805104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hu M.C., Shi M., Zhang J., Pastor J., Nakatani T., Lanske B., Razzaque M.S., Rosenblatt K.P., Baum M.G., Kuro-o M., Moe O.W. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J. 2010;24:3438–3450. doi: 10.1096/fj.10-154765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Imura A., Iwano A., Tohyama O., Tsuji Y., Nozaki K., Hashimoto N., Fujimori T., Nabeshima Y. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–147. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 11.Kurosu H., Ogawa Y., Miyoshi M., Yamamoto M., Nandi A., Rosenblatt K.P., Baum M.G., Schiavi S., Hu M.C., Moe O.W., Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin A., David V., Quarles L.D. Regulation and function of the FGF23/klotho endocrine pathways. Physiol Rev. 2012;92:131–155. doi: 10.1152/physrev.00002.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakatani T., Sarraj B., Ohnishi M., Densmore M.J., Taguchi T., Goetz R., Mohammadi M., Lanske B., Razzaque M.S. In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (Fgf23)-mediated regulation of systemic phosphate homeostasis. FASEB J. 2009;23:433–441. doi: 10.1096/fj.08-114397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urakawa I., Yamazaki Y., Shimada T., Iijima K., Hasegawa H., Okawa K., Fujita T., Fukumoto S., Yamashita T. Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature. 2006;444:770–774. doi: 10.1038/nature05315. [DOI] [PubMed] [Google Scholar]
- 15.Hruska K.A., Teitelbaum S.L. Renal osteodystrophy. N Engl J Med. 1995;333:166–174. doi: 10.1056/NEJM199507203330307. [DOI] [PubMed] [Google Scholar]
- 16.Mazzaferro S., Pasquali M., Pirro G., Rotondi S., Tartaglione L. The bone and the kidney. Arch Biochem Biophys. 2010;503:95–102. doi: 10.1016/j.abb.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 17.Lim K., Lu T.S., Molostvov G., Lee C., Lam F.T., Zehnder D., Hsiao L.L. Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation. 2012;125:2243–2255. doi: 10.1161/CIRCULATIONAHA.111.053405. [DOI] [PubMed] [Google Scholar]
- 18.Hu M.C., Shi M., Zhang J., Quinones H., Griffith C., Kuro-o M., Moe O.W. Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol. 2011;22:124–136. doi: 10.1681/ASN.2009121311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fliser D., Kollerits B., Neyer U., Ankerst D.P., Lhotta K., Lingenhel A., Ritz E., Kronenberg F., Kuen E., Konig P., Kraatz G., Mann J.F., Muller G.A., Kohler H., Riegler P. Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: the Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol. 2007;18:2600–2608. doi: 10.1681/ASN.2006080936. [DOI] [PubMed] [Google Scholar]
- 20.Gutierrez O., Isakova T., Rhee E., Shah A., Holmes J., Collerone G., Juppner H., Wolf M. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16:2205–2215. doi: 10.1681/ASN.2005010052. [DOI] [PubMed] [Google Scholar]
- 21.Isakova T., Xie H., Yang W., Xie D., Anderson A.H., Scialla J., Wahl P., Gutierrez O.M., Steigerwalt S., He J., Schwartz S., Lo J., Ojo A., Sondheimer J., Hsu C.Y., Lash J., Leonard M., Kusek J.W., Feldman H.I., Wolf M. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305:2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsson T., Nisbeth U., Ljunggren O., Juppner H., Jonsson K.B. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- 23.Lipkin G.W., Kendall R.G., Russon L.J., Turney J.H., Norfolk D.R., Brownjohn A.M. Erythropoietin deficiency in acute renal failure. Nephrol Dial Transplant. 1990;5:920–922. doi: 10.1093/ndt/5.11.920. [DOI] [PubMed] [Google Scholar]
- 24.Zhang F., Laneuville P., Gagnon R.F., Morin B., Brox A.G. Effect of chronic renal failure on the expression of erythropoietin message in a murine model. Exp Hematol. 1996;24:1469–1474. [PubMed] [Google Scholar]
