Abstract
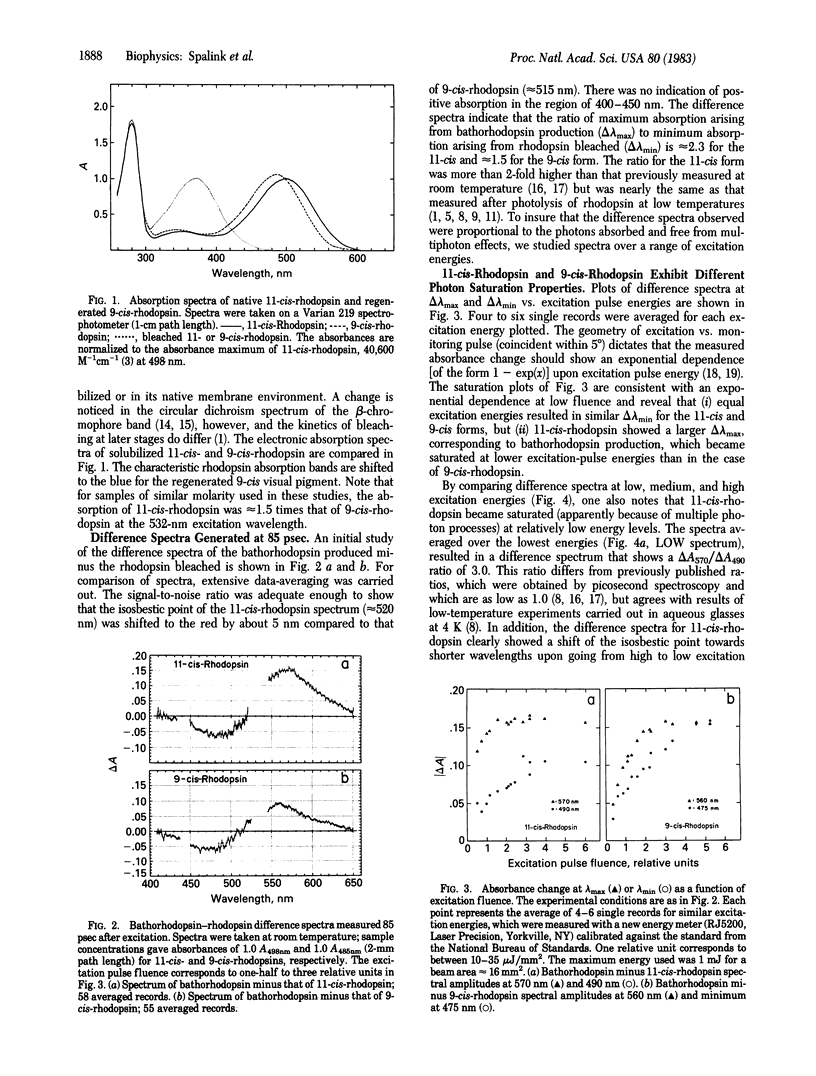
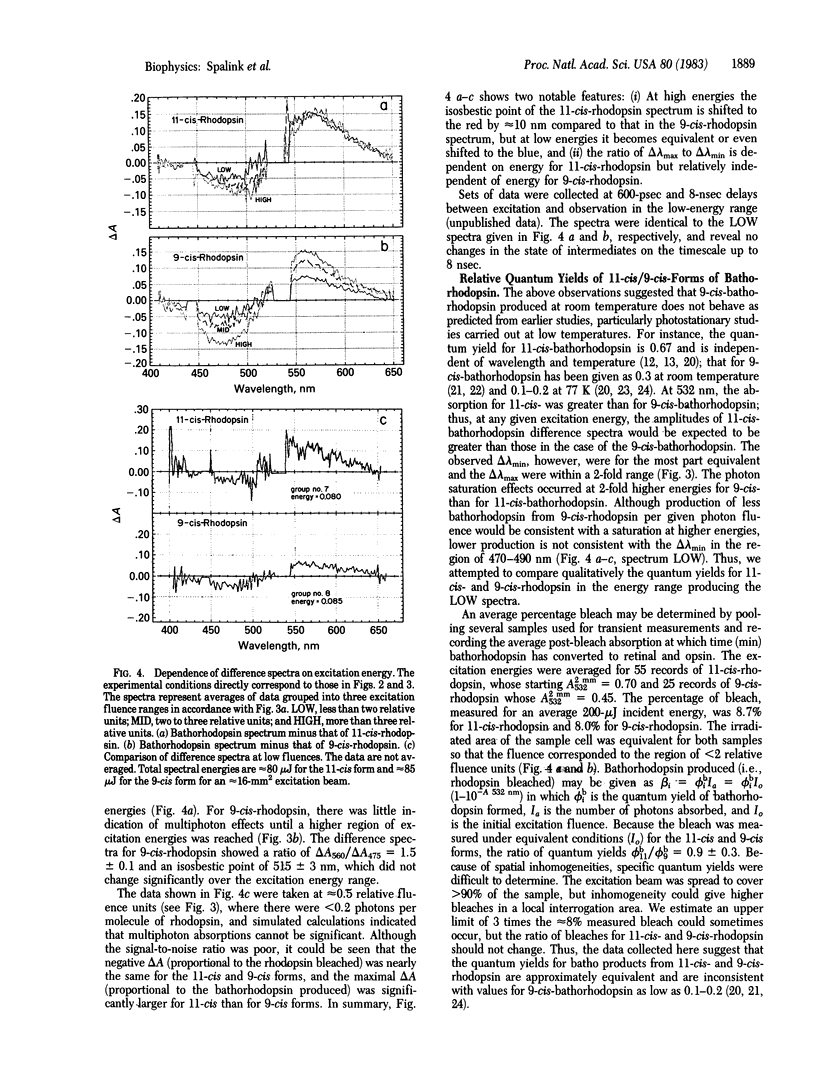
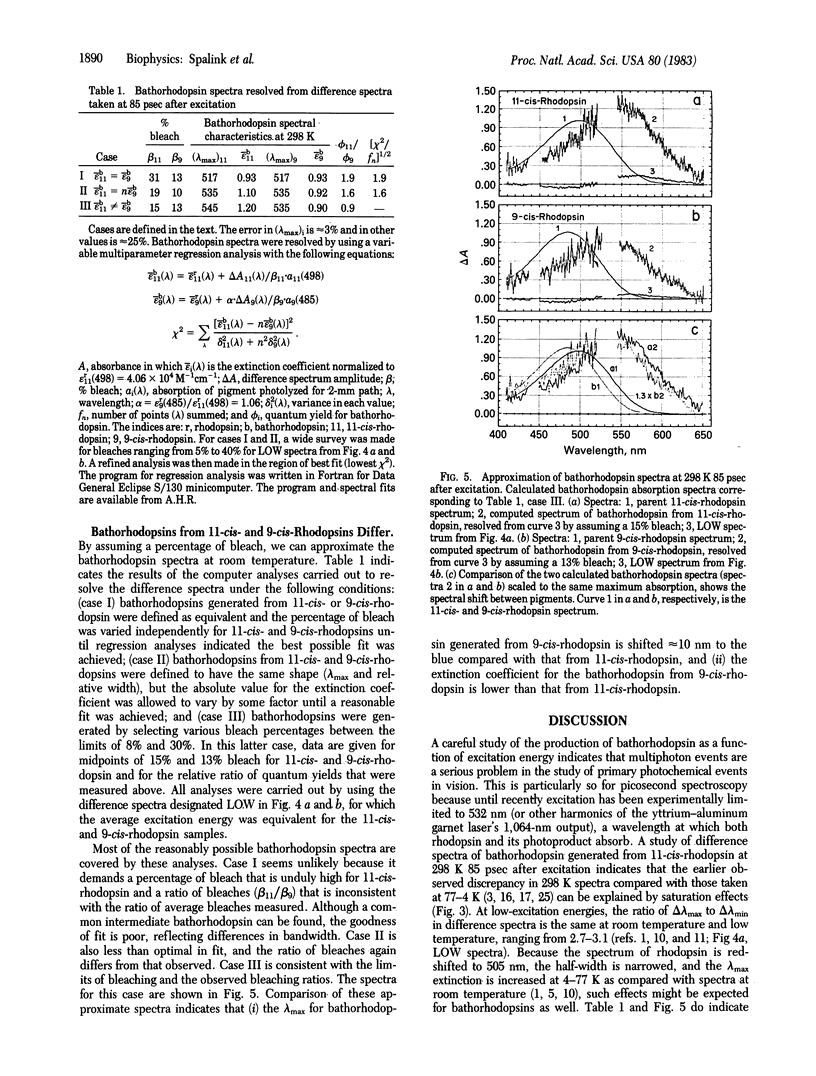
Bathorhodopsin-rhodopsin difference spectra of native 11-cis-rhodopsin and regenerated 9-cis-rhodopsin were measured at room temperature with a double-beam laser spectrophotometer after excitation at 532 nm. A detailed analysis of data obtained at 85 psec after excitation suggests that the bathorhodopsins generated from 11-cis- and 9-cis-rhodopsin differ in their extinction coefficients and that their absorption maxima are shifted in wavelength by about 10 nm from one another. The ratio of quantum yields for photochemical production of the 11-cis-bathorhodopsin and the 9-cis-bathorhodopsin approximates 1. Implications that the early photochemical processes in vision are more complex than previously considered are explored.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Applebury M. L., Rentzepis P. M. Picosecond spectroscopy of visual pigments. Methods Enzymol. 1982;81:354–368. doi: 10.1016/s0076-6879(82)81052-5. [DOI] [PubMed] [Google Scholar]
- Applebury M. L. The primary processes of vision: a view from the experimental side. Photochem Photobiol. 1980 Oct;32(4):425–431. doi: 10.1111/j.1751-1097.1980.tb03784.x. [DOI] [PubMed] [Google Scholar]
- Applebury M. L., Zuckerman D. M., Lamola A. A., Jovin T. M. Rhodopsin. Purification and recombination with phospholipids assayed by the metarhodopsin I leads to metarhodopsin II transition. Biochemistry. 1974 Aug 13;13(17):3448–3458. doi: 10.1021/bi00714a005. [DOI] [PubMed] [Google Scholar]
