Abstract
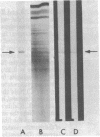
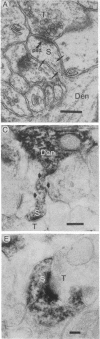
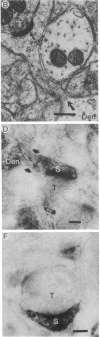
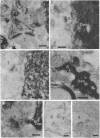
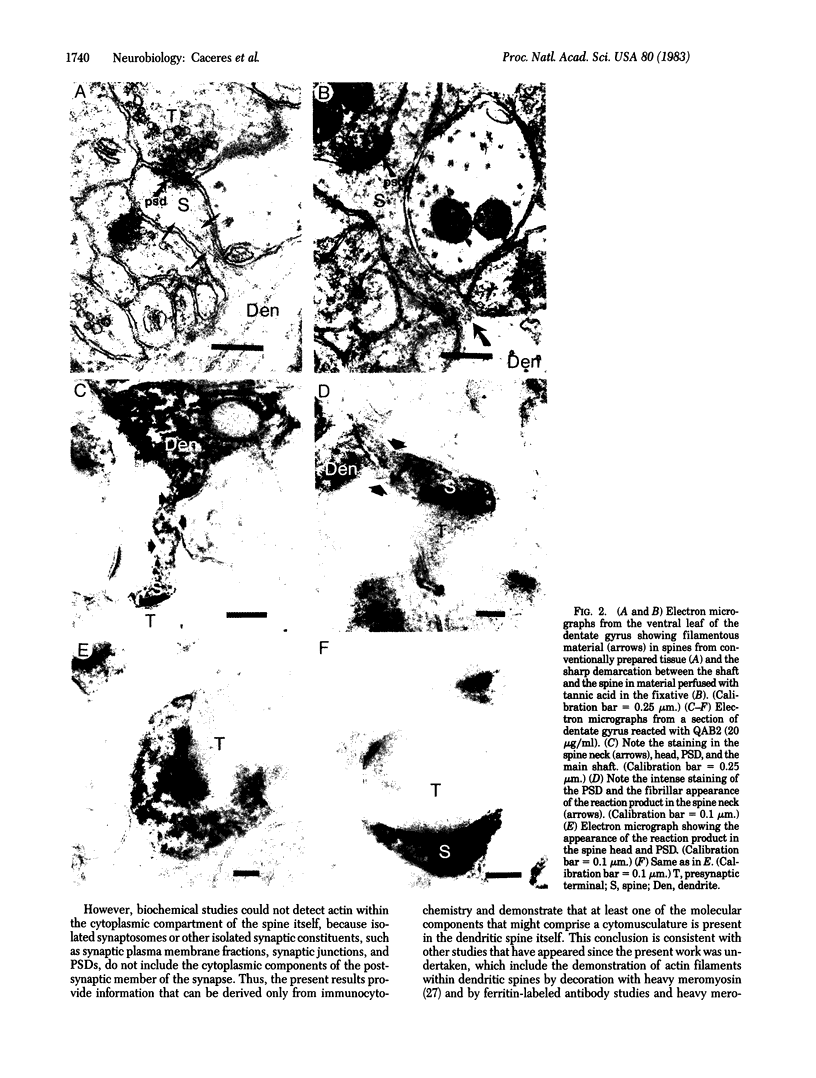
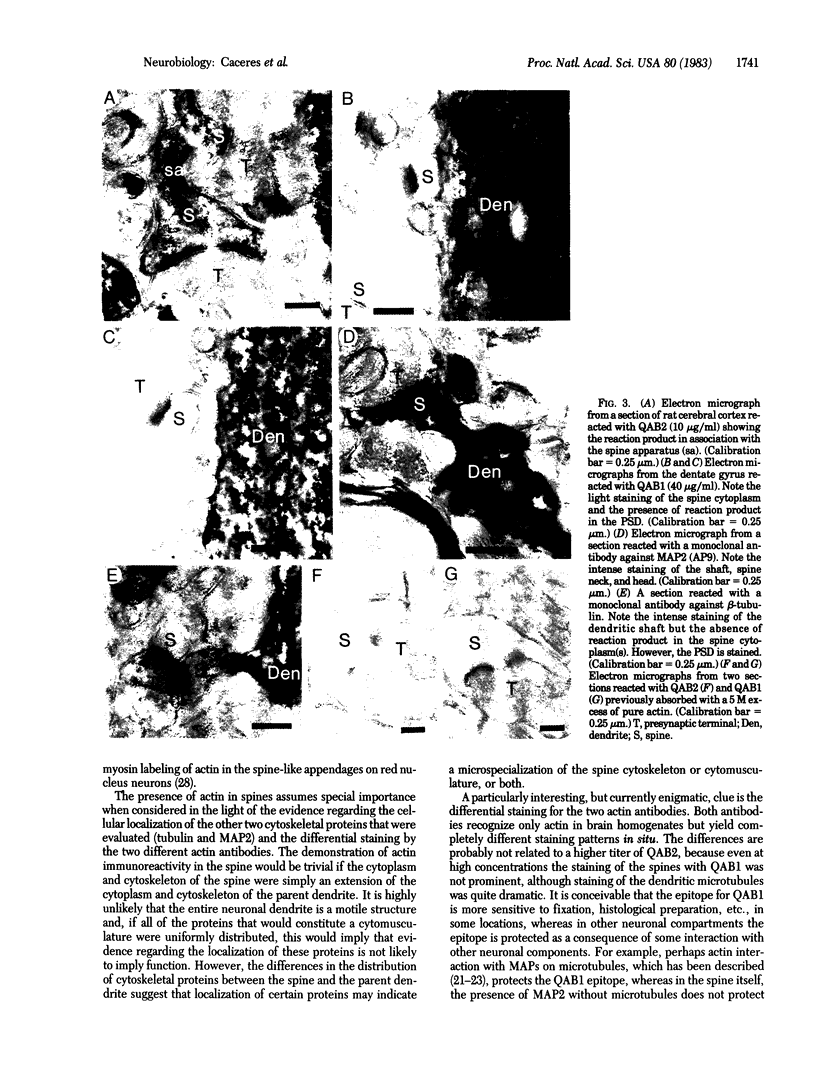
To determine whether dendritic spines contain actin, we evaluated the immunocytochemical localization of actin in the hippocampal formation and cerebral cortex of the rat. Monoclonal hybridoma antibodies were prepared against adult quail breast muscle actin. The culture supernatant of two cell lines (QAB1 and QAB2) was examined. Both antibodies bound only actin in crude brain homogenates, and neither exhibited species specificity. Electron microscopic analyses of sections reacted with QAB1 revealed staining of postsynaptic densities and dendritic microtubules but little staining of the cytoplasmic compartment of spines. However, sections reacted with QAB2 exhibited staining at the cytoplasmic compartment of spines as well as the sites stained by QAB1. We also evaluated the immunocytochemical distribution of beta-tubulin and high molecular weight microtubule-associated protein (MAP2) utilizing monoclonal antibodies. MAP2 was found in the dendritic spine as well as in the parent dendrite. However, beta-tubulin was found only in the postsynaptic density and in the microtubules of the parent dendrite. The combined results indicate that actin is present in the spine along with MAP2 and that there is a difference in the actin (or the state of actin) in the spine in comparison with other neuronal compartments.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berard D. R., Burgess J. W., Coss R. G. Plasticity of dendritic spine formation: a state-dependent stochastic process. Int J Neurosci. 1981;13(2-3):93–98. doi: 10.3109/00207458109043306. [DOI] [PubMed] [Google Scholar]
- Blomberg F., Cohen R. S., Siekevitz P. The structure of postsynaptic densities isolated from dog cerebral cortex. II. Characterization and arrangement of some of the major proteins within the structure. J Cell Biol. 1977 Jul;74(1):204–225. doi: 10.1083/jcb.74.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coss R. G., Globus A. Spine stems on tectal interneurons in jewel fish are shortened by social stimulation. Science. 1978 May 19;200(4343):787–790. doi: 10.1126/science.644322. [DOI] [PubMed] [Google Scholar]
- Ey P. L., Prowse S. J., Jenkin C. R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978 Jul;15(7):429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- Fifková E., Delay R. J. Cytoplasmic actin in neuronal processes as a possible mediator of synaptic plasticity. J Cell Biol. 1982 Oct;95(1):345–350. doi: 10.1083/jcb.95.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fifková E., Van Harreveld A. Long-lasting morphological changes in dendritic spines of dentate granular cells following stimulation of the entorhinal area. J Neurocytol. 1977 Apr;6(2):211–230. doi: 10.1007/BF01261506. [DOI] [PubMed] [Google Scholar]