- 25.Forster R.E., Jurutka P.W., Hsieh J.C., Haussler C.A., Lowmiller C.L., Kaneko I., Haussler M.R., Kerr Whitfield G. Vitamin D receptor controls expression of the anti-aging klotho gene in mouse and human renal cells. Biochem Biophys Res Commun. 2011;414:557–562. doi: 10.1016/j.bbrc.2011.09.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kawaguchi H., Manabe N., Miyaura C., Chikuda H., Nakamura K., Kuro-o M. Independent impairment of osteoblast and osteoclast differentiation in klotho mouse exhibiting low-turnover osteopenia. J Clin Invest. 1999;104:229–237. doi: 10.1172/JCI5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohnishi M., Nakatani T., Lanske B., Razzaque M.S. In vivo genetic evidence for suppressing vascular and soft-tissue calcification through the reduction of serum phosphate levels, even in the presence of high serum calcium and 1,25-dihydroxyvitamin d levels. Circ Cardiovasc Genet. 2009;2:583–590. doi: 10.1161/CIRCGENETICS.108.847814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kurosu H., Yamamoto M., Clark J.D., Pastor J.V., Nandi A., Gurnani P., McGuinness O.P., Chikuda H., Yamaguchi M., Kawaguchi H., Shimomura I., Takayama Y., Herz J., Kahn C.R., Rosenblatt K.P., Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuro-o M. Klotho as a regulator of oxidative stress and senescence. Biol Chem. 2008;389:233–241. doi: 10.1515/BC.2008.028. [DOI] [PubMed] [Google Scholar]
- 30.Hosokawa K., Arai F., Yoshihara H., Nakamura Y., Gomei Y., Iwasaki H., Miyamoto K., Shima H., Ito K., Suda T. Function of oxidative stress in the regulation of hematopoietic stem cell-niche interaction. Biochem Biophys Res Commun. 2007;363:578–583. doi: 10.1016/j.bbrc.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Lepperdinger G. Inflammation and mesenchymal stem cell aging. Curr Opin Immunol. 2011;23:518–524. doi: 10.1016/j.coi.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okada S., Yoshida T., Hong Z., Ishii G., Hatano M., Kuro-o M., Nabeshima Y., Nabeshima Y., Tokuhisa T. Impairment of B lymphopoiesis in precocious aging (klotho) mice. Int Immunol. 2000;12:861–871. doi: 10.1093/intimm/12.6.861. [DOI] [PubMed] [Google Scholar]
- 33.Witkowski J.M., Soroczynska-Cybula M., Bryl E., Smolenska Z., Jozwik A. Klotho: a common link in physiological and rheumatoid arthritis-related aging of human CD4(+) lymphocytes. J Immunol. 2007;178:771–777. doi: 10.4049/jimmunol.178.2.771. [DOI] [PubMed] [Google Scholar]
- 34.Mendez-Ferrer S., Michurina T.V., Ferraro F., Mazloom A.R., Macarthur B.D., Lira S.A., Scadden D.T., Ma'ayan A., Enikolopov G.N., Frenette P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen Y., Nilsson S.K. Bone, microenvironment and hematopoiesis. Curr Opin Hematol. 2012;19:250–255. doi: 10.1097/MOH.0b013e328353c714. [DOI] [PubMed] [Google Scholar]
- 36.Arai F., Suda T. Regulation of hematopoietic stem cells in the osteoblastic niche. Adv Exp Med Biol. 2007;602:61–67. doi: 10.1007/978-0-387-72009-8_8. [DOI] [PubMed] [Google Scholar]
- 37.Calvi L.M., Adams G.B., Weibrecht K.W., Weber J.M., Olson D.P., Knight M.C., Martin R.P., Schipani E., Divieti P., Bringhurst F.R., Milner L.A., Kronenberg H.M., Scadden D.T. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 38.Visnjic D., Kalajzic Z., Rowe D.W., Katavic V., Lorenzo J., Aguila H.L. Hematopoiesis is severely altered in mice with an induced osteoblast deficiency. Blood. 2004;103:3258–3264. doi: 10.1182/blood-2003-11-4011. [DOI] [PubMed] [Google Scholar]
- 39.Zhu J., Emerson S.G. A new bone to pick: osteoblasts and the haematopoietic stem-cell niche. Bioessays. 2004;26:595–599. doi: 10.1002/bies.20052. [DOI] [PubMed] [Google Scholar]
