Background: OXA-1 and OXA-24 are class D β-lactamases that resist clinically used inhibitors.
Results: Spectroscopic methods and kinetic measurements show that penem drug candidates are good inhibitors of OXA-1 but are rapidly hydrolyzed by OXA-24.
Conclusion: An active site water in OXA-24 aids the reversible carboxylation of Lys-84, enabling many reaction cycles.
Significance: Understanding the mechanism of class D β-lactamases is vital for drug development.
Keywords: Crystallography, Enzyme Mechanisms, Physical Methods, Raman Spectroscopy, UV Spectroscopy, OXA-1, OXA-24, Carboxylated Lysine, Penems
Abstract
The catalytic efficiency of class D β-lactamases depends critically on an unusual carboxylated lysine as the general base residue for both the acylation and deacylation steps of the enzyme. Microbiological and biochemical studies on the class D β-lactamases OXA-1 and OXA-24 showed that the two enzymes behave differently when reacting with two 6-methylidene penems (penem 1 and penem 3): the penems are good inhibitors of OXA-1 but act more like substrates for OXA-24. UV difference and Raman spectroscopy revealed that the respective reaction mechanisms are different. The penems form an unusual intermediate, a 1,4-thiazepine derivative in OXA-1, and undergo deacylation followed by the decarboxylation of Lys-70, rendering OXA-1 inactive. This inactivation could not be reversed by the addition of 100 mm NaHCO3. In OXA-24, under mild conditions (enzyme:inhibitor = 1:4), only hydrolyzed products were detected, and the enzyme remained active. However, under harsh conditions (enzyme:inhibitor = 1:2000), OXA-24 was inhibited via decarboxylation of Lys-84; however, the enzyme could be reactivated by the addition of 100 mm NaHCO3. We conclude that OXA-24 not only decarboxylates with difficulty but also recarboxylates with ease; in contrast, OXA-1 decarboxylates easily but recarboxylates with difficulty. Structural analysis of the active site indicates that a crystallographic water molecule may play an important role in carboxylation in OXA-24 (an analogous water molecule is not found in OXA-1), supporting the suggestion that a water molecule in the active site of OXA-24 can lower the energy barrier for carboxylation significantly.
Introduction
β-Lactamase production is the most important mechanism by which Gram-negative pathogens including Acinetobacter baumannii and Pseudomonas aeruginosa become resistant to β-lactam antibiotics. Based on their protein sequence similarities, they are divided into four major classes (Classes A–D) (1). Class A, C, and D enzymes involve an active site serine to hydrolyze β-lactams, whereas class B enzymes are zinc-dependent hydrolases. Unlike the majority of class A enzymes, which have been extensively studied, class D β-lactamases confer a higher level resistance to a broad spectrum of β-lactam inhibitors and are the least understood class (2, 3).
The class D β-lactamases are characterized by the presence of a unique carboxylated lysine in the active site that participates in catalysis. Although carboxylated lysine has also been found in other enzymes, such as ribulose-bisphosphate carboxylase/oxygenase (4), urease (5), and phosphotriesterase (6), it mainly serves in a structural role. The carboxylated lysine in class D enzymes plays a similar role as the general base (Glu-166) in SHV-1 class A β-lactamase (7). The formation of the carboxylated lysine is reversible (8). Low pH or mutation of hydrophobic residues surrounding the carboxylated lysine, such as Val-117 (OXA-1)3 or Trp-154 (OXA-10), results in decarboxylation of that lysine and loss of enzyme activity, notably deacylation (9–12), whereas the addition of bicarbonate can reactivate the enzyme by recarboxylation of the lysine (8, 12, 13).
OXA-1 and OXA-24 are two class D enzymes exhibiting resistance to the clinically available β-lactamase inhibitors (tazobactam, sulbactam, and clavulanate). They are both monomeric and are related based on three aspects: 1) ∼30% sequence homology, 2) similar folded structures, and 3) highly conserved active site residues (>95%) (using the following Protein Data Bank codes: OXA-1, 1M6K (2); OXA-24, 3G4P (14)). However, they show differing affinities for β-lactam-based inhibitors. OXA-1 is the most common of the class D β-lactamases and is found in up to 10% of Escherichia coli and Pseudomonas aeruginosa (2, 15). Its closely related variants (e.g. OXA-15, OXA-18, and OXA-19), resulting from point mutations and plasmid transfer, have arisen with enhanced capability to hydrolyze imipenem, aztreonam, and third generation cephalosporins, such as cefotaxime and ceftriaxone (15, 16). Found in A. baumannii, OXA-24 is a class D carbapenem-hydrolyzing enzyme that also possesses extended spectrum cephalosporinase activity (3, 17). Both OXA-1 and OXA-24 cause serious problems in nosocomial infections, such as bloodstream infections, wound infections, and ventilator-associated pneumonia (18, 19). Thus, the need to develop potent inhibitors of these enzymes is an urgent priority; to achieve this, it is imperative to understand the properties of these enzymes and how they work.
In this study, the catalytic properties of OXA-1 and OXA-24 were evaluated using two methylidene penems (penems 1 and 3; see Fig. 1). Bethel et al. (20) in 2008 proposed that penems inactivate OXA-1 β-lactamase efficiently by forming an unusual acyl-enzyme complex. Here, our results show that penem inhibitors have a high affinity for both OXA-1 and OXA-24 enzymes. However, they are effective inhibitors of OXA-1 but act more like substrates for OXA-24. UV difference (UVD) and Raman spectroscopies show that the reaction pathways are different when penems react with OXA-1 or OXA-24. Existing structural analysis of the active site indicates that a crystallographic water molecule may play an important role in carboxylation in OXA-24 (an analogous water molecule is not found in OXA-1), providing further support for the computational model by Schlegel and co-workers (21), who showed that a water molecule in the OXA-10 active site can lower the energy barrier for carboxylation.
