Abstract
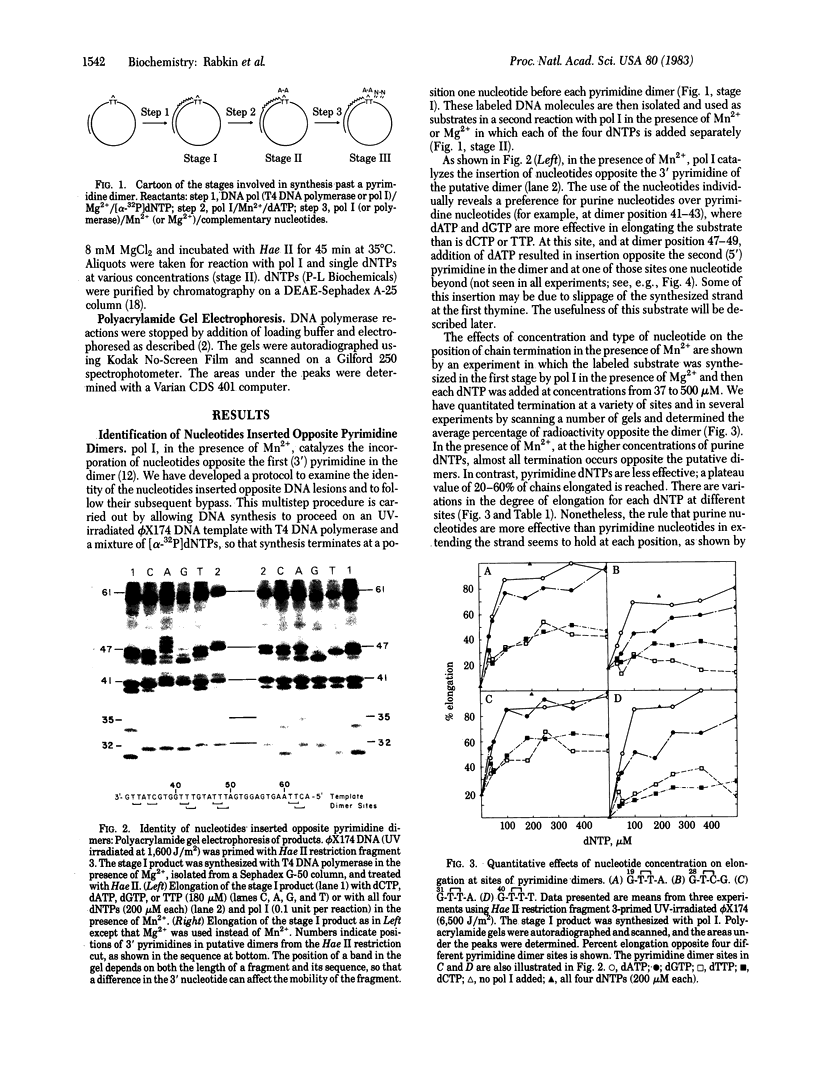
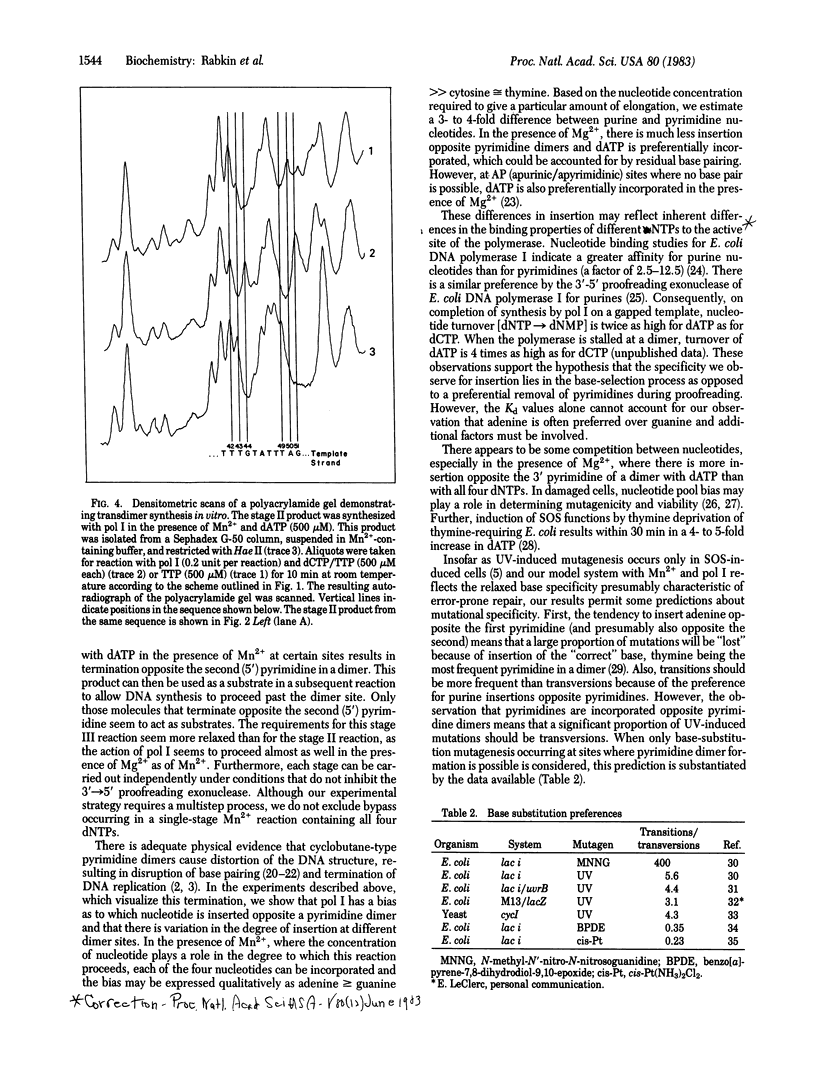
A variety of DNA polymerases, synthesizing in vitro on an UV-irradiated phi X174 DNA template, terminate synthesis one nucleotide before the 3' pyrimidines of putative dimers on the template. We have devised a system using Escherichia coli DNA polymerase I (Klenow fragment) that can synthesize past at least some of these dimers. The bypass is carried out in a multistep process--first, the incorporation of nucleotides opposite the pyrimidines in the dimer and, then, the addition of nucleotides complementary to the bases distal to the dimer. The insertion of a nucleotide opposite the first (3') pyrimidine of a putative dimer in the presence of Mn2+ occurs in a concentration-dependent fashion with a 3- to 4-fold preference for purine nucleotides over pyrimidine nucleotides. In the presence of Mg2+, insertion is less frequent. Correlation of these results with in vivo mutation data suggests a role for the polymerase in determining the spectrum of base substitution mutagenesis in SOS induced cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brouwer J., van de Putte P., Fichtinger-Schepman A. M., Reedijk J. Base-pair substitution hotspots in GAG and GCG nucleotide sequences in Escherichia coli K-12 induced by cis-diamminedichloroplatinum (II). Proc Natl Acad Sci U S A. 1981 Nov;78(11):7010–7014. doi: 10.1073/pnas.78.11.7010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciarrocchi G., Pedrini A. M. Determination of pyrimidine dimer unwinding angle by measurement of DNA electrophoretic mobility. J Mol Biol. 1982 Feb 25;155(2):177–183. doi: 10.1016/0022-2836(82)90445-4. [DOI] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H. Genetic studies of the lac repressor. IV. Mutagenic specificity in the lacI gene of Escherichia coli. J Mol Biol. 1977 Dec 15;117(3):577–606. doi: 10.1016/0022-2836(77)90059-6. [DOI] [PubMed] [Google Scholar]
- Drake J. W., Baltz R. H. The biochemistry of mutagenesis. Annu Rev Biochem. 1976;45:11–37. doi: 10.1146/annurev.bi.45.070176.000303. [DOI] [PubMed] [Google Scholar]