- 40.Cho K.A., Joo S.Y., Han H.S., Ryu K.H., Woo S.Y. Osteoclast activation by receptor activator of NF-kappaB ligand enhances the mobilization of hematopoietic progenitor cells from the bone marrow in acute injury. Int J Mol Med. 2010;26:557–563. doi: 10.3892/ijmm_00000499. [DOI] [PubMed] [Google Scholar]
- 41.Kollet O., Dar A., Shivtiel S., Kalinkovich A., Lapid K., Sztainberg Y., Tesio M., Samstein R.M., Goichberg P., Spiegel A., Elson A., Lapidot T. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 42.Levesque J.P., Helwani F.M., Winkler I.G. The endosteal 'osteoblastic' niche and its role in hematopoietic stem cell homing and mobilization. Leukemia. 2010;24:1979–1992. doi: 10.1038/leu.2010.214. [DOI] [PubMed] [Google Scholar]
- 43.Lymperi S., Ersek A., Ferraro F., Dazzi F., Horwood N.J. Inhibition of osteoclast function reduces hematopoietic stem cell numbers in vivo. Blood. 2011;117:1540–1549. doi: 10.1182/blood-2010-05-282855. [DOI] [PubMed] [Google Scholar]
- 44.Mansour A., Abou-Ezzi G., Sitnicka E., Jacobsen S.E., Wakkach A., Blin-Wakkach C. Osteoclasts promote the formation of hematopoietic stem cell niches in the bone marrow. J Exp Med. 2012;209:537–549. doi: 10.1084/jem.20110994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Adams G.B., Chabner K.T., Alley I.R., Olson D.P., Szczepiorkowski Z.M., Poznansky M.C., Kos C.H., Pollak M.R., Brown E.M., Scadden D.T. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- 46.Jeanson N.T., Scadden D.T. Vitamin D receptor deletion leads to increased hematopoietic stem and progenitor cells residing in the spleen. Blood. 2010;116:4126–4129. doi: 10.1182/blood-2010-04-280552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang H.T., Zon L.I. Regulation of stem cells in the zebra fish hematopoietic system. Cold Spring Harb Symp Quant Biol. 2008;73:111–118. doi: 10.1101/sqb.2008.73.029. [DOI] [PubMed] [Google Scholar]
- 48.Jin H., Xu J., Wen Z. Migratory path of definitive hematopoietic stem/progenitor cells during zebrafish development. Blood. 2007;109:5208–5214. doi: 10.1182/blood-2007-01-069005. [DOI] [PubMed] [Google Scholar]
- 49.Murayama E., Kissa K., Zapata A., Mordelet E., Briolat V., Lin H.F., Handin R.I., Herbomel P. Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity. 2006;25:963–975. doi: 10.1016/j.immuni.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 50.Koulnis M., Pop R., Porpiglia E., Shearstone J.R., Hidalgo D., Socolovsky M. Identification and analysis of mouse erythroid progenitors using the CD71/TER119 flow-cytometric assay. J Vis Exp. 2011;54 doi: 10.3791/2809. pii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li S.A., Watanabe M., Yamada H., Nagai A., Kinuta M., Takei K. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct. 2004;29:91–99. doi: 10.1247/csf.29.91. [DOI] [PubMed] [Google Scholar]
- 52.Ohata Y., Arahori H., Namba N., Kitaoka T., Hirai H., Wada K., Nakayama M., Michigami T., Imura A., Nabeshima Y., Yamazaki Y., Ozono K. Circulating levels of soluble alpha-Klotho are markedly elevated in human umbilical cord blood. J Clin Endocrinol Metab. 2011;96:E943–E947. doi: 10.1210/jc.2010-2357. [DOI] [PubMed] [Google Scholar]
- 53.Wang Y., Sun Z. Current understanding of klotho. Ageing Res Rev. 2009;8:43–51. doi: 10.1016/j.arr.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanaka T., Kato H., Kojima I., Ohse T., Son D., Tawakami T., Yatagawa T., Inagi R., Fujita T., Nangaku M. Hypoxia and expression of hypoxia-inducible factor in the aging kidney. J Gerontol A Biol Sci Med Sci. 2006;61:795–805. doi: 10.1093/gerona/61.8.795. [DOI] [PubMed] [Google Scholar]