- Aton B., Doukas A. G., Narva D., Callender R. H., Dinur U., Honig B. Resonance Raman studies of the primary photochemical event in visual pigments. Biophys J. 1980 Jan;29(1):79–94. doi: 10.1016/S0006-3495(80)85119-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch G. E., Applebury M. L., Lamola A. A., Rentzepis P. M. Formation and decay of prelumirhodopsin at room temperatures. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2802–2806. doi: 10.1073/pnas.69.10.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dartnall H. J. The photosensitivities of visual pigments in the presence of hydroxylamine. Vision Res. 1968 Apr;8(4):339–358. doi: 10.1016/0042-6989(68)90104-1. [DOI] [PubMed] [Google Scholar]
- Ebrey T. G., Yoshizawa T. The circular dichroism of rhodopsin and lumirhodopsin. Exp Eye Res. 1973 Dec 24;17(6):545–556. doi: 10.1016/0014-4835(73)90084-5. [DOI] [PubMed] [Google Scholar]
- Eyring G., Curry B., Mathies R., Fransen R., Palings I., Lugtenburg J. Interpretation of the resonance Raman spectrum of bathorhodopsin based on visual pigment analogues. Biochemistry. 1980 May 27;19(11):2410–2418. doi: 10.1021/bi00552a020. [DOI] [PubMed] [Google Scholar]
- GRELLMANN K. H., LIVINGSTON R., PRATT D. A flashphotolytic investigation of rhodopsin at low temperatures. Nature. 1962 Mar 31;193:1258–1260. doi: 10.1038/1931258a0. [DOI] [PubMed] [Google Scholar]
