Abstract
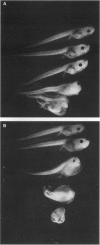
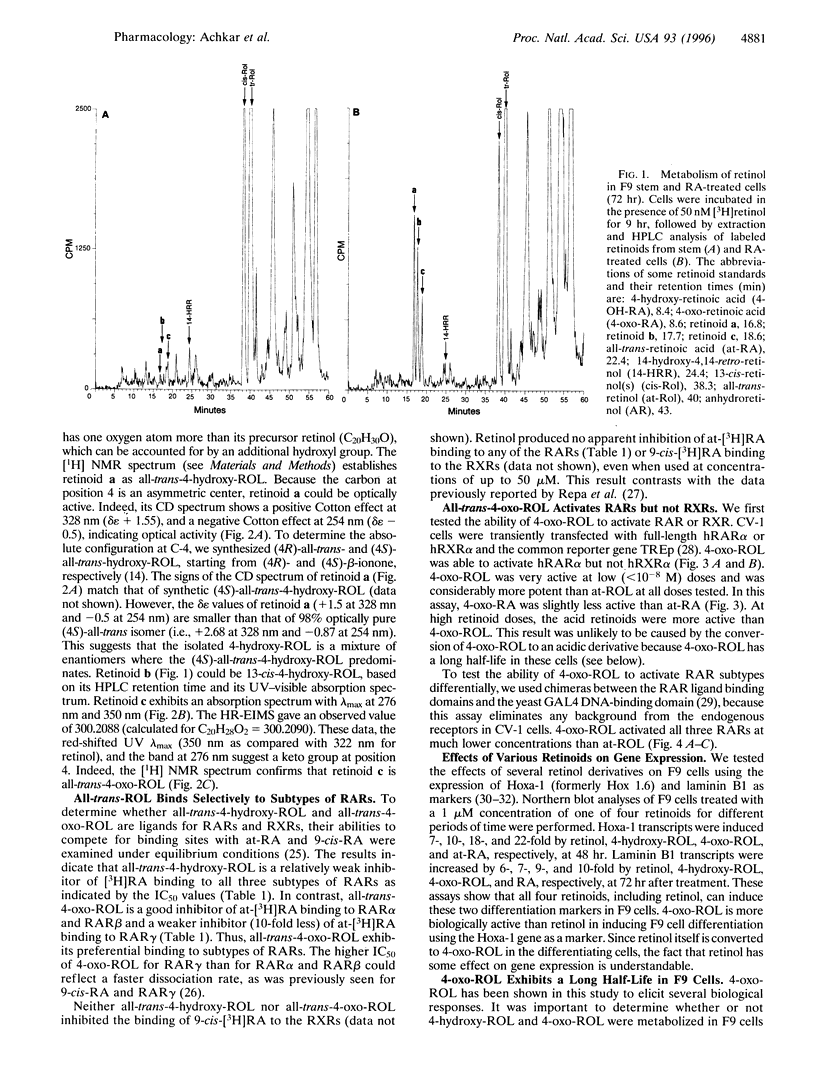
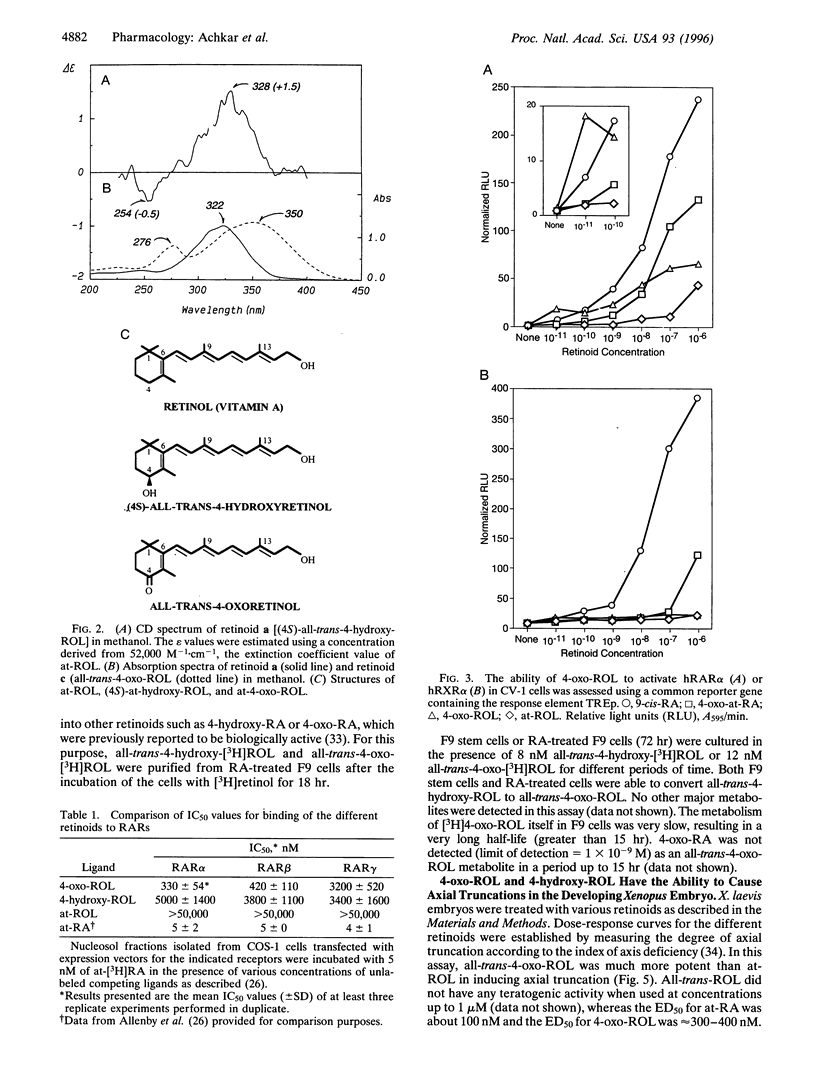
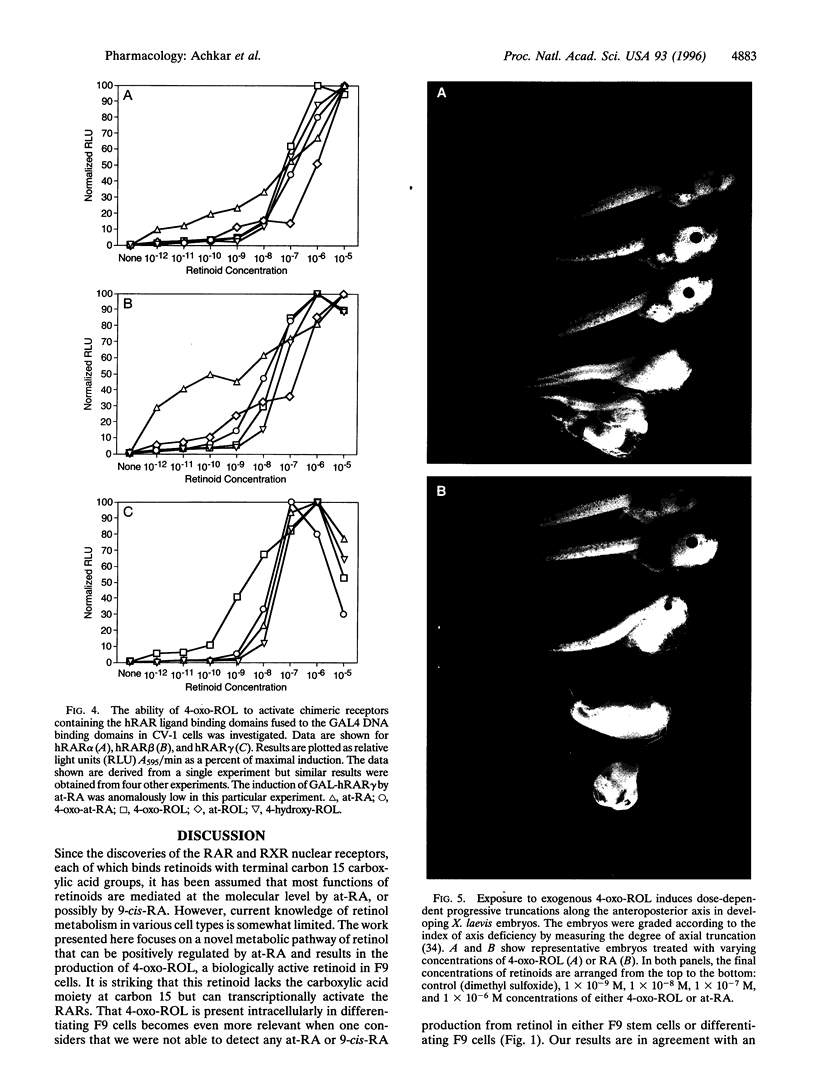
All-trans-retinoic acid (at-RA) induces cell differentiation in a wide variety of cell types, including F9 embryonic teratocarcinoma cells, and can influence axial pattern formation during embryonic development. We now identify a novel retinoid synthetic pathway in differentiating F9 cells that results in the intracellular production of 4-oxoretinol (4-oxo-ROL) from retinol (vitamin A). Approximately 10-15% of the total retinol in the culture is metabolized to 4-hydroxyretinol and 4-oxo-ROL by the at-RA-treated, differentiating F9 cells over an 18-hr period, but no detectable metabolism of all-trans-retinol to at-RA or 9-cis-retinoic acid is observed in these cells. Remarkably, we show that 4-oxo-ROL can bind and activate transcription of the retinoic acid receptors whereas all-trans-retinol shows neither activity. Low doses of 4-oxo-ROL (e.g., 10(-9) or 10(-10 M) can activate the retinoic acid receptors even though, unlike at-RA, 4-oxo-ROL does not contain an acid moiety at the carbon 15 position. 4-oxo-ROL does not bind or transcriptionally activate the retinoid X receptors. Treatment of F9 cells with 4-oxo-ROL induces differentiation without conversion to the acid and 4-oxo-ROL is active in causing axial truncation when administered to Xenopus embryos at the blastula stage. Thus, 4-oxo-ROL is a natural, biologically active retinoid that is present in differentiated F9 cells. Our data suggest that 4-oxo-ROL may be a novel signaling molecule and regulator of cell differentiation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allenby G., Bocquel M. T., Saunders M., Kazmer S., Speck J., Rosenberger M., Lovey A., Kastner P., Grippo J. F., Chambon P. Retinoic acid receptors and retinoid X receptors: interactions with endogenous retinoic acids. Proc Natl Acad Sci U S A. 1993 Jan 1;90(1):30–34. doi: 10.1073/pnas.90.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allenby G., Janocha R., Kazmer S., Speck J., Grippo J. F., Levin A. A. Binding of 9-cis-retinoic acid and all-trans-retinoic acid to retinoic acid receptors alpha, beta, and gamma. Retinoic acid receptor gamma binds all-trans-retinoic acid preferentially over 9-cis-retinoic acid. J Biol Chem. 1994 Jun 17;269(24):16689–16695. [PubMed] [Google Scholar]
