Abstract
The specific and direct contribution of the stress-activated serine kinase c-Jun N-terminal kinase (JNK) in the development of oxidative stress-induced insulin resistance of the glucose transport system in mammalian skeletal muscle is not fully understood. We assessed the specific role of JNK in the development of insulin resistance caused by in vitro exposure of rat soleus muscle to low levels (30–40 μM) of the oxidant hydrogen peroxide (H2O2) for up to 6 h. Oxidant exposure caused significant (p < 0.05) decreases in insulin-stimulated glucose transport activity (up to 42%) and Akt Ser473 phosphorylation (up to 67%), and increased (up to 74%) phosphorylation (Thr183/Tyr185) of JNK1 and JNK2/3 isoforms. Importantly, insulin-stimulated glucose transport activity in the presence of H2O2 was moderately improved with the selective JNK inhibitor SP600125. These results indicate that activation of the serine kinase JNK contributes, at least in part, to oxidative stress-induced insulin resistance in isolated mammalian skeletal muscle.
Keywords: Hydrogen peroxide, soleus muscle, glucose transport, Akt serine phosphorylation, SP600125
Introduction
A primary site of impaired insulin action leading to the development of type 2 diabetes is skeletal muscle, affecting mainly the glucose transport system (Abdul-Ghani and DeFronzo, 2010; DeFronzo, 1997, 2004, 2009; DeFronzo and Ferannini, 1991; Henriksen et al., 2011; Zierath et al., 2000). While the aetiology of insulin resistance in skeletal muscle is multi-factorial, the intracellular mechanisms underlying insulin resistance all seem to affect the translocation of GLUT-4-containing vesicles to the plasma membrane by impairing the functionality or protein expression of elements of the canonical insulin signalling pathway (Abdul-Ghani and DeFronzo, 2010; DeFronzo 2004, 2009; Henriksen et al., 2011; Holloszy and Hansen, 1996).
One important cause of insulin resistance in skeletal muscle is oxidative stress, the overproduction of reactive oxygen species (ROS), such as hydrogen peroxide (H2O2) (Davies, 1995; Diamond-Stanic et al. 2011; Evans et al., 2002; Henriksen et al., 2011; Sies, 1997). ROS are normally produced in cells by the mitochondria or by NADPH oxidase, but cellular compensatory mechanisms, such as superoxide dismutases and peroxidases, maintain them at low levels (Davies, 1995; Evans et al., 2002; Sies, 1997). When these antioxidant mechanisms begin to falter, ROS levels increase and oxidative stress occurs. Excess ROS can lead to various cellular dysfunctions, including insulin resistance of the glucose transport system (Henriksen et al., 2011).
Oxidants can engage various stress-activated serine kinases, such as glycogen synthase kinase-3 (GSK-3) and p38 mitogen-activated protein kinase (p38 MAPK), as demonstrated previously in cultured muscles cells (Blair et al., 1999; Cirialdi et al., 2007; Nikoulina et al., 2002) and in isolated mammalian skeletal muscle, such as rat soleus (Diamond-Stanic et al., 2011; Dokken et al., 2008). An additional important stress-activated kinase, c-Jun N-terminal kinase (JNK), has also been shown previously to be associated with oxidative-stress induced insulin resistance (Hirosumi et al., 2002; Solinas and Karin, 2010), but its direct contribution to insulin resistance in isolated mammalian skeletal muscle under highly defined incubation conditions of oxidant excess has not been quantified. When activated, these various serine kinases can phosphorylate numerous substrates, ultimately interfering with or deactivating critical insulin signalling elements, such as IRS-1 and Akt (Archuleta et al., 2009; Blair et al., 1999; Diamond-Stanic et al., 2011; Dokken et al., 2008; Evans et al., 2002; Henriksen et al., 2011).