- Griffith L. M., Pollard T. D. The interaction of actin filaments with microtubules and microtubule-associated proteins. J Biol Chem. 1982 Aug 10;257(15):9143–9151. [PubMed] [Google Scholar]
- Kelly P. T., Cotman C. W. Synaptic proteins. Characterization of tubulin and actin and identification of a distinct postsynaptic density polypeptide. J Cell Biol. 1978 Oct;79(1):173–183. doi: 10.1083/jcb.79.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFountain J. R., Jr, Zobel C. R., Thomas H. R., Galbreath C. Fixation and staining of F-actin and microfilaments using tannic acid. J Ultrastruct Res. 1977 Jan;58(1):78–86. doi: 10.1016/s0022-5320(77)80009-9. [DOI] [PubMed] [Google Scholar]
- Lasek R. J. The dynamic ordering of neuronal cytoskeletons. Neurosci Res Program Bull. 1981 Feb;19(1):7–32. [PubMed] [Google Scholar]
- LeBeux Y. J., Willemot J. An ultrastructural study of the microfilaments in rat brain by means of E-PTA staining and heavy meromyosin labeling. II. The synapses. Cell Tissue Res. 1975 Jun 27;160(1):37–68. doi: 10.1007/BF00219841. [DOI] [PubMed] [Google Scholar]
- Lee K. S., Schottler F., Oliver M., Lynch G. Brief bursts of high-frequency stimulation produce two types of structural change in rat hippocampus. J Neurophysiol. 1980 Aug;44(2):247–258. doi: 10.1152/jn.1980.44.2.247. [DOI] [PubMed] [Google Scholar]
- Matus A., Ackermann M., Pehling G., Byers H. R., Fujiwara K. High actin concentrations in brain dendritic spines and postsynaptic densities. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7590–7594. doi: 10.1073/pnas.79.23.7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matus A., Bernhardt R., Hugh-Jones T. High molecular weight microtubule-associated proteins are preferentially associated with dendritic microtubules in brain. Proc Natl Acad Sci U S A. 1981 May;78(5):3010–3014. doi: 10.1073/pnas.78.5.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall W., Rinzel J. Branch input resistance and steady attenuation for input to one branch of a dendritic neuron model. Biophys J. 1973 Jul;13(7):648–687. doi: 10.1016/S0006-3495(73)86014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattilaro R. F., Dentler W. L., LeCluyse E. L. Microtubule-associated proteins (MAPs) and the organization of actin filaments in vitro. J Cell Biol. 1981 Aug;90(2):467–473. doi: 10.1083/jcb.90.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]