- 55.Kapitsinou P.P., Liu Q., Unger T.L., Rha J., Davidoff O., Keith B., Epstein J.A., Moores S.L., Erickson-Miller C.L., Haase V.H. Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood. 2010;116:3039–3048. doi: 10.1182/blood-2010-02-270322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Colvin G.A., Lambert J.F., Dooner M.S., Cerny J., Quesenberry P.J. Murine allogeneic in vivo stem cell homing(,) J Cell Physiol. 2007;211:386–391. doi: 10.1002/jcp.20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Plett P.A., Frankovitz S.M., Orschell-Traycoff C.M. In vivo trafficking, cell cycle activity, and engraftment potential of phenotypically defined primitive hematopoietic cells after transplantation into irradiated or nonirradiated recipients. Blood. 2002;100:3545–3552. doi: 10.1182/blood.V100.10.3545. [DOI] [PubMed] [Google Scholar]
- 58.Wang Z., Bunting K.D. Hematopoietic stem cell transplant into non-myeloablated W/Wv mice to detect steady-state engraftment defects. Methods Mol Biol. 2008;430:171–181. doi: 10.1007/978-1-59745-182-6_12. [DOI] [PubMed] [Google Scholar]
- 59.Ohnishi M., Nakatani T., Lanske B., Razzaque M.S. Reversal of mineral ion homeostasis and soft-tissue calcification of klotho knockout mice by deletion of vitamin D 1alpha-hydroxylase. Kidney Int. 2009;75:1166–1172. doi: 10.1038/ki.2009.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liang Y., Van Zant G., Szilvassy S.J. Effects of aging on the homing and engraftment of murine hematopoietic stem and progenitor cells. Blood. 2005;106:1479–1487. doi: 10.1182/blood-2004-11-4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Linton P.J., Dorshkind K. Age-related changes in lymphocyte development and function. Nat Immunol. 2004;5:133–139. doi: 10.1038/ni1033. [DOI] [PubMed] [Google Scholar]
- 62.Miller J.P., Allman D. Linking age-related defects in B lymphopoiesis to the aging of hematopoietic stem cells. Semin Immunol. 2005;17:321–329. doi: 10.1016/j.smim.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 63.Morrison S.J., Wandycz A.M., Akashi K., Globerson A., Weissman I.L. The aging of hematopoietic stem cells. Nat Med. 1996;2:1011–1016. doi: 10.1038/nm0996-1011. [DOI] [PubMed] [Google Scholar]
- 64.Sharpless N.E., DePinho R.A. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8:703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- 65.Sudo K., Ema H., Morita Y., Nakauchi H. Age-associated characteristics of murine hematopoietic stem cells. J Exp Med. 2000;192:1273–1280. doi: 10.1084/jem.192.9.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Warren L.A., Rossi D.J. Stem cells and aging in the hematopoietic system. Mech Ageing Dev. 2009;130:46–53. doi: 10.1016/j.mad.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kuro O.M. Phosphate and Klotho. Kidney Int Suppl. 2011;121:S20–S23. doi: 10.1038/ki.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Torres P.U., Prie D., Molina-Bletry V., Beck L., Silve C., Friedlander G. Klotho: an antiaging protein involved in mineral and vitamin D metabolism. Kidney Int. 2007;71:730–737. doi: 10.1038/sj.ki.5002163. [DOI] [PubMed] [Google Scholar]
- 69.Suzuki H., Amizuka N., Oda K., Noda M., Ohshima H., Maeda T. Histological and elemental analyses of impaired bone mineralization in klotho-deficient mice. J Anat. 2008;212:275–285. doi: 10.1111/j.1469-7580.2008.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Paredes-Gamero E.J., Leon C.M., Borojevic R., Oshiro M.E., Ferreira A.T. Changes in intracellular Ca2+ levels induced by cytokines and P2 agonists differentially modulate proliferation or commitment with macrophage differentiation in murine hematopoietic cells. J Biol Chem. 2008;283:31909–31919. doi: 10.1074/jbc.M801990200. [DOI] [PubMed] [Google Scholar]