FIGURE 1.
Chemical structures of penem inhibitors (penem 1 and penem 3), comparators (tazobactam and BRL 42715), and penam sulfone inhibitor (SA-1-204).
EXPERIMENTAL PROCEDURES
Genetic Constructs and Host Strains
The blaOXA-1 gene was cloned from plasmid RGN238 into pET12a(+)-KM as described previously (2). Plasmid RGN238blaOXA-1 was maintained in E. coli DH10B cells (Invitrogen). This host strain was used for minimum inhibitory concentration (MIC) determinations. For protein purification, blaOXA-1 was cloned in the modified vector pET12a(+)-KM described previously and was expressed in E. coli BL21(DE3) cells (Stratagene, La Jolla, CA) (16).
For large scale protein expression and β-lactamase characterization, the blaOXA-24 gene was cloned into the pET24a(+) vector (Novagen, Madison, WI) according to the following method. Using the Gene-Amp XL PCR kit (Applied Biosystems), high fidelity amplification of blaOXA-24 without leader peptide sequence from the OXA-24/pIM-1-RA clone designed by Héritier et al. (22) was performed with primers OXA-24FOR and OXA-24REV listed in Table 1. The cycling conditions used were 95 °C for 30 s, 55 °C for 1 min, and 72 °C for 1 min for 25 cycles after which there was final extension at 72 °C for 10 min. A restriction digest of the pET24a(+) vector was done using NdeI and BamHI. The amplification product was purified using the QIAquick gel extraction kit (Qiagen, Venlo, Netherlands) and digested using NdeI and BamHI. This product was ligated to the digested pET24a(+) vector and electroporated into E. coli DH10B. The resulting construct was sequenced with pET24a(+) primers T7 promoter primer and T7 terminator primer. After sequencing verification, the construct was transformed into E. coli BL21(DE3) cells for protein expression.
TABLE 1.
Primer sequences
| Function and primer | Sequence (5′ to 3′) |
|---|---|
| Cloning primers | |
| OXA-24FOR | CATATGTCTATTAAAACTAAATCTGA |
| OXA-24REV | GGATCCTTAAATGATTCCAAGA |
| OXA-24LDR | CATATGAAAAAATTTATACTTCCTATATTC |
| Sequencing (Cy5-labeled) primers | |
| M13 universal | GTAAAACGACGGCCAG |
| M13 reverse | CAGGAAACAGCTATGAC |
| T7 promoter | TAATACGACTCACTATAGGG |
| T7 terminator | GCTAGTTATTGCTCAGCGG |
For MIC determinations, blaOXA-24 was cloned into the pBC SK(+) phagemid vector (Stratagene) as described previously (23). blaOXA-24/pBC SK(+) sequence was verified using M13 universal and M13 reverse primers.
Inhibitors
The inhibitors penem 1 and penem 3 (Fig. 1) were gifts from Wyeth Pharmaceuticals (Madison, NJ). Their chemical syntheses were described previously (24). A stock solution of the inhibitor at 20 mm in 10 mm HEPES buffer (pH 7.5) was prepared for “soak-in” and “soak-out” experiments with the protein crystals and for UV absorbance studies. Only water was used in the rapid mix-rapid freeze experiment to avoid the interference of salt signal. Potency was verified using the colorimetric β-lactamase substrate nitrocefin (BD Biosciences; λmax = 482 nm; ϵ = 17,400 m−1 cm−1).
Antibiotic Susceptibility
The MICs of E. coli DH10B expressing blaOXA-1 or blaOXA-24 β-lactamases were determined in Mueller-Hinton agar supplemented with 20 mm NaHCO3 using a Steers replicator, which delivered 10 μl of Mueller-Hinton broth containing 104 colony-forming units/spot. The penem inhibitors were tested at the concentration of 4 μg/ml partnered with piperacillin (Sigma) at concentrations of 1–2048 μg/ml. As a comparator inhibitor, tazobactam (Chem-Impex, Wood Dale, IL) was used at the concentration of 4 μg/ml. Break points for susceptibility and resistance were defined by the Clinical Laboratory Standards Institute and interpreted with criteria published in 2005 (Clinical and Laboratory Standards Institute Standard M100-S15) (25, 26).
Protein Isolation, Purification, and Crystallization
OXA-1 or OXA-24 was purified as described previously (20, 27). The concentration of the protein was measured by a Bio-Rad protein assay, and the purity of the enzyme was evaluated by SDS-PAGE. Following purification, OXA-1 β-lactamase was crystallized using the protocol described in Sun et al. (2) with a protein concentration of 9 mg/ml. Crystals were grown by the hanging drop vapor diffusion method in a crystallization solution containing 0.05 m HEPES (pH 7.5) and 15% PEG 8000. OXA-24 β-lactamase was crystallized using the protocol of Bou et al. (14). Briefly, OXA-24 was concentrated to 6 mg/ml in 10 mm HEPES buffer (pH 7.5). Crystals were grown by the hanging drop vapor diffusion method in a crystallization solution containing 0.1 m HEPES (pH 7.5), 0.1 m sodium acetate, and 28% PEG 2000.
UVD Spectroscopy
In our protocol, each spectrum included absorbance at wavelengths (λ) from 200 to 600 nm. The spectrum of only β-lactamase (20 μm) or inhibitor (20 or 80 μm) was taken separately. Then OXA-1 and OXA-24 (20 μm for both) were incubated with penem 1 (20 or 80 μm) in a ratio of 1:1 or 1:4 (E:I), and UV absorbance spectra were taken at 30-s intervals; the length of the experiment was 30 min. To obtain UV difference spectra, an apo-β-lactamase spectrum was subtracted from the protein-inhibitor complex spectra at varying time intervals following addition of inhibitor. The nitrocefin (NCF) assay uses nitrocefin as a chromogenic substrate to monitor the enzyme activity in solution. When OXA-1 or OXA-24 react with NCF, NCF is hydrolyzed and turns red (λmax = 482 nm); when the enzyme is inhibited, NCF does not react and remains yellow (λmax = 382 nm).