While the direct contributions of GSK-3 and p38 MAPK to the aetiology of oxidant-induced insulin resistance in mammalian skeletal muscle have been previously demonstrated and quantified (Dokken et al., 2008; Diamond-Stanic et al., 2011), the direct effects of JNK in the development of oxidative stress-induced insulin resistance in mammalian skeletal muscle are not fully understood. Therefore, this study was designed to assess the specific impact of JNK in the development of insulin resistance caused by in vitro exposure of mammalian skeletal muscle to low levels of a known oxidant, H2O2. In the present study, isolated soleus strips from lean Zucker rats with normal insulin sensitivity were used as the model of mammalian skeletal muscle. The effect of in vitro exposure to low levels (30–40 μM) of H2O2 on basal and insulin-stimulated glucose transport activity and Akt functionality were performed to measure the degree of insulin resistance induced by this oxidant. Moreover, the impact of this oxidant stress on the activation of JNK isoforms (JNK1 and JNK2/3) was determined. Finally, a selective JNK inhibitor, the anthrapyrazolone SP600125 (Bennett et al., 2001; Li et al., 2005), was used to assess the specific role of JNK in the development of this oxidant-induced insulin resistance in mammalian skeletal muscle.
Methods
Animals
All procedures used were approved by the Institutional Animal Care and Use Committee at the University of Arizona. Female lean Zucker rats (Harlan, Indianapolis, IN) were used at 6–8 weeks of age (body weights of 130–150 g). Animals were housed in a temperature-controlled (20–22°C) room with a 12:12 h light–dark cycle, and the animals had free access to chow (Teklad 7001, Madison, WI) and water. At 5 pm the evening before each experiment, animals were restricted to 4 g of chow, which was consumed immediately. Experiments commenced the next morning between 8 and 9 am.
Muscle incubations and exposure to H2O2 and SP600125
Animals were deeply anaesthetized with pentobarbital sodium (50 mg/kg), and soleus muscle strips (~25–35 mg) were prepared for in vitro incubation in the unmounted state. Muscles were initially incubated for 2–6 h at 37°C in oxygenated (95% O2/5% CO2) Krebs–Henseleit buffer (KHB) containing 8 mM glucose, 32 mM mannitol, and 0.1% bovine serum albumin (Sigma Chemical, St Louis, MO), with or without 5 mU/ml insulin (Humulin, Eli Lilly, Indianapolis, IN) and/or 50 mU/ml glucose oxidase (MP Biomedicals, Solon, OH). The incubation medium was changed after every 2 h of treatment. The H2O2 level in the medium was measured spectrophotometrically (Diamond-Stanic et al., 2011; Kim et al., 2006) and reached 30–40 μM. In a second set of experiments, muscle strips were incubated for 6 h in the presence or absence of insulin with or without glucose oxidase and/or the selective JNK inhibitor SP600125 (Bennet et al., 2001; Li et al., 2005) (10 μM; EMD Chemicals, Gibbtown, NJ).
Assessment of glucose transport activity
Glucose transport activity was assessed by determination of the intracellular accumulation of 2-deoxyglucose (2-DG, 1 mM) as described previously (Henriksen and Jacob, 1995). Briefly, after the initial incubation period, the muscles were rinsed for 10 min at 37°C in 3 ml of oxygenated KHB containing 40 mM mannitol, 0.1% BSA, and insulin, glucose oxidase, and/or SP600125, if present previously. Following the rinse period, muscles were transferred to 2 ml of KHB containing 1 mM 2-deoxy-[1,2-3H]glucose (0.3 mCi/mmol; Sigma Chemical), 39 mM [U-14C]mannitol (0.8 mCi/mmol; ICN Radiochemicals, Irvine, CA), 0.1% BSA, and insulin, glucose oxidase, and/or SP600125, if previously present, and incubated for 20 min at 37°C. At the end of this final incubation period, muscles were removed and quickly frozen in liquid nitrogen, weighed, and placed in 0.5 ml of 0.5 mM NaOH. After the muscles were completely solubilized, 5 ml of scintillation cocktail were added, and the specific intracellular accumulation of 2-[3H]DG was determined as described previously (Hansen et al., 1994).