- 71.Chang W.H., Tu C., Chen T.H., Bikle D., Shoback D. The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Sci Signal. 2008;1:1–13. doi: 10.1126/scisignal.1159945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu J.Y., Purton L.E., Rodda S.J., Chen M., Weinstein L.S., McMahon A.P., Scadden D.T., Kronenberg H.M. Osteoblastic regulation of B lymphopoiesis is mediated by Gs{alpha}-dependent signaling pathways. Proc Natl Acad Sci U S A. 2008;105:16976–16981. doi: 10.1073/pnas.0802898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhu J., Garrett R., Jung Y., Zhang Y., Kirn N., Wang J., Joe G.J., Hexner E., Choi Y., Taichman R.S., Emerson S.G. Osteoblasts support B-lymphocyte commitment and differentiation from hematopoietic stem cells. Blood. 2007;109:3706–3712. doi: 10.1182/blood-2006-08-041384. [DOI] [PubMed] [Google Scholar]
- 74.Mansour A., Anginot A., Mancini S.J., Schiff C., Carle G.F., Wakkach A., Blin-Wakkach C. Osteoclast activity modulates B-cell development in the bone marrow. Cell Res. 2011;21:1102–1115. doi: 10.1038/cr.2011.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sweeney E., Roberts D., Jacenko O. Altered matrix at the chondro-osseous junction leads to defects in lymphopoiesis. Ann N Y Acad Sci. 2011;1237:79–87. doi: 10.1111/j.1749-6632.2011.06227.x. [DOI] [PubMed] [Google Scholar]
- 76.Sweeney E., Roberts D., Lin A., Guldberg R., Jacenko O. Defective endochondral ossification-derived matrix and bone cells alter the lymphopoietic niche in collagen x mouse models. Stem Cells Dev. 2013;22:2581–2595. doi: 10.1089/scd.2012.0387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haase V.H. Hypoxic regulation of erythropoiesis and iron metabolism. Am J Physiol Renal Physiol. 2010;299:F1–F13. doi: 10.1152/ajprenal.00174.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jelkmann W. Regulation of erythropoietin production. J Physiol. 2011;589:1251–1258. doi: 10.1113/jphysiol.2010.195057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sugiura H., Yoshida T., Mitobe M., Shiohira S., Nitta K., Tsuchiya K. Recombinant human erythropoietin mitigates reductions in renal klotho expression. Am J Nephrol. 2010;32:137–144. doi: 10.1159/000315864. [DOI] [PubMed] [Google Scholar]
- 80.Rankin E.B., Wu C., Khatri R., Wilson T.L., Andersen R., Araldi E., Rankin A.L., Yuan J., Kuo C.J., Schipani E., Giaccia A.J. The HIF signaling pathway in osteoblasts directly modulates erythropoiesis through the production of EPO. Cell. 2012;149:63–74. doi: 10.1016/j.cell.2012.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Arai F., Suda T. Maintenance of quiescent hematopoietic stem cells in the osteoblastic niche. Ann N Y Acad Sci. 2007;1106:41–53. doi: 10.1196/annals.1392.005. [DOI] [PubMed] [Google Scholar]
- 82.Rossi D.J., Bryder D., Weissman I.L. Hematopoietic stem cell aging: mechanism and consequence. Exp Gerontol. 2007;42:385–390. doi: 10.1016/j.exger.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Waterstrat A., Van Zant G. Effects of aging on hematopoietic stem and progenitor cells. Curr Opin Immunol. 2009;21:408–413. doi: 10.1016/j.coi.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 84.Anour R., Andrukhova O., Ritter E., Zeitz U., Erben R.G. Klotho lacks a vitamin D independent physiological role in glucose homeostasis, bone turnover, and steady-state PTH secretion in vivo. PLoS One. 2012;7:e31376. doi: 10.1371/journal.pone.0031376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Aucella F., Gatta G., Vigilante M., Scalzulli R.P., Mantuano S., Carotenuto M., Stallone C. Calcitriol increases burst forming unit-erythroid (BFU-E) in vitro proliferation in chronic uremia: synergic effect with DNA recombinant erythropoietin (rHu-Epo) Minerva Urol Nefrol. 2001;53:1–5. [PubMed] [Google Scholar]