Raman Spectroscopy
The Raman microscope system has been described previously (28, 29). A single OXA-1 or OXA-24 crystal was transferred from the mother liquor solution to a 4-μl drop of 0.05 m HEPES (pH 7.5) and 15% PEG 8000 (for OXA-1) or a drop of 0.1 m HEPES (pH 7.5), 0.1 m NaOAc, and 28% PEG 2000 (for OXA-24). After obtaining spectra of the apoprotein crystals, inhibitors were soaked into the drop to achieve a final volume of 5 μl and a final inhibitor concentration of 5 mm. Spectra were then acquired every 2–3 min after addition of the inhibitors. To obtain difference spectra, an apo-β-lactamase spectrum was subtracted from the protein-inhibitor spectra at varying time intervals following addition of inhibitor (27).
To study the reaction in solution at early time points, we used a slightly modified KinTek instrument model RQF-3 (30). Reactions were initiated by mixing OXA-1 or OXA-24 enzyme (2.5 mg/ml) with penem inhibitors in a 1:2 molar ratio and quenched at 1 s. The sample after reactions was examined using a Raman microscope (31). For a longer time scale reaction, we also used a hand mixing system that incubated enzyme with inhibitor at a 1:2 ratio for 5 and 30 s, and then the reaction was quenched by injecting into the isopentane solution surrounded by liquid nitrogen.
Kinetics
Steady-state kinetics were performed on an Agilent 8453 diode array spectrophotometer (Palo Alto, CA) in 50 mm sodium phosphate buffer (pH 7.2) supplemented with 20 mm NaHCO3. Vmax and Km were determined from initial steady-state velocities for NCF. The kinetic parameters were obtained using iterative non-linear least square fit of the data to the Henri-Michaelis equation using Origin 8.1 (OriginLab, Northampton, MA) according to Equation 1.
The overall mechanism for β-lactamase-inhibitor reaction is shown in Scheme 1. We determined the Ki for the penems by measuring initial steady-state velocities in the presence of a constant concentration of enzyme with increasing concentrations of inhibitor against the indicator substrate NCF (100 μm). Assuming a competitive mode of inhibition under these conditions, initial velocity (v0) measurements immediately after mixing yield a Ki that closely approximates Km as represented by Equation 2.
Ki values were corrected for nitrocefin affinity (Km = 8.3 μm for OXA-1 and Km = 28 μm for OXA-24) according to Equation 3.
IC50, defined as the inhibitor concentration resulting in a reduction of NCF (100 μm) hydrolysis by 50%, was determined by measurements of initial velocities after 5-min preincubation of enzyme with inhibitor.
SCHEME 1.
Reaction scheme of β-lactamase-inhibitor interactions. P, product; M-M complex, Michaelis-Menten complex.
Turnover numbers (tn) or partition ratios (kcat/kinact) (kcat refers to hydrolytic efficiency for inhibitors as shown in Scheme 1) were determined as the ratio of inhibitor concentration to enzyme concentration necessary to decrease enzyme activity by 95% (32). The turnover numbers were determined after a 24-h incubation with increasing concentrations of the inhibitor. Incubations were done in a final volume of 300 μl, and 25 μl of this reaction mixture were added to a 1-ml final volume to determine the residual enzyme activity using 100 μm nitrocefin.
Quantum Mechanical Calculations
Ab initio quantum mechanical calculations were performed on the Case Western Reserve University cluster facility to predict the Raman spectra of penems and model intermediate compounds using Gaussian 03 (33). Calculations were performed at the density functional theory level using the 6-31+G(d) basis set. Density functional theory calculations were performed with Becke's three parameters hybrid method using the correlation functional of Lee, Yang, and Parr (B3LYP) (34, 35). The vibrations giving rise to the most intense calculated peaks could be visualized using “GaussView,” revealing which molecular vibrations contribute to the peaks.
RESULTS
Kinetic Data for Penems 1 and 3 Reacting with OXA-1 and OXA-24
Antibiotic Susceptibility
To first determine whether penem inhibitors can be used as effective partners with clinical antibiotics, we performed microbiological assays to evaluate their ability to lower the MICs. To establish a comparison, we used piperacillin, a broad spectrum penicillin family member, with the penems at a concentration of 4 μg/ml. We also used tazobactam at the same concentration as a comparator β-lactamase inhibitor. Tazobactam in combination with piperacillin became available in the clinic in the United States in 1993 and does extend the activity of piperacillin against most class A β-lactamase-producing strains. Against E. coli DH10B lacking OXA-1 or OXA-24 expression, the piperacillin MICs are 8 μg/ml, well within the susceptible range for piperacillin (Clinical and Laboratory Standards Institute guidelines) (36). In the bacterial strain where OXA-1 or OXA-24 is expressed, a high level piperacillin resistance was observed (Table 2; the piperacillin MIC is 512 μg/ml for OXA-1 and 1024 μg/ml for OXA-24). When tazobactam was combined with piperacillin at the concentration of 4 μg/ml, we did not detect a reduction in MICs for OXA-24 (1024 μg/ml; Table 2), and we detected only a slight reduction for OXA-1 (256 μg/ml and no significant inhibition with piperacillin). This is consistent with the observation that the current clinically used β-lactamase inhibitors (tazobactam, sulbactam, and clavulanate) are not effective against class D β-lactamases (14, 20, 27). Before measuring the inhibitory activity of penem inhibitors combined with piperacillin, we first tested whether penem 1 or penem 3 possesses any intrinsic antibiotic activity against bacterial strains. The results showed that the MICs for penem 1 or penem 3 alone are >1024 μg/ml, indicating that alone they do not bear any inhibitory activity.