Determination of signalling protein expression and functionality
In some experiments, muscles were frozen after the initial incubation period, weighed, and stored at −80°C until analysis. Muscles were homogenized in eight volumes of ice-cold lysis buffer (50 mM HEPES, 150 mM NaCl, 20 mM Na pyrophosphate, 20 mM β-glycerophosphate, 10 mM NaF, 2 mM Na3VO4, 2 mM EDTA, 1% Triton X-100, 10% glycerol, 1 mM MgCl2, 1 mM CaCl2, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 0.5 μg/ml pepstatin, and 2 mM PMSF). Homogenates were incubated on ice for 20 min and then centrifuged at 13,000 g for 20 min at 4°C. Total protein concentration was determined using the BCA method (Pierce, Rockford, IL). Samples containing equal amounts of total protein were separated by SDS–PAGE on 10% or 12% polyacrylamide gels and transferred to nitrocellulose. Membranes were incubated overnight with antibodies against phosphorylated Akt Ser473 (Cell Signaling Technology, Danvers, MA), for 72 h with antibodies against phosphorylated JNK Thr183/Tyr185 (Cell Signaling), or overnight with antibodies against total Akt or total JNK (Cell Signaling). The membranes were then incubated with secondary goat anti-rabbit antibody conjugated with horseradish peroxidase (HRP) (Chemicon, Temecula, CA) or anti-mouse antibody conjugated with HRP (Santa Cruz Biotechnology, Santa Cruz, CA). Proteins were visualized using a Bio-Rad Chemidoc XRS instrument (Bio-Rad Laboratories, Hercules, CA) using the SuperSignal West Femto Maximum Sensitivity Western blot detection substrate (Pierce). Band density was quantified using the Bio-Rad Quantity One software.
Statistical analysis
All values are expressed as means ± SEM for 4–5 muscles/group. Paired Student’s t-tests were used to assess the specific effects of H2O2 or the JNK inhibitor SP600125 on group means. A p-value of p < 0.05 was considered to be statistically significant.
Results
Effects of low-level oxidant stress on glucose transport activity
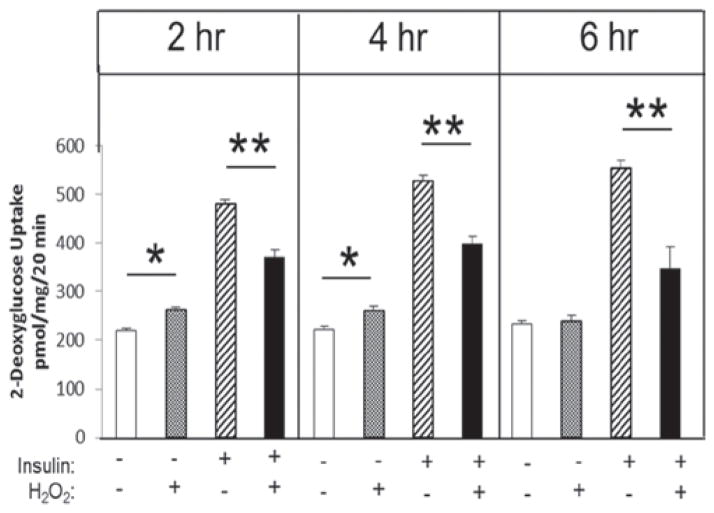
Soleus muscle strips were incubated in 30–40 μM H2O2 in the absence or presence of insulin for 2, 4 or 6 h. In the absence of insulin, the H2O2 significantly (p < 0.05) increased basal glucose transport activity at 2 and 4 h, but not at 6 h (Figure 1). However, oxidant-induced decreases in insulin-stimulated glucose transport occurred at 2 h (23%), 4 h (25%) and 6 h (42%) (all p < 0.05).
Figure 1.
Time course of the effect of low-level H2O2 on glucose transport activity in the absence or presence of insulin in isolated rat soleus muscle. *p < 0.05 vs. no H2O2; **p < 0.05 vs. insulin without H2O2.
Effect of low-level oxidant stress on insulin signalling
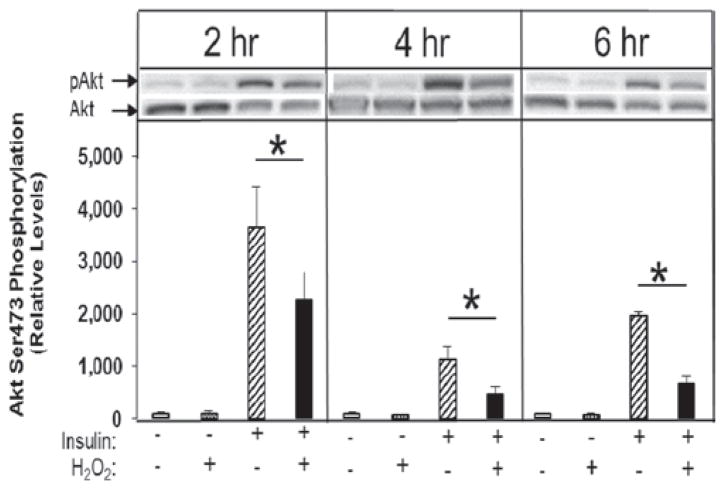
The H2O2 had no effect on the basal phosphorylation of Akt Ser473 at any time point, but did inhibit insulin-stimulated phosphorylation of Akt Ser473 by 37, 57, and 67% (p < 0.05) at 2, 4, and 6 h, respectively (Figure 2).