- Boylan J. F., Gudas L. J. Overexpression of the cellular retinoic acid binding protein-I (CRABP-I) results in a reduction in differentiation-specific gene expression in F9 teratocarcinoma cells. J Cell Biol. 1991 Mar;112(5):965–979. doi: 10.1083/jcb.112.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boylan J. F., Gudas L. J. The level of CRABP-I expression influences the amounts and types of all-trans-retinoic acid metabolites in F9 teratocarcinoma stem cells. J Biol Chem. 1992 Oct 25;267(30):21486–21491. [PubMed] [Google Scholar]
- Buck J., Derguini F., Levi E., Nakanishi K., Hämmerling U. Intracellular signaling by 14-hydroxy-4,14-retro-retinol. Science. 1991 Dec 13;254(5038):1654–1656. doi: 10.1126/science.1749937. [DOI] [PubMed] [Google Scholar]
- Buck J., Grün F., Derguini F., Chen Y., Kimura S., Noy N., Hämmerling U. Anhydroretinol: a naturally occurring inhibitor of lymphocyte physiology. J Exp Med. 1993 Aug 1;178(2):675–680. doi: 10.1084/jem.178.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M., Achkar C., Gudas L. J. Enzymatic conversion of retinaldehyde to retinoic acid by cloned murine cytosolic and mitochondrial aldehyde dehydrogenases. Mol Pharmacol. 1994 Jul;46(1):88–96. [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Gubler M. L., Sherman M. I. Metabolism of retinoids by embryonal carcinoma cells. J Biol Chem. 1985 Aug 15;260(17):9552–9558. [PubMed] [Google Scholar]
- Heyman R. A., Mangelsdorf D. J., Dyck J. A., Stein R. B., Eichele G., Evans R. M., Thaller C. 9-cis retinoic acid is a high affinity ligand for the retinoid X receptor. Cell. 1992 Jan 24;68(2):397–406. doi: 10.1016/0092-8674(92)90479-v. [DOI] [PubMed] [Google Scholar]
- LaRosa G. J., Gudas L. J. An early effect of retinoic acid: cloning of an mRNA (Era-1) exhibiting rapid and protein synthesis-independent induction during teratocarcinoma stem cell differentiation. Proc Natl Acad Sci U S A. 1988 Jan;85(2):329–333. doi: 10.1073/pnas.85.