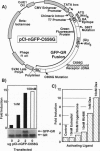
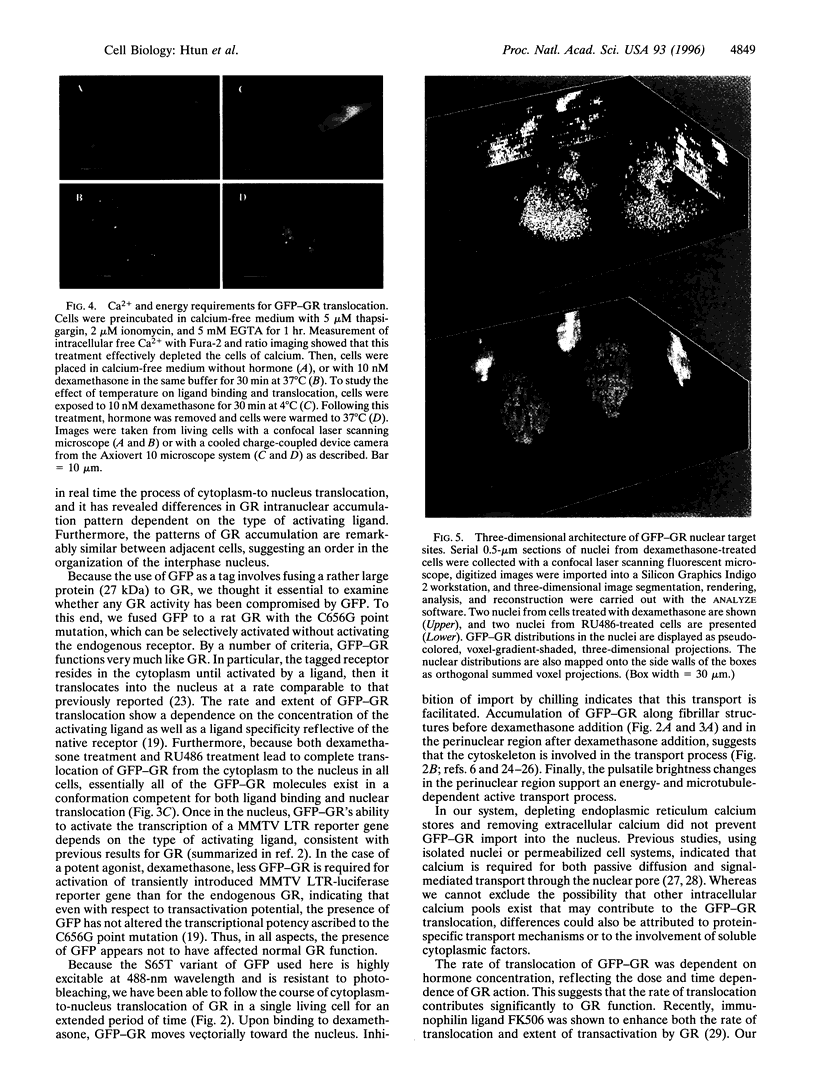
Abstract
A highly fluorescent mutant form of the green fluorescent protein (GFP) has been fused to the rat glucocorticoid receptor (GR). When GFP-GR is expressed in living mouse cells, it is competent for normal transactivation of the GR-responsive mouse mammary tumor virus promoter. The unliganded GFP-GR resides in the cytoplasm and translocates to the nucleus in a hormone-dependent manner with ligand specificity similar to that of the native GR receptor. Due to the resistance of the mutant GFP to photobleaching, the translocation process can be studied by time-lapse video microscopy. Confocal laser scanning microscopy showed nuclear accumulation in a discrete series of foci, excluding nucleoli. Complete receptor translocation is induced with RU486 (a ligand with little agonist activity), although concentration into nuclear foci is not observed. This reproducible pattern of transactivation-competent GR reveals a previously undescribed intranuclear architecture of GR target sites.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akner G., Wikström A. C., Gustafsson J. A. Subcellular distribution of the glucocorticoid receptor and evidence for its association with microtubules. J Steroid Biochem Mol Biol. 1995 Jan;52(1):1–16. doi: 10.1016/0960-0760(94)00155-f. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Becker P. B., Gloss B., Schmid W., Strähle U., Schütz G. In vivo protein-DNA interactions in a glucocorticoid response element require the presence of the hormone. Nature. 1986 Dec 18;324(6098):686–688. doi: 10.1038/324686a0. [DOI] [PubMed] [Google Scholar]
- Brasch K., Ochs R. L. Nuclear remodeling in response to steroid hormone action. Int Rev Cytol. 1995;159:161–194. doi: 10.1016/s0074-7696(08)62107-5. [DOI] [PubMed] [Google Scholar]
- Carson-Jurica M. A., Schrader W. T., O'Malley B. W. Steroid receptor family: structure and functions. Endocr Rev. 1990 May;11(2):201–220. doi: 10.1210/edrv-11-2-201. [DOI] [PubMed] [Google Scholar]
- Carter K. C., Bowman D., Carrington W., Fogarty K., McNeil J. A., Fay F. S., Lawrence J. B. A three-dimensional view of precursor messenger RNA metabolism within the mammalian nucleus. Science. 1993 Feb 26;259(5099):1330–1335. doi: 10.1126/science.8446902. [DOI] [PubMed] [Google Scholar]
- Chakraborti P. K., Garabedian M. J., Yamamoto K. R., Simons S. S., Jr Creation of "super" glucocorticoid receptors by point mutations in the steroid binding domain. J Biol Chem. 1991 Nov 25;266(33):22075–22078. [PubMed] [Google Scholar]
- Chalfie M., Tu Y., Euskirchen G., Ward W. W., Prasher D. C. Green fluorescent protein as a marker for gene expression. Science. 1994 Feb 11;263(5148):802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook P. R. A chromomeric model for nuclear and chromosome structure. J Cell Sci. 1995 Sep;108(Pt 9):2927–2935. doi: 10.1242/jcs.108.9.2927. [DOI] [PubMed] [Google Scholar]
- DeFranco D. B., Qi M., Borror K. C., Garabedian M. J., Brautigan D. L. Protein phosphatase types 1 and/or 2A regulate nucleocytoplasmic shuttling of glucocorticoid receptors. Mol Endocrinol. 1991 Sep;5(9):1215–1228. doi: 10.1210/mend-5-9-1215. [DOI] [PubMed] [Google Scholar]