Figure 2.
Time course of the effect of low-level H2O2 on basal and insulin-stimulated Akt Ser473 phosphorylation in isolated rat soleus muscle. *p < 0.05 vs. insulin without H2O2.
Effect of low-level oxidant stress on engagement of JNK
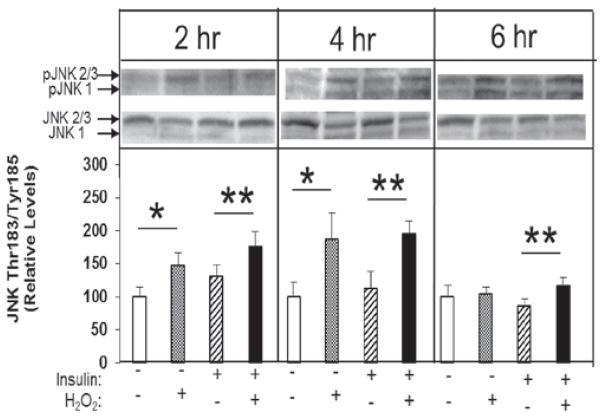
The responses to the oxidant intervention for phosphorylation of JNK are shown in Figure 3. For the final analysis, data from the JNK1 and JNK2/3 isoforms were pooled. At 2 and 4 h, there were significant (46% and 86%, respectively, both p < 0.05) overall increases in JNK phosphorylation in the presence of H2O2 under basal conditions. This effect, however, had disappeared by 6 h. At all time points, there were significant increases in JNK phosphorylation induced by H2O2 in the presence of insulin: 35% at 2 h, 74% at 4 h, and 55% at 6 h (all p < 0.05). Insulin alone had no effect on this parameter at any time point.
Figure 3.
Time course of the effects of low-level H2O2 on Thr183/Tyr185 phosphorylation of JNK 1 and JNK 2/3 isoforms in the absence or presence of insulin in isolated rat soleus muscle. *p < 0.05 vs. no H2O2; **p < 0.05 vs. insulin without H2O2.
Specific role of JNK in oxidant-stress-induced insulin resistance
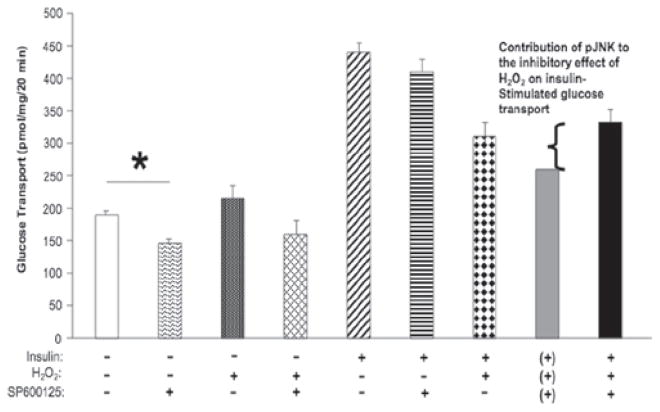
To determine if the activation of JNK contributes to this H2O2-induced insulin resistance in isolated mammalian skeletal muscle, the selective JNK inhibitor, SP600125 (10 μM), was utilized in 6-h incubations. Treatment with SP600125 significantly (p < 0.05) decreased basal glucose transport activity (Figure 4, first and second bars from left), and this effect of SP600125 was maintained in the presence of H2O2 alone (Figure 4, third and fourth bars from left). SP600125 caused a small, but statistically insignificant, decrease in insulin-stimulated glucose transport activity (Figure 4, fifth and sixth bars from the left), likely a carryover from the effect of SP600125 on basal glucose transport activity. In muscles that were incubated with insulin, H2O2, and SP600125 in combination, the rate of glucose transport activity measured experimentally (332 ± 15 pmol/mg muscle/20 min) (Figure 4, ninth bar from left) was considerably greater than the theoretical value (261 pmol/mg muscle/20 min) (Figure 4, eighth bar from left) calculated by accounting for the decreases in insulin-stimulated glucose transport activity due to H2O2 or SP600125 individually. This theoretical additive value assumes that H2O2 and SP600125 impaired insulin-stimulated glucose transport activity through separate pathways and that JNK did not have a direct effect on oxidative-stress induced insulin resistance. The theoretical additive value was calculated as the rate of insulin-stimulated glucose transport activity (440 pmol/mg muscle/20 min) minus the effect of H2O2 on insulin-stimulated glucose transport activity (137 pmol/mg muscle/20 min) and also minus the decrease in basal glucose transport due to SP600125 (42 pmol/mg muscle/20 min). Since the actual experimental value is greater than this theoretical additive value, these data are consistent with the interpretation that JNK mediates, at least in part (~30%), the effects of the oxidant stress on insulin-stimulated glucose transport activity in skeletal muscle.