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa G. J., Gudas L. J. Early retinoic acid-induced F9 teratocarcinoma stem cell gene ERA-1: alternate splicing creates transcripts for a homeobox-containing protein and one lacking the homeobox. Mol Cell Biol. 1988 Sep;8(9):3906–3917. doi: 10.1128/mcb.8.9.3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo M. A., Lasker J. M., Raucy J. L., Kim C. I., Black M., Lieber C. S. Metabolism of retinol and retinoic acid by human liver cytochrome P450IIC8. Arch Biochem Biophys. 1989 Feb 15;269(1):305–312. doi: 10.1016/0003-9861(89)90112-4. [DOI] [PubMed] [Google Scholar]
- Leo M. A., Lieber C. S. New pathway for retinol metabolism in liver microsomes. J Biol Chem. 1985 May 10;260(9):5228–5231. [PubMed] [Google Scholar]
- Levin A. A., Sturzenbecker L. J., Kazmer S., Bosakowski T., Huselton C., Allenby G., Speck J., Kratzeisen C., Rosenberger M., Lovey A. 9-cis retinoic acid stereoisomer binds and activates the nuclear receptor RXR alpha. Nature. 1992 Jan 23;355(6358):359–361. doi: 10.1038/355359a0. [DOI] [PubMed] [Google Scholar]
- McClean S. W., Ruddel M. E., Gross E. G., DeGiovanna J. J., Peck G. L. Liquid-chromatographic assay for retinol (vitamin A) and retinol analogs in therapeutic trials. Clin Chem. 1982 Apr;28(4 Pt 1):693–696. [PubMed] [Google Scholar]
- Nervi C., Grippo J. F., Sherman M. I., George M. D., Jetten A. M. Identification and characterization of nuclear retinoic acid-binding activity in human myeloblastic leukemia HL-60 cells. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5854–5858. doi: 10.1073/pnas.86.15.5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nervi C., Poindexter E. C., Grignani F., Pandolfi P. P., Lo Coco F., Avvisati G., Pelicci P. G., Jetten A. M. Characterization of the PML-RAR alpha chimeric product of the acute promyelocytic leukemia-specific t(15;17) translocation. Cancer Res. 1992 Jul 1;52(13):3687–3692. [PubMed] [Google Scholar]
- Ostrowski J., Hammer L., Roalsvig T., Pokornowski K., Reczek P. R. The N-terminal portion of domain E of retinoic acid receptors alpha and beta is essential for the recognition of retinoic acid and various analogs. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):1812–1816. doi: 10.1073/pnas.92.6.1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmann T., Rangarajan P. N., Umesono K., Evans R. M. Determinants for selective RAR and TR recognition of direct repeat HREs. Genes Dev. 1993 Jul;7(7B):1411–1422. doi: 10.1101/gad.7.7b.1411. [DOI] [PubMed] [Google Scholar]