- Eisenstadt E., Warren A. J., Porter J., Atkins D., Miller J. H. Carcinogenic epoxides of benzo[a]pyrene and cyclopenta[cd]pyrene induce base substitutions via specific transversions. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1945–1949. doi: 10.1073/pnas.79.6.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund P. T., Huberman J. A., Jovin T. M., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXX. Binding of triphosphates to deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):3038–3044. [PubMed] [Google Scholar]
- Englund P. T. The 3'-terminal nucleotide sequences of T7 DNA. J Mol Biol. 1972 May 14;66(2):209–224. doi: 10.1016/0022-2836(72)90474-3. [DOI] [PubMed] [Google Scholar]
- Ganesan A. K. Persistence of pyrimidine dimers during post-replication repair in ultraviolet light-irradiated Escherichia coli K12. J Mol Biol. 1974 Jul 25;87(1):103–119. doi: 10.1016/0022-2836(74)90563-4. [DOI] [PubMed] [Google Scholar]
- Hayes F. N., Williams D. L., Ratlift R. L., Varghese A. J., Rupert C. S. Effect of a single thymine photodimer on the oligodeoxythymidylate-polydeoxyadenylate interaction. J Am Chem Soc. 1971 Sep;93(19):4940–4942. doi: 10.1021/ja00748a065. [DOI] [PubMed] [Google Scholar]
- Huberman J. A., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXXV. A 3'-hydroxylribonucleotide binding site of Escherichia coli deoxyribonucleic acid polymerase. J Biol Chem. 1970 Oct 25;245(20):5326–5334. [PubMed] [Google Scholar]
- Johns H. E. Dosimetry in photochemistry. Photochem Photobiol. 1968 Dec;8(6):547–563. doi: 10.1111/j.1751-1097.1968.tb05898.x. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A., Loeb L. A. On the fidelity of DNA replication. Effect of divalent metal ion activators and deoxyrionucleoside triphosphate pools on in vitro mutagenesis. J Biol Chem. 1979 Jul 10;254(13):5718–5725. [PubMed] [Google Scholar]
- Lawrence C. W. Are pyrimidine dimers non-instructive lesions? Mol Gen Genet. 1981;182(3):511–513. doi: 10.1007/BF00293945. [DOI] [PubMed] [Google Scholar]
- Lawrence C. W., Christensen R. B. Absence of relationship between UV-induced reversion frequency and nucleotide sequence at the CYC1 locus of yeast. Mol Gen Genet. 1979;177(1):31–38. doi: 10.1007/BF00267250. [DOI] [PubMed] [Google Scholar]