TABLE 2.
Minimum inhibitory concentration of laboratory isolates
According to the Clinical Laboratory Standards Institute, MIC break points for piperacillin and piperacillin/tazobactam are as follows: ≤8 μg/ml, susceptible; 8–16 μg/ml, intermediate; ≥32 μg/ml, resistant.
| Laboratory isolate | MICs |
|||
|---|---|---|---|---|
| Piperacillin | Piperacillin/tazobactama | Piperacillin/penem 1 | Piperacillin/penem 3 | |
| μg/ml | ||||
| E. coli DH10B | 8 | 8 | 8 | 8 |
| E. coli blaOXA-1 | 512 | 256 | 8 | 8 |
| E. coli blaOXA-24 | 1024 | 1024 | 1024 | 1024 |
a The concentration of tazobactam, penem 1, and penem 3 is 4 μg/ml.
Penems combined with piperacillin resulted in significant differences between OXA-1 and OXA-24. In OXA-1, a noticeable reduction in MICs by penem 1 or penem 3 was observed (512 to 8 μg/ml for both). However, in OXA-24, the MIC is not affected in the presence of penem 1 or penem 3, which shows that the two inhibitors are not effective against OXA-24 β-lactamase.
Kinetic Parameters
To further demonstrate that OXA-1 and OXA-24 β-lactamases behave differently with penem inhibitors, we performed kinetic assays to observe the properties and activities of penems 1 and 3. Table 3 shows the Ki and IC50 of the penem compounds with the enzymes OXA-1 and OXA-24. The data suggest that penem 1 and penem 3 are good inhibitors against both OXA-1 and OXA-24 because their Ki and IC50 values are very low (at nm level). However, closer examination shows that the IC50 value is much lower than Ki in OXA-1 but higher in OXA-24. This suggests that, in OXA-24, the two penem inhibitors to some extent undergo subsequent hydrolysis before forming the stable acyl-enzyme complex. Thus, next we aimed to measure the turnover number for both enzymes. In Table 3, the results show that tn for OXA-24 is ∼450 times higher than that for OXA-1 (900 versus 2). Considering that the periplasmic concentration of the OXA-10 β-lactamase in two clinical strains of Pseudomonas is about 4–15 μm (8), if OXA-1 or OXA-24 is at a similar concentration level as OXA-10, it would not be possible to inhibit OXA-24 under physiological conditions because of the high amounts of penems required. In summary, penems 1 and 3 are effective inhibitors for OXA-1 but not for OXA-24.
TABLE 3.
Kinetic parameters of inhibition
Ki for the penems was determined by measuring initial steady-state velocities in the presence of a constant concentration of enzyme with increasing concentrations of inhibitors against nitrocefin (100 μm); the value was then corrected for nitrocefin affinity. IC50 was determined by measurements of inhibitor concentration that reduces the initial velocities by 50% after 5-min preincubation of enzyme with inhibitor. tn was determined as the ratio of inhibitor concentration to enzyme concentration necessary to decrease enzyme activity by 95% after 24 h.
| Inhibitors | Enzymes |
|||||
|---|---|---|---|---|---|---|
| OXA-1 |
OXA-24 |
|||||
| Ki | IC50 | tn | Ki | IC50 | tn | |
| nm | nm | nm | nm | |||
| Penem 1 | 50 ± 8 | 30 ± 8 | 2 | 30 ± 2 | 150 ± 30 | 900 |
| Penem 3 | 380 ± 70 | 60 ± 5 | 2 | 150 ± 20 | 160 ± 40 | 925 |
Spectroscopic Evidence for Different Reaction Schemes in OXA-1 and OXA-24
UVD Spectroscopy: the Role of Carboxylated Lysine
Further evidence that OXA-1 and OXA-24 react differently with penem inhibitors was also obtained by UVD spectroscopy, which has been widely used to provide insight into the nature of reactive intermediates or products formed during β-lactamase inactivation processes (37–39). We incubated penem 1 with OXA-1 or OXA-24 at different ratios to see whether the reaction is stoichiometric. The data for penem 3 are not shown, but they are similar to the data for penem 1.
Fig. 2 shows the reaction between penem 1 and OXA-1 or OXA-24 at a 1:1 or 1:4 ratio (E:I). The peak at 280 nm represents the unreacted compounds and is assigned to an electronic transition located in the conjugated region involving the double bonds in the bicyclic ring and the methylenic double bond at C6 position, extending to the carbonyl group in the β-lactam ring. At a 1:1 ratio, all penem 1 molecules have been consumed because the 280 nm peak disappears, only leaving the product spectrum (Fig. 2A). However, at a 1:4 ratio, almost three-fourths of the penem 1 remained unreacted in OXA-1 (Fig. 2B, red line), and at this time, the enzyme is no longer active against nitrocefin (not shown). In OXA-24, the spectrum for the 1:4 ratio shows a pattern similar to that of the 1:1 ratio in that all penem 1 is hydrolyzed (Fig. 2B, blue line). The enzyme is still active.
FIGURE 2.

UV difference spectrum of OXA-1 and OXA-24 reacting with penem 1. The intense peak at 280 nm represents unreacted penem 1 (black line). The concentration of enzyme is 20 μm for OXA-1 and OXA-24. The concentration of inhibitor is 20 μm (1:1) or 80 μm (1:4). After the enzyme is incubated with penem 1 inhibitor, the spectrum is recorded every 30 s for 30 min. The spectra shown in the figure are the UV difference spectra at 1 min (the other spectra overlap together because the reaction occurs rapidly in solution). A, OXA-1 or OXA-24 reacting with penem 1 at a 1:1 ratio (E:I). B, OXA-1 or OXA-24 with penem 1 at a 1:4 ratio.