Figure 4.
Effects of JNK inhibitor SP600125 on H2O2-induced inhibition of insulin-stimulated glucose transport activity at 6 h in isolated rat soleus muscle. The grey bar indicates the theoretic value due to the additive effects of SP600125 and H2O2 on insulin-stimulated 2DG uptake (the theoretical conditions are indicated by the (+) symbols below the grey bar). The theoretical additive value (261 pmol/mg muscle/20 min) was calculated as the rate of insulin-stimulated glucose transport activity (440 pmol/mg muscle/20 min) minus the effect of H2O2 on insulin-stimulated glucose transport activity (137 pmol/mg muscle/20 min) and also minus the decrease in basal glucose transport due to SP600125 (42 pmol/mg muscle/20 min). *p < 0.05 vs. no additions.
Discussion
The purpose of the present study was to assess, for the first time, the direct role of the stress-activated serine kinase JNK in the development of insulin resistance in mammalian skeletal muscle in response to an in vitro oxidant stress under highly defined incubation conditions (Figures 1 and 2). The results of this study show that JNK activation (Figure 3) is necessary, at least in part, for the ability of low levels (30–40 μM) of the oxidant H2O2 to induce insulin resistance of glucose transport activity in an isolated rat skeletal muscle preparation (Figure 4).
While previous studies have shown that JNK activation is associated with oxidative stress-induced insulin resistance (Blair et al., 1999; Hirosumi et al., 2002; Solinas and Karin, 2010), we have demonstrated this relationship in isolated mammalian skeletal muscle (Figure 3), with direct exposure of this tissue to the oxidant H2O2 causing an increase in JNK phosphorylation and therefore activation. Indeed, all three JNK isoforms present in skeletal muscle (JNK1, JNK2, and JNK3) were engaged by this low-level oxidant stress (Figure 3). Our findings are consistent with those from a previous study using the L6 myotube cell line, in which JNK activation was increased as much as 8-fold in the presence of high concentrations of H2O2 (Blair et al., 1999). Moreover, the selective inhibition of JNK using the compound SP600125 (Bennett et al., 2001; Li et al., 2005) in the presence of both insulin and H2O2 resulted in a partial reversal of the insulin resistance of glucose transport activity (Figure 4). These latter results provide solid evidence supporting an important role of JNK in oxidative stress-induced insulin resistance in mammalian skeletal muscle.
It is important to note that JNK is not the only serine kinase that is activated by oxidative stress or that can have an effect on insulin resistance in mammalian skeletal muscle. The activation of other serine kinases, such as GSK-3 (Archuleta et al., 2009; Dokken et al., 2008) and p38 MAPK (Archuleta et al., 2009; Blair et al., 1999; Diamond-Stanic et al., 2011; Kim et al., 2006), by oxidative stress is also mechanistically connected with the development of insulin resistance of glucose transport acivity. Interestingly, none of these serine kinases, including JNK, can account for more than 25–30% of the insulin resistance caused by H2O2 (Figure 4; Archuleta et al., 2009; Diamond-Stanic et al., 2011; Dokken et al., 2008; Henriksen, 2010; Henriksen et al., 2011). It is possible that oxidative stress induces insulin resistance in mammalian skeletal muscle by simultaneously engaging all of these pathways, and maybe others that have not been extensively studied in this context, such as p70S6 kinase and IKK-β. Previous studies that have shown the direct roles of GSK-3 and p38 MAPK (Archuleta et al., 2009; Diamond-Stanic et al., 2011; Dokken et al., 2008) by using selective inhibitors, including the present study (Figure 4), suggest that using multiple serine kinase inhibitors in combination could potentially normalize insulin action under conditions of oxidative stress; this approach should be evaluated in future investigations.