- 86.Moore D.C., Carter D.L., Bhandal A.K., Studzinski G.P. Inhibition by 1,25 dihydroxyvitamin D3 of chemically induced erythroid differentiation of K562 leukemia cells. Blood. 1991;77:1452–1461. [PubMed] [Google Scholar]
- 87.Bogdanova A., Makhro A., Wang J., Lipp P., Kaestner L. Calcium in red blood cells: a perilous balance. Int J Mol Sci. 2013;14:9848–9872. doi: 10.3390/ijms14059848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Benderro G.F., LaManna J.C. Kidney EPO expression during chronic hypoxia in aged mice. Adv Exp Med Biol. 2013;765:9–14. doi: 10.1007/978-1-4614-4989-8_2. [DOI] [PubMed] [Google Scholar]
- 89.Ershler W.B., Sheng S., McKelvey J., Artz A.S., Denduluri N., Tecson J., Taub D.D., Brant L.J., Ferrucci L., Longo D.L. Serum erythropoietin and aging: a longitudinal analysis. J Am Geriatr Soc. 2005;53:1360–1365. doi: 10.1111/j.1532-5415.2005.53416.x. [DOI] [PubMed] [Google Scholar]
- 90.Gal-Moscovici A., Sprague S.M. Role of vitamin D deficiency in chronic kidney disease. J Bone Miner Res. 2007;22(Suppl 2):V91–V94. doi: 10.1359/jbmr.07s203. [DOI] [PubMed] [Google Scholar]
- 91.Alon D.B., Chaimovitz C., Dvilansky A., Lugassy G., Douvdevani A., Shany S., Nathan I. Novel role of 1,25(OH)(2)D(3) in induction of erythroid progenitor cell proliferation. Exp Hematol. 2002;30:403–409. doi: 10.1016/s0301-472x(02)00789-0. [DOI] [PubMed] [Google Scholar]
- 92.Blazsek I., Farabos C., Quittet P., Labat M.L., Bringuier A.F., Triana B.K., Machover D., Reynes M., Misset J.L. Bone marrow stromal cell defects and 1 alpha,25-dihydroxyvitamin D3 deficiency underlying human myeloid leukemias. Cancer Detect Prev. 1996;20:31–42. [PubMed] [Google Scholar]
- 93.Dzierzak E., Speck N.A. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol. 2008;9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sugiyama D., Inoue-Yokoo T., Fraser S.T., Kulkeaw K., Mizuochi C., Horio Y. Embryonic regulation of the mouse hematopoietic niche. ScientificWorldJournal. 2011;11:1770–1780. doi: 10.1100/2011/598097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A: Klotho mRNA expression in WT mouse tissues. RNA (1 μg) was reverse transcribed and amplified by real-time quantitative RT-PCR using specific primers for klotho and HPRT (as an internal control). Klotho mRNA expression in the kidney (n = 3), BM (n = 3), spleen (n = 3), and fetal liver (n = 3) is represented as the fold change relative to HPRT. B and C: Graphic representation of flow cytometry analysis of the intermediate erythroid populations Ery-A (WT, n = 10; klotho−/−, n = 9) (B) and Ery-B (WT, n = 10; klotho−/−, n = 7) (C) in the BM of 6-week-old WT and klotho−/− mice. D: Spleen weight to body weight ratio of 6-week-old WT (n = 7) and klotho−/− (n = 7) mice. E: Number of cells counted in homogenized spleens from 6-week-old WT (n = 7) and klotho−/− (n = 6) mice. F: Quantitative PCR analysis of HIF-1α gene expression in kidney (WT, n = 6; klotho−/−, n = 5), liver (WT, n = 5; klotho−/−, n = 4), and BM (WT, n = 7; klotho−/−, n = 6) from 6-week-old WT and klotho−/− mice. Data represent the means ± SEM. ∗∗P < 0.01, ∗∗∗P < 0.001, and ∗∗∗∗P < 0.0001.
A–C: Flow cytometry profiles of peripheral blood (PB) (A), spleen (B), and BM (C) 20 hours after transplantation of WT or klotho−/− BM cells (CD45.2; Ly5.2) into nonirradiated B6.SJL (CD45.1; Ly5.1) recipient mice. SSC, side scatter. D: Graphic representation of flow cytometry (n = 5 for each set). E: Number of cells counted in homogenized fetal livers (FL) from E15.5 WT (n = 6) and klotho−/− (n = 7) mice. Data represent the means ± SEM. ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗∗P < 0.0001.