The unique peaks of the hydrolyzed product spectra in OXA-1 (255 and 375 nm; Fig. 2A) and OXA-24 (351 nm; Fig. 2A) suggest that the reaction undergoes different pathways. Based on the studies by Bethel et al. (20), we propose a reaction mechanism for penem 1 and OXA-1 (Scheme 2, modified from Bethel et al. (20)). Previous studies of compound BRL 42715 (see Fig. 1), which has a structure similar to penem 1, showed that its reaction with a base (sodium methoxide in methanol) results in the formation of a seven-membered thiazepine with chromophores at 253 and 370 nm (40), which is consistent with the peaks in UVD spectroscopy of OXA-1. In addition, the NMR studies by Bethel et al. (20) also support the conclusion that a similar intermediate, the 1,4-thiazepine derivative, is formed during the reaction between penem 1 and OXA-1. Both of these serve as the experimental foundation for our proposed mechanism in Scheme 2 and support the existence of a seven-membered thiazepine in the reaction between penem 1 and OXA-1.
SCHEME 2.
Proposed mechanism for penem 1 and OXA-1.
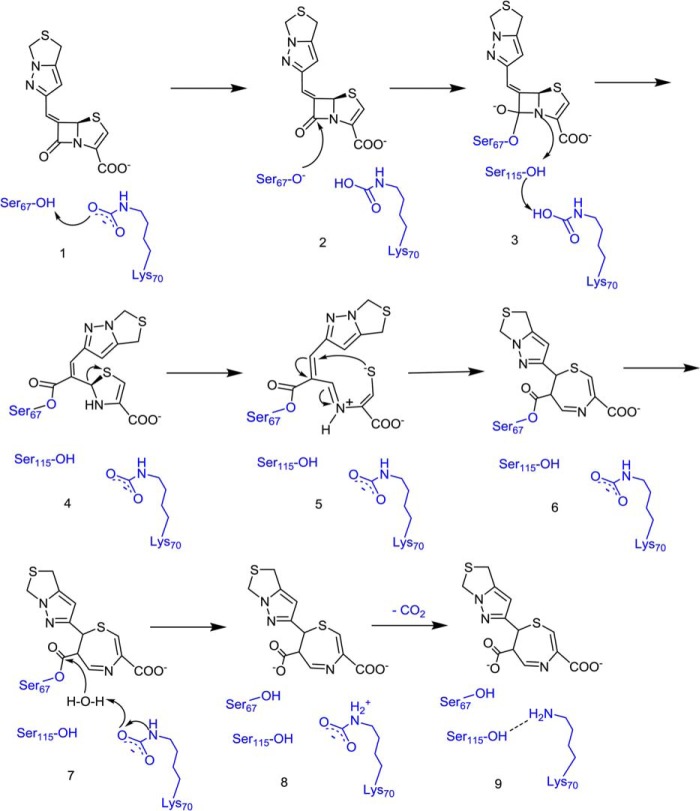
For OXA-24, in terms of the turnover number (900 for penem 1), the situation mostly mimics the reaction pathway for penicillin hydrolyzed by OXA-24 β-lactamase (14). Under normal conditions, penem inhibitors are treated as substrates of the enzyme. Thus, we propose another mechanism for penem 1 and OXA-24 (Scheme 3; adapted from the mechanism for penicillin hydrolysis (14)). In contrast to the reaction in OXA-1, the carboxylated Lys-84 in the active site of OXA-24 utilizes a catalytic water molecule to deacylate the Ser-81. As a result, the penem 1 inhibitor is hydrolyzed, and the enzyme is regenerated because Lys-84 is not decarboxylated (as discussed below) and hydrolyzes the next arriving inhibitor molecule.
SCHEME 3.
Proposed mechanism for penem 1 and OXA-24.
To confirm the role of carboxylation and decarboxylation of the active site lysine, we undertook assays with NCF and bicarbonate. The nitrocefin assay shows that OXA-24 can be inhibited by a high concentration of penem 1 (E:I = 1:2000), although the addition of 100 mm NaHCO3 can reactivate the enzyme because nitrocefin is hydrolyzed again (Fig. 3A). A recent study in our laboratory shows that one penam sulfone inhibitor, SA-1-204 (Fig. 1), can effectively inhibit OXA-24 by decarboxylating the Lys-84 in the active site; however, the enzyme is recarboxylated and becomes active again after adding 100 mm NaHCO3 as a source of CO2 molecules in the solution (27). These findings together indicate that, in OXA-24, a high concentration of penem 1 causes the decarboxylation of Lys-84, which has been shown to be critical for the deacylation of the enzyme (10, 27).
FIGURE 3.

Recarboxylation of the lysine can restore the enzyme activity in OXA-24 but not in OXA-1. A, black line, 1 μm OXA-24 was incubated with 100 μm NCF in 10 mm HEPES buffer (pH 7.5); red line, 1 μm OXA-24 was first incubated with 2 mm penem 1, and then 100 μm NCF was added; blue line, reagents were added in the order of 1 μm OXA-24, 2 mm penem 1, 100 μm NCF, and 100 mm NaHCO3. B, black line, 1 μm OXA-1 was incubated with 100 μm NCF in 10 mm HEPES buffer (pH 7.5); red line, 1 μm OXA-1 was first incubated with 4 μm penem 1, and then 100 μm NCF was added; blue line, reagents were added in the order of 1 μm OXA-1, 4 μm penem 1, 100 μm NCF, and 100 mm NaHCO3.
In contrast, the nitrocefin assay shows that a low concentration of penem 1 effectively inhibits OXA-1, but nitrocefin does not react in a mixture of OXA-1 and penem 1 (E:I = 1:4) after treatment with 100 mm NaHCO3 (Fig. 3B). Considering that OXA-1 and OXA-24 belong to class D β-lactamases that use a carboxylated lysine side chain (Lys-70 and Lys-84, respectively) to aid catalysis, we hypothesize that penem 1 can also cause the essentially irreversible decarboxylation of OXA-1.