- Gametchu B., Harrison R. W. Characterization of a monoclonal antibody to the rat liver glucocorticoid receptor. Endocrinology. 1984 Jan;114(1):274–279. doi: 10.1210/endo-114-1-274. [DOI] [PubMed] [Google Scholar]
- Giordano T., Howard T. H., Coleman J., Sakamoto K., Howard B. H. Isolation of a population of transiently transfected quiescent and senescent cells by magnetic affinity cell sorting. Exp Cell Res. 1991 Jan;192(1):193–197. doi: 10.1016/0014-4827(91)90175-t. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber U. F., Gerace L. Depletion of calcium from the lumen of endoplasmic reticulum reversibly inhibits passive diffusion and signal-mediated transport into the nucleus. J Cell Biol. 1995 Jan;128(1-2):5–14. doi: 10.1083/jcb.128.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim R., Cubitt A. B., Tsien R. Y. Improved green fluorescence. Nature. 1995 Feb 23;373(6516):663–664. doi: 10.1038/373663b0. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H., Okret S., Wikström A. C., Gustafsson J. A., Shaper J. H. Binding of the glucocorticoid receptor to the rat liver nuclear matrix. The role of disulfide bond formation. J Biol Chem. 1986 Sep 15;261(26):11962–11967. [PubMed] [Google Scholar]
- Ktistaki E., Ktistakis N. T., Papadogeorgaki E., Talianidis I. Recruitment of hepatocyte nuclear factor 4 into specific intranuclear compartments depends on tyrosine phosphorylation that affects its DNA-binding and transactivation potential. Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9876–9880. doi: 10.1073/pnas.92.21.9876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanz R. B., Rusconi S. A conserved carboxy-terminal subdomain is important for ligand interpretation and transactivation by nuclear receptors. Endocrinology. 1994 Nov;135(5):2183–2195. doi: 10.1210/endo.135.5.7956941. [DOI] [PubMed] [Google Scholar]
- Lefebvre P., Berard D. S., Cordingley M. G., Hager G. L. Two regions of the mouse mammary tumor virus long terminal repeat regulate the activity of its promoter in mammary cell lines. Mol Cell Biol. 1991 May;11(5):2529–2537. doi: 10.1128/mcb.11.5.2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L. A view of interphase chromosomes. Science. 1990 Dec 14;250(4987):1533–1540. doi: 10.1126/science.2274784. [DOI] [PubMed] [Google Scholar]
- Nelson W. G., Pienta K. J., Barrack E. R., Coffey D. S. The role of the nuclear matrix in the organization and function of DNA. Annu Rev Biophys Biophys Chem. 1986;15:457–475. doi: 10.1146/annurev.bb.15.060186.002325. [DOI] [PubMed] [Google Scholar]
- Niman H. L., Houghten R. A., Walker L. E., Reisfeld R. A., Wilson I. A., Hogle J. M., Lerner R. A. Generation of protein-reactive antibodies by short peptides is an event of high frequency: implications for the structural basis of immune recognition. Proc Natl Acad Sci U S A. 1983 Aug;80(16):4949–4953. doi: 10.1073/pnas.80.16.4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning Y. M., Sánchez E. R. Potentiation of glucocorticoid receptor-mediated gene expression by the immunophilin ligands FK506 and rapamycin. J Biol Chem. 1993 Mar 25;268(9):6073–6076. [PubMed] [Google Scholar]