- LeClerc J. E., Istock N. L. Specificity of UV mutagenesis in the lac promoter of M13lac hybrid phage DNA. Nature. 1982 Jun 17;297(5867):596–598. doi: 10.1038/297596a0. [DOI] [PubMed] [Google Scholar]
- Little J. W., Mount D. W. The SOS regulatory system of Escherichia coli. Cell. 1982 May;29(1):11–22. doi: 10.1016/0092-8674(82)90085-x. [DOI] [PubMed] [Google Scholar]
- Loeb L. A., Kunkel T. A. Fidelity of DNA synthesis. Annu Rev Biochem. 1982;51:429–457. doi: 10.1146/annurev.bi.51.070182.002241. [DOI] [PubMed] [Google Scholar]
- Meuth M. Sensitivity of a mutator gene in Chinese hamster ovary cell to deoxynucleoside triphosphate pool alterations. Mol Cell Biol. 1981 Jul;1(7):652–660. doi: 10.1128/mcb.1.7.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. D., Bose K. K., Rabkin S. D., Strauss B. S. Sites of termination of in vitro DNA synthesis on ultraviolet- and N-acetylaminofluorene-treated phi X174 templates by prokaryotic and eukaryotic DNA polymerases. Proc Natl Acad Sci U S A. 1981 Jan;78(1):110–114. doi: 10.1073/pnas.78.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. D., Rabkin S. D., Strauss B. S. Termination of vitro DNA synthesis at AAF adducts in the DNA. Nucleic Acids Res. 1980 Oct 10;8(19):4473–4484. doi: 10.1093/nar/8.19.4473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhard J., Munch-Petersen A. Studies on the acid-soluble nucleotide pool in thymine-requiring mutants of Escherichia coli during thymine starvation. II. Changes in the amounts of deoxycytidine triphosphate and deoxyadenosine triphosphate in Escherichia coli 15 T-A-U. Biochim Biophys Acta. 1966 Jan 18;114(1):61–71. doi: 10.1016/0005-2787(66)90253-x. [DOI] [PubMed] [Google Scholar]
- Orgel A., Orgel L. E. Induction of mutations in bacteriophage T4 with divalent manganese. J Mol Biol. 1965 Dec;14(2):453–457. doi: 10.1016/s0022-2836(65)80195-4. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Howard-Flanders P. Discontinuities in the DNA synthesized in an excision-defective strain of Escherichia coli following ultraviolet irradiation. J Mol Biol. 1968 Jan 28;31(2):291–304. doi: 10.1016/0022-2836(68)90445-2. [DOI] [PubMed] [Google Scholar]