Raman Studies of Penem 1-OXA-1/OXA-24 Reactions in Solution
Using the rapid mix-rapid freeze protocol developed in our laboratory (30), we examined the reaction between OXA-1 or OXA-24 and penem 1 at 1 s after mixing. A ratio of 1:2 (E:I) was used. For OXA-1, we expect to see the inhibited complex (tn is 2; Table 3), but for OXA-24, we expect to see predominantly hydrolyzed product because tn is about 900 (Table 3). These predictions are supported by the UVD spectra discussed in the previous section.
Fig. 4 compares the Raman spectrum of free penem 1 in aqueous solution with the spectrum of the freeze-dried reaction mixtures obtained 1 s after mixing and flash freezing. We first used ab initio quantum mechanical calculations to help interpret the spectrum of the substrate (Fig. 4, upper trace), which serves as a basis for the following analysis. Based on Gaussian calculations, the peak at 1687 cm−1 in the spectrum of unreacted penem 1 is assigned to the methylenic double bond coupled to the carbonyl group in the β-lactam ring (Table 4), which is expected to change markedly when the β-lactam ring opens or the hybridization at C6 changes. Another characteristic feature due to the carbonyl group (C=O) in the intact lactam ring is at 1757 cm−1. The disappearance of this peak suggests the opening of the lactam ring due to acylation of the enzyme (41).
FIGURE 4.

Raman difference of spectra of reactions between penem 1 and OXA-1 or OXA-24 in solution. The unreacted penem 1 spectrum (upper trace; 10 mm in H2O) is shown. Enzyme (86 μm) and inhibitor (172 μm) were incubated at a ratio of 1:2 (E:I). The reactions were quenched by liquid nitrogen after 1 s. The ice was then freeze-dried and characterized by Raman microscopy. 4(2) and 9(2), species 4 and 9 in Scheme 2; C(3) and F(3), species C and F in Scheme 3; C5-S1, C–S bond of the thiazole ring attached to the β-lactam ring.
TABLE 4.
Raman peak assignments for the major peaks in penem 1 complexed with OXA-1 or OXA-24 difference spectra shown in Fig. 4
The structures of the proposed intermediates in the reaction pathway were sent to the High Performance Computing Cluster at Case Western Reserve University for calculating the theoretical Raman spectrum.

a The values in this column represent the peaks from the spectrum of OXA-1/OXA-24 reaction with penem 1 in Fig. 4.
For OXA-1 reacting with penem 1, we see evidence for two species. Quantum mechanical calculations indicate that the band at 1630 cm−1 is from species 4 in Scheme 2 and is essentially due to the methylenic double bond stretch coupled to the ester bond (-O-C(=O)-C=C-) in acyl-enzymes (Table 4), although this mode is partially delocalized over adjacent conjugated bonds. Chemical structures including this acrylic group (R1-O-C(=O)-C=C-R2) all give rise to intense Raman peak around 1628 cm−1 (data not shown). This assignment is strengthened by the observation that a similar mode occurs at 1645 cm−1 for the reaction with OXA-24 (Fig. 4). The latter mode is due to hydrolyzed species E and/or F in Scheme 3 (see below). Carey and co-workers (42) reported in the 1980s that the ethylenic stretch in α,β conjugated molecules, such as cinnamic acid and furylacryloyl acid, increases by 10–15 cm−1 upon ionization of the acid group (see Table 2 in Ref. 42). Species E and F (Scheme 3) are the ionized forms, and species 4 (Scheme 2) is the neutral ester form. Important evidence for the second species comes from the broad unresolved band around 1500 cm−1 (Fig. 4). The quantum mechanical calculations for the seven-membered ring seen in Scheme 2 show two intense features near 1510 and 1492 cm−1 (Table 4). These features arise from the double bonds in the seven-membered ring and are not resolved in Fig. 4. Meanwhile, we do not detect a mode characterizing an ester near 1725 cm−1. This argues that we are detecting the species with the ionized -COO− (species 9) and not the ester-like acyl-enzyme (species 6). However, we cannot definitively argue in favor of species 9 because in some instances the Raman spectrum of acrylic acid ester, the C=O feature, has low intensity. Interestingly, a characteristic C–S stretch is also present at 715 cm−1, giving additional support to the formation of the seven-membered ring. It is noteworthy that Ke et al. (43) have detected the same seven-membered ring species in the x-ray structure of SHV-1 class A β-lactamase complexed with penem 1.
The reason we have evidence for two species from the OXA-1 reaction is that with a tn of 2 we expect to produce a population of product species 9 in Scheme 2 and a population of covalently inhibited stable acyl-enzyme (species 4 in Scheme 2). This analysis predicts that Lys-70 is decarboxylated after the first cycle, and thus, in the second cycle, species 4 is bound irreversibly. An underlying hypothesis is that acylation can occur without the participation of Lys-70, but that this residue is essential for deacylation.
The solution data for OXA-24 and penem 1 are expected to be very different because the tn is high for OXA-24 (900; Table 3); we should see a lot of penem 1 transformed into hydrolyzed product. This expectation is confirmed by the Gaussian calculations. The latter show that species F (Scheme 3) has an intense Raman band near 1645 cm−1 as seen in Fig. 4. This is due to the C=C stretch in molecule F where -COO− shifts new C=C 10–15 cm−1 to a higher wave number than in the neutral molecule C as discussed above and listed in Table 4. The calculations also reproduce the intense Raman band near 716 cm−1 from the thiazole five-membered ring of species F (Scheme 3) and support the presence of the thiazole ring.
Raman Analysis of Penem 1 Reaction with OXA-1/OXA-24 Single Crystals
Whereas the solution studies had an enzyme:inhibitor ratio of 1:2, the crystals suspended in a hanging drop containing inhibitor have access to a huge excess of inhibitors (10 mm in a 5-μl drop). For OXA-1 and penem 1, it is again likely that the enzyme goes through a maximum of two cycles.
Fig. 5A shows the Raman difference spectra of the penem 1 reaction in OXA-1 single crystals (underneath the spectrum of unreacted penem 1 in aqueous solution). The results show that the reaction occurs slowly because there is no intense Raman signal at 3 min (Fig. 5A), indicating that the penem 1 molecule has not entered the crystal and the active site of OXA-1 by that time. At 15 min, some new peaks appear, e.g. at 1656 cm−1. At 30 min, another peak at 1628 cm−1 intensifies and remains stable for up to 1 h; this is assigned below. The intense peak at 1656 cm−1 is assigned to the protonated imine (-C=NH+-) of species 5 (Scheme 2). This protonated imine, due to the opening of the five-membered ring, is a common intermediate in sulbactam, tazobactam, and clavulanate reactions with SHV-1 β-lactamase that also give rise to an intense peak at 1656 cm−1 (44). On the basis of the calculations discussed above, we predict the seven-member ring product (species 9; Scheme 2) will generate two intense peaks around 1490 and 1500 cm−1. These were detected in solution (above), but they are not obvious in Fig. 5A. The reason may be due to low abundance or fast release. Considering that there are still intense substrate peaks together with the intermediate peaks under soak-in conditions, to see what species is finally left in the active site of OXA-1, we performed a soak-out experiment. By immersing the reacted crystal in holding solution containing no substrate, we remove non-covalently bound substrates or products, leaving the covalently bound species in the active site. After 1-h incubation with penem 1, we transferred the crystal to a new hanging drop without any inhibitor molecule and took the spectrum of the crystal. In the soak-out experiment, the peaks at 1761, 1689, and 1654 cm−1 disappear; only the 1629 cm−1 peak remains, strongly suggesting that the species at 1629 cm−1 represents an entity covalently bound in the active site (Fig. 5A).
FIGURE 5.

Raman difference spectra of OXA-1 or OXA-24 single crystal reaction with penem 1. A, Raman difference spectrum of OXA-1 and penem 1 at 3, 15, and 30 min in the presence of PEG 8000. A control spectrum of penem 1 (upper trace) was first recorded in the presence of PEG. After penem 1 was soaked in, the spectra were taken at the above indicated time points. B, Raman difference spectrum of OXA-24 and penem 1 at 4, 15, and 30 min in the presence of PEG 2000. A control spectrum of penem 1 (upper trace) was first recorded in the presence of PEG. After penem 1 was soaked in, the spectra were taken at the above indicated time points.
In accord with the solution studies (above), the 1629 cm−1 feature is assigned to the stable acyl-enzyme (species 4 in Scheme 2) that resists hydrolysis because Lys-70 is decarboxylated in the first cycle of the reaction. Thus, it appears that species 4 in reaction Scheme 2 is the final stable product in the crystal after 30-min soak-in and 30-min soak-out.
In the crystal reaction for OXA-24, we predict that the reaction cycle will occur many times because the tn for penem 1 is ≈900 (Table 3). Fig. 5B shows the reaction between penem 1 and OXA-24 single crystals. As for OXA-1, penem 1 enters the active site slowly because at 4 min there are no intense substrate or intermediate peaks. At 15 min, a broad peak at 1628 cm−1 appears, and another intense peak at 1647 cm−1 is present at 30 min. As described above, based on the quantum mechanical calculations, the peak at 1647 cm−1 is due to the released species F (Scheme 3), which is produced in large amounts due to multiple reaction cycles. Again, to remove the unreacted substrate and detect the covalently bound species in the active site, the soak-out experiment was conducted. Interestingly, we saw the 1628 cm−1 peak, the same peak as in the active site of OXA-1. This indicates that, after penem 1 reacts with OXA-1 or OXA-24, they both have the same species finally remaining, covalently bound, in the active site. We observe the 1628 cm−1 band due to species C in Scheme 3 because in OXA-24 Lys-84 has finally been decarboxylated after about 900 cycles, and acyl-enzyme species C can no longer be hydrolyzed. The same hypothesis applies as that for OXA-1: acylation is still possible without a carboxylated lysine, but deacylation cannot occur or occurs very slowly at best.
DISCUSSION
The results discussed above indicate that OXA-1 and OXA-24 react differently with the penem inhibitors. The deacylating lysine in both enzymes can be decarboxylated by penem 1; OXA-1 resists recarboxylation and thus cannot hydrolyze the acyl-enzyme. An unusual finding in our work is that, at modest conditions, penems 1 and 3 appear to be ineffective against OXA-24. Based on the kinetic data and the reactions in crystal and in solution, we propose that penem 1 causes decarboxylation of Lys-70 in the active site of OXA-1 in the first cycle, rendering the enzyme inactive. The next arriving molecule forms the acyl-enzyme complex (species 4; Scheme 2) where carboxylation of Lys-70 is not essential for acylation (10, 45), but it does not react further because the enzyme loses the deacylation function following decarboxylation of Lys-70. However, decarboxylation of Lys-84 in the active site of OXA-24 occurs only under harsh conditions. The enzyme remains active and hydrolyzes penem 1 again and again. The difference in the nitrocefin assay indicates that OXA-1 and OXA-24 differ in both decarboxylation and recarboxylation steps. OXA-24 not only decarboxylates with difficulty but also recarboxylates with ease. In contrast, OXA-1 decarboxylates easily but recarboxylates with difficulty. We now discuss evidence from the literature to explain the difference between OXA-1 and OXA-24.