- Pijnappel W. W., Hendriks H. F., Folkers G. E., van den Brink C. E., Dekker E. J., Edelenbosch C., van der Saag P. T., Durston A. J. The retinoid ligand 4-oxo-retinoic acid is a highly active modulator of positional specification. Nature. 1993 Nov 25;366(6453):340–344. doi: 10.1038/366340a0. [DOI] [PubMed] [Google Scholar]
- Repa J. J., Hanson K. K., Clagett-Dame M. All-trans-retinol is a ligand for the retinoic acid receptors. Proc Natl Acad Sci U S A. 1993 Aug 1;90(15):7293–7297. doi: 10.1073/pnas.90.15.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts E. S., Vaz A. D., Coon M. J. Role of isozymes of rabbit microsomal cytochrome P-450 in the metabolism of retinoic acid, retinol, and retinal. Mol Pharmacol. 1992 Feb;41(2):427–433. [PubMed] [Google Scholar]
- Scharf S. R., Gerhart J. C. Axis determination in eggs of Xenopus laevis: a critical period before first cleavage, identified by the common effects of cold, pressure and ultraviolet irradiation. Dev Biol. 1983 Sep;99(1):75–87. doi: 10.1016/0012-1606(83)90255-5. [DOI] [PubMed] [Google Scholar]
- Tate B. F., Allenby G., Janocha R., Kazmer S., Speck J., Sturzenbecker L. J., Abarzúa P., Levin A. A., Grippo J. F. Distinct binding determinants for 9-cis retinoic acid are located within AF-2 of retinoic acid receptor alpha. Mol Cell Biol. 1994 Apr;14(4):2323–2330. doi: 10.1128/mcb.14.4.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesono K., Giguere V., Glass C. K., Rosenfeld M. G., Evans R. M. Retinoic acid and thyroid hormone induce gene expression through a common responsive element. Nature. 1988 Nov 17;336(6196):262–265. doi: 10.1038/336262a0. [DOI] [PubMed] [Google Scholar]
- Vasios G. W., Gold J. D., Petkovich M., Chambon P., Gudas L. J. A retinoic acid-responsive element is present in the 5' flanking region of the laminin B1 gene. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9099–9103. doi: 10.1073/pnas.86.23.9099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. B., Napoli J. L. Metabolism of retinoic acid and retinol during differentiation of F9 embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4658–4662. doi: 10.1073/pnas.82.14.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]