- Rupp W. D., Wilde C. E., 3rd, Reno D. L., Howard-Flanders P. Exchanges between DNA strands in ultraviolet-irradiated Escherichia coli. J Mol Biol. 1971 Oct 14;61(1):25–44. doi: 10.1016/0022-2836(71)90204-x. [DOI] [PubMed] [Google Scholar]
- SETLOW R. B., SWENSON P. A., CARRIER W. L. THYMINE DIMERS AND INHIBITION OF DNA SYNTHESIS BY ULTRAVIOLET IRRADIATION OF CELLS. Science. 1963 Dec 13;142(3598):1464–1466. doi: 10.1126/science.142.3598.1464. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Friedmann T., Air G. M., Barrell B. G., Brown N. L., Fiddes J. C., Hutchison C. A., 3rd, Slocombe P. M., Smith M. The nucleotide sequence of bacteriophage phiX174. J Mol Biol. 1978 Oct 25;125(2):225–246. doi: 10.1016/0022-2836(78)90346-7. [DOI] [PubMed] [Google Scholar]
- Sedgwick S. G. Misrepair of overlapping daughter strand gaps as a possible mechanism for UV induced mutagenesis in UVR strains of Escherichia coli: a general model for induced mutagenesis by misrepair (SOS repair) of closely spaced DNA lesions. Mutat Res. 1976 Dec;41(2-3):185–200. doi: 10.1016/0027-5107(76)90091-9. [DOI] [PubMed] [Google Scholar]
- Setlow R. B., Carrier W. L. Pyrimidine dimers in ultraviolet-irradiated DNA's. J Mol Biol. 1966 May;17(1):237–254. doi: 10.1016/s0022-2836(66)80105-5. [DOI] [PubMed] [Google Scholar]
- Strauss B., Rabkin S., Sagher D., Moore P. The role of DNA polymerase in base substitution mutagenesis on non-instructional templates. Biochimie. 1982 Aug-Sep;64(8-9):829–838. doi: 10.1016/s0300-9084(82)80138-7. [DOI] [PubMed] [Google Scholar]
- Tabak H. F., Flavell R. A. A method for the recovery of DNA from agarose gels. Nucleic Acids Res. 1978 Jul;5(7):2321–2332. doi: 10.1093/nar/5.7.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd P. A., Glickman B. W. Mutational specificity of UV light in Escherichia coli: indications for a role of DNA secondary structure. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4123–4127. doi: 10.1073/pnas.79.13.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg G., Ullman B., Martin D. W., Jr Mutator phenotypes in mammalian cell mutants with distinct biochemical defects and abnormal deoxyribonucleoside triphosphate pools. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2447–2451. doi: 10.1073/pnas.78.4.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