While the kinase inhibitor used in this study, SP600125, as well as other kinase inhibitors used in previous studies (Archuleta et al., 2009; Diamond-Stanic et al., 2011; Dokken et al., 2005, 2008; Kim et al., 2006), have been shown to have metabolic benefits, caution should be expressed before fully accepting their usefulness. Whereas the benefits seem obvious for skeletal muscle, it is very possible that these inhibitors, when administered systemically (Dokken and Henriksen, 2006), will act on many other tissues and cells in the experimental subject. It is possible that these same serine kinases that contribute to oxidative stress-induced insulin resistance in skeletal muscle could have normal regulatory functions in other cells. Inhibiting these regulatory functions could be detrimental overall and the costs might outweigh the benefits. The in vivo use of these kinase inhibitors should be carefully studied before approving their use as a treatment for any disease state.
In conclusion, the results of the present study indicate that activation of the serine kinase JNK does directly contribute to oxidative stress-induced insulin resistance in isolated slow-twitch skeletal muscle. However, like other stress-activated serine kinases studied under these same experimental conditions, such as GSK-3 and p38 MAPK, the engagement of JNK alone cannot account completely for the induction of insulin resistance by a low-level oxidant stress (H2O2) in this rat skeletal muscle preparation. It is likely that the simultaneous engagement of numerous stress-activated serine kinases (including JNK, GSK-3, and p38 MAPK, as well as others) must be stimulated by this oxidant stress in order to fully induce insulin resistance of the glucose transport system in mammalian skeletal muscle. This latter speculation should be addressed in a future investigation.
Footnotes
Declaration of interest
The study was supported by grants DK063967 and T32 HL07249 from the National Institutes of Health, by the Guenther J. Dietze Foundation, by the Undergraduate Biology Research Program at the University of Arizona (funded by the Howard Hughes Medical Institute), and by the Committee on Higher Education of the Government of Thailand. The authors declare no conflicts of interest.
References
- Abdul-Ghani MA, DeFronzo RA. Pathogenesis of insulin resistance in skeletal muscle. J Biomed Biotechnol. 2010;2010:Article ID 476279, 19. doi: 10.1155/2010/476279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archuleta TL, Lemieux AM, Saengsirisuwan V, Teachey MK, Lindborg KA, Kim JS, Henriksen EJ. Oxidant stress-induced loss of IRS-1 and IRS-2 proteins in rat skeletal muscle: role of p38 MAPK. Free Radic Biol Med. 2009;47:1486–93. doi: 10.1016/j.freeradbiomed.2009.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BL, Sasaki DT, Murray BW, et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci. 2001;98:13681–6. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair AS, Hajduch E, Litherland GJ, Hundal HS. Regulation of glucose transport and glycogen synthesis in L6 muscle cells during oxidative stress: Evidence for cross talk between the insulin and SAPK2/p38 mitogen activated-protein kinase signaling pathways. J Biol Chem. 1999;274:36293–9. doi: 10.1074/jbc.274.51.36293. [DOI] [PubMed] [Google Scholar]
- Cirialdi TP, Nikoulina SE, Bandukwalam RA, Carter L, Henry RR. Role of glycogen synthase kinase-3α in insulin action in cultured human muscle cells. Endocrinology. 2007;148:4393–9. doi: 10.1210/en.2006-0932. [DOI] [PubMed] [Google Scholar]
- Davies KJ. Oxidative stress: the paradox of aerobic life. Biochem Soc Symp. 1995;61:1–31. doi: 10.1042/bss0610001. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Ferrannini E. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–94. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA. Pathogenesis of type 2 diabetes: metabolic and molecular implications for identifying diabetes genes. Diab Rev. 1997;5:177–269. [Google Scholar]