Previous studies by Mobashery and co-workers (46, 47) showed that the BlaR1 sensor, a signal transducer found in Staphylococcus aureus bacterium, has a serine and carboxylated lysine motif in the active site. Its protein sequence and overall folding indicate that it is evolutionarily related to class D β-lactamases. When the sensor reacts with antibiotics, it forms an acyl-enzyme complex, and the Lys-62 undergoes decarboxylation, switching the receptor to the “on” state and continuously inducing the expression of the β-lactamase (47–50). To find out why BlaR1 sensor decarboxylates easily compared with OXA enzymes, Birck et al. (47) in a related study used the x-ray coordinates of OXA-10 as a model to calculate the energy barrier to decarboxylation. Before the decarboxylation occurs, the carboxylated lysine is protonated following active site serine acylation (47, 51, 52). Based on their calculations on OXA-10 (Fig. 6), if the protonation occurs on the oxygen in the -NH-COO− of the side chain, there is a huge barrier (∼40 kcal/mol) for the decarboxylation of Lys-70. However, if the protonation is on the ζ-nitrogen in the NH of the side chain, there is no barrier for the decarboxylation. Thus, in BlaR1 sensor, if ζ-nitrogen protonation can be prevented, Lys-62 will remain carboxylated, and the acyl-enzyme can be hydrolyzed. Based on this consideration, Birck et al. (47) changed the Lys-62 in BlaR1 to S-(4-butanoate)-cysteine by chemical mutagenesis (53) to see whether it is sufficient to convert the BlaR1 sensor from a susceptible receptor to an antibiotic-resistant enzyme (51). The results show that the cysteine derivative does not undergo decarboxylation, and the variant hydrolyzes the acyl-enzymes formed from a broad spectrum of antibiotics (51). Kinetic studies indicate that this behavior is reproduced in OXA-10 (8). Returning to the OXA-1 and OXA-24 systems, we postulate that, in OXA-1, it is the ζ-nitrogen of Lys-70 that undergoes protonation in the deacylation process, and this brings about decarboxylation (Scheme 2, species 7, 8, and 9). Although the Ser-67 is free now and can react with the next penem 1 molecule, forming the acyl-enzyme complex, the Lys-70 is inactive and loses the deacylation function because of the decarboxylation. Previous studies have also shown that BlaR1 sensor shares more similar properties with OXA-1 than with OXA-24 (47, 54, 55). In OXA-24, we postulate that it is the COO− that undergoes protonation (Scheme 3, species D); thus, the enzyme is still active and can hydrolyze the next penem 1 molecule.
FIGURE 6.

Quantum mechanical/molecular mechanical calculations using the x-ray coordinates of the OXA-10 active site reveal that the protonation of the ζ-nitrogen leads to a barrierless decarboxylation of the lysine carbamate (modified from Birck et al. (47)).
Regarding the recarboxylation process, we found that OXA-24 can be reactivated by the addition of 100 mm NaHCO3, but OXA-1 cannot. According to the calculation results by Schlegel and co-workers on OXA-10 β-lactamase (21), the barrier for unassisted carboxylation of neutral lysine is as high as 30 kcal/mol. However, if the recarboxylation is assisted by a water molecule close enough (2.4–2.8 Å) to the lysine in the active site, the energy barrier is much lower (exothermic with a barrier of 14 kcal/mol). This led us to examine the active sites of both enzymes to determine whether there is a water molecule that exists near the lysine in the active site of OXA-24 but not in OXA-1. Fig. 7 shows the positions of the water molecules surrounding the lysine residue in the active sites of OXA-1 and OXA-24. In OXA-1, the closest water molecule is 4.6 Å away from Lys-70; in OXA-24, it is 2.6 Å away. Thus, there is a water molecule in OXA-24 that can be utilized to catalyze the recarboxylation. This water molecule, together with Trp-167, can form H-bonds with the CO2 group (2.6 and 2.9 Å, respectively), positioning the CO2 in the right place, ready for the attack from ζ-nitrogen in Lys-84 to form the carboxylated lysine seen in Fig. 7. A detailed model for the reaction scheme during lysine carboxylation is shown in Li et al. (21). The x-ray structure of OXA-24 complexed with inhibitor LN-1-255 also indicates that the bulky side group in the inhibitor seems to force out the water molecule, leading to deacylation deficiency (14). Considering that the CO2 concentration is about 1.3 mm in cells (56), OXA-24 has easy access to the CO2 group and becomes fully carboxylated. This may explain in part why clinical strains harboring blaOXA-24 genes have multiple drug resistance to β-lactams.
FIGURE 7.

The water position in the active site of OXA-1 (A) and OXA-24 (B). The water molecule is shown as a red sphere. Selected interacting residues are labeled, and hydrogen bonds are indicated by dashed lines. The Protein Data Bank codes are as follows: OXA-1, 1M6K; OXA-24, 3G4P.
CONCLUSIONS
In conclusion, the two variants of class D β-lactamase, OXA-1 and OXA-24, are found to react differently with 6-methyledene penems. This is ascribed to the differential stability of the carboxylated lysine in the active site. The catalytic difference between OXA-1 and OXA-24 also provides us new insight into the inactivation mechanism by OXA carbapenemases. Compared with OXA-1, OXA-24 is not only difficult to decarboxylate but also can easily be recarboxylated. This may also be consistent with their relative pathogenic effects as the pathogen harboring blaOXA-24 (A. baumannii) has been more problematic in the clinic. Based on these findings, a novel inhibitor design of class D β-lactamase should aim to accelerate the decarboxylation step and also importantly retard the recarboxylation step unlike the traditional strategy in class A and C β-lactamases of blocking Glu-166 (or its homologue)-assisted hydrolysis.
Acknowledgments
We thank Dr. Mary Barkley (Department of Chemistry, Case Western Reserve University) for the loan of the KinTek system. We also acknowledge the use of the High Performance Computing Cluster