- Ogawa H., Inouye S., Tsuji F. I., Yasuda K., Umesono K. Localization, trafficking, and temperature-dependence of the Aequorea green fluorescent protein in cultured vertebrate cells. Proc Natl Acad Sci U S A. 1995 Dec 5;92(25):11899–11903. doi: 10.1073/pnas.92.25.11899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten A. D., Sanders M. M., McKnight G. S. The MMTV LTR promoter is induced by progesterone and dihydrotestosterone but not by estrogen. Mol Endocrinol. 1988 Feb;2(2):143–147. doi: 10.1210/mend-2-2-143. [DOI] [PubMed] [Google Scholar]
- Perrot-Applanat M., Lescop P., Milgrom E. The cytoskeleton and the cellular traffic of the progesterone receptor. J Cell Biol. 1992 Oct;119(2):337–348. doi: 10.1083/jcb.119.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard D., Yamamoto K. R. Two signals mediate hormone-dependent nuclear localization of the glucocorticoid receptor. EMBO J. 1987 Nov;6(11):3333–3340. doi: 10.1002/j.1460-2075.1987.tb02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirollet F., Job D., Margolis R. L., Garel J. R. An oscillatory mode for microtubule assembly. EMBO J. 1987 Nov;6(11):3247–3252. doi: 10.1002/j.1460-2075.1987.tb02642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasher D. C. Using GFP to see the light. Trends Genet. 1995 Aug;11(8):320–323. doi: 10.1016/s0168-9525(00)89090-3. [DOI] [PubMed] [Google Scholar]
- Pruschy M., Ju Y., Spitz L., Carafoli E., Goldfarb D. S. Facilitated nuclear transport of calmodulin in tissue culture cells. J Cell Biol. 1994 Dec;127(6 Pt 1):1527–1536. doi: 10.1083/jcb.127.6.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherrer L. C., Pratt W. B. Association of the transformed glucocorticoid receptor with a cytoskeletal protein complex. J Steroid Biochem Mol Biol. 1992 Mar;41(3-8):719–721. doi: 10.1016/0960-0760(92)90411-b. [DOI] [PubMed] [Google Scholar]
- Tsai M. J., O'Malley B. W. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y., Harootunian A. T. Practical design criteria for a dynamic ratio imaging system. Cell Calcium. 1990 Feb-Mar;11(2-3):93–109. doi: 10.1016/0143-4160(90)90063-z. [DOI] [PubMed] [Google Scholar]
- Wang S., Hazelrigg T. Implications for bcd mRNA localization from spatial distribution of exu protein in Drosophila oogenesis. Nature. 1994 Jun 2;369(6479):400–403. doi: 10.1038/369400a0. [DOI] [PubMed] [Google Scholar]
- Yokota H., van den Engh G., Hearst J. E., Sachs R. K., Trask B. J. Evidence for the organization of chromatin in megabase pair-sized loops arranged along a random walk path in the human G0/G1 interphase nucleus. J Cell Biol. 1995 Sep;130(6):1239–1249. doi: 10.1083/jcb.130.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Steensel B., Brink M., van der Meulen K., van Binnendijk E. P., Wansink D. G., de Jong L., de Kloet E. R., van Driel R. Localization of the glucocorticoid receptor in discrete clusters in the cell nucleus. J Cell Sci. 1995 Sep;108(Pt 9):3003–3011. doi: 10.1242/jcs.108.9.3003. [DOI] [PubMed] [Google Scholar]