- DeFronzo RA. Pathogenesis of type 2 diabetes mellitus. Med Clin North Am. 2004;88:787–835. doi: 10.1016/j.mcna.2004.04.013. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58:773–95. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond-Stanic MK, Marchionne EM, Teachey MK, Durazo DE, Kim JS, Henriksen EJ. Critical role of transient p38 MAPK activation in skeletal muscle insulin resistance caused by low-level in vitro oxidant stress. Biochem Biophys Res Commun. 2011;405:439–444. doi: 10.1016/j.bbrc.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokken BB, Henriksen EJ. Chronic selective glycogen synthase kinase-3 inhibition enhances glucose disposal and muscle insulin action in pre-diabetic obese Zucker rats. Am J Physiol Endocrinol Metab. 2006;291:E207–13. doi: 10.1152/ajpendo.00628.2005. [DOI] [PubMed] [Google Scholar]
- Dokken BB, Sloniger JA, Henriksen EJ. Acute selective glycogen synthase kinase-3 inhibition enhances insulin signaling in prediabetic insulin-resistant rat skeletal muscle. Am J Physiol Endocrinol Metab. 2005;288:E1188–94. doi: 10.1152/ajpendo.00547.2004. [DOI] [PubMed] [Google Scholar]
- Dokken BB, Saengsirisuwan V, Kim JS, Teachey MK, Henriksen EJ. Oxidative stress-induced insulin resistance in rat skeletal muscle: role of glycogen synthase kinase-3. Am J Physiol Endocrinol Metab. 2008;294:E615–21. doi: 10.1152/ajpendo.00578.2007. [DOI] [PubMed] [Google Scholar]
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocrin Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- Hansen PA, Gulve EA, Holloszy JO. Suitability of 2-deoxyglucose for in vitro measurement of glucose transport activity in skeletal muscle. J Appl Physiol. 1994;76:979–85. doi: 10.1152/jappl.1994.76.2.979. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ, Jacob S. Effects of captopril on glucose transport activity in skeletal muscle of obese Zucker rats. Metabolism. 1995;44:267–72. doi: 10.1016/0026-0495(95)90276-7. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ. Dysregulation of glycogen synthase kinase-3 and the etiology of insulin resistance and type 2 diabetes. Curr Diab Rev. 2010;6:285–93. doi: 10.2174/157339910793360888. [DOI] [PubMed] [Google Scholar]
- Henriksen EJ, Diamond-Stanic MK, Marchionne EM. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med. 2011;51:993–9. doi: 10.1016/j.freeradbiomed.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 420:333–6. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- Holloszy JO, Hansen PA. Regulation of glucose transport into skeletal muscle. Rev Physiol Biochem Pharmacol. 1996;128:99–193. doi: 10.1007/3-540-61343-9_8. [DOI] [PubMed] [Google Scholar]
- Kim JS, Saengsirisuwan V, Sloniger JA, Teachey MK, Henriksen EJ. Stimulation of muscle glucose transport by an oxidant stress: roles of insulin signaling and p38 MAP kinase. Free Radic Biol Med. 2006;41:818–24. doi: 10.1016/j.freeradbiomed.2006.05.031. [DOI] [PubMed] [Google Scholar]
- Li Y-P, Chen Y, John J, Moylan J, Jin B, Mann DL, Reid MB. TNF-alpha acts via p38 MAPK to stimulate expression of the ubiquitin ligase atrogin1/MAFbx in skeletal muscle. FASEB J. 2005;19:362–70. doi: 10.1096/fj.04-2364com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikoulina SE, Cirialdi TP, Mudaliar S, Carter L, Johnson K, Henry RR. Inhibition of glycogen synthase kinase-3 improves insulin action and glucose metabolism in human skeletal muscle. Diabetes. 2002;51:2190–98. doi: 10.2337/diabetes.51.7.2190. [DOI] [PubMed] [Google Scholar]
- Sies H. Oxidative stress: Oxidants and antioxidants. Exp Physiol. 1997;82:291–5. doi: 10.1113/expphysiol.1997.sp004024. [DOI] [PubMed] [Google Scholar]
- Solinas G, Karin M. JNK1 and IKKβ: molecular links between obesity and metabolic dysfunction. FASEB J. 2010;24:2596–611. doi: 10.1096/fj.09-151340. [DOI] [PubMed] [Google Scholar]
- Zierath JR, Krook A, Wallberg-Henriksson H. Insulin action and insulin resistance in human skeletal muscle. Diabetologia. 2000;43:821–35. doi: 10.1007/s001250051457. [DOI] [PubMed] [Google Scholar]