- HUBBARD R. Retinene isomerase. J Gen Physiol. 1956 Jul 20;39(6):935–962. doi: 10.1085/jgp.39.6.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubbard R., Kropf A. THE ACTION OF LIGHT ON RHODOPSIN. Proc Natl Acad Sci U S A. 1958 Feb;44(2):130–139. doi: 10.1073/pnas.44.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley J. B., Ebrey T. G., Honig B., Ottolenghi M. Temperature and wavelength effects on the photochemistry of rhodopsin, isorhodopsin, bacteriorhodopsin and their photoproducts. Nature. 1977 Dec 8;270(5637):540–542. doi: 10.1038/270540a0. [DOI] [PubMed] [Google Scholar]
- KROPF A., HUBBARD R. The mechanism of bleaching rhodopsin. Ann N Y Acad Sci. 1959 Nov 12;74(2):266–280. doi: 10.1111/j.1749-6632.1958.tb39550.x. [DOI] [PubMed] [Google Scholar]
- Mao B., Ebrey T. G., Crouch R. Bathoproducts of rhodopsin, isorhodopsin I, and isorhodopsin II. Biophys J. 1980 Feb;29(2):247–256. doi: 10.1016/S0006-3495(80)85129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monger T. G., Alfano R. R., Callender R. H. Photochemistry of rhodopsin and isorhodopsin investigated on a picosecond time scale. Biophys J. 1979 Jul;27(1):105–115. doi: 10.1016/S0006-3495(79)85205-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters K., Applebury M. L., Rentzepis P. M. Primary photochemical event in vision: proton translocation. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3119–3123. doi: 10.1073/pnas.74.8.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulkes M., Lewis A., Marcus M. A. Resonance Raman spectroscopy of squid and bovine visual pigments: the primary photochemistry in visual transduction. Biochemistry. 1978 Oct 31;17(22):4712–4722. doi: 10.1021/bi00615a018. [DOI] [PubMed] [Google Scholar]
- Sundstrom V., Rentzepis P. M., Peters K., Applebury M. L. Kinetics of rhodopsin at room temperature measured by picosecond spectroscopy. Nature. 1977 Jun 16;267(5612):645–646. doi: 10.1038/267645a0. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Callender R. H. Primary photochemistry and photoisomerization of retinal at 77 degrees K in cattle and squid rhodopsins. Biophys J. 1981 May;34(2):261–270. doi: 10.1016/S0006-3495(81)84848-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga F., Kawamura S., Yoshizawa T. Analysis by spectral difference of the orientational change of the rhodopsin chromophore during bleaching. Vision Res. 1976;16(6):633–641. doi: 10.1016/0042-6989(76)90011-0. [DOI] [PubMed] [Google Scholar]
- Vetterlein D., Cassman M. Different expressions of cooperativity in the kinetics of two forms of cytoplasmic malic dehydrogenase. Biochemistry. 1974 Jul 30;13(16):3243–3250. doi: 10.1021/bi00713a009. [DOI] [PubMed] [Google Scholar]
- WALD G., BROWN P. K. The molar extinction of rhodopsin. J Gen Physiol. 1953 Nov 20;37(2):189–200. doi: 10.1085/jgp.37.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell W. H., Yudd A. P., Nakanishi K. Letter: Micellar effects on the photochemistry of rhodopsin. J Am Chem Soc. 1976 Jan 7;98(1):238–239. doi: 10.1021/ja00417a041. [DOI] [PubMed] [Google Scholar]
- YOSHIZAWA T., WALD G. Pre-lumirhodopsin and the bleaching of visual pigments. Nature. 1963 Mar 30;197:1279–1286. doi: 10.1038/1971279a0. [DOI] [PubMed] [Google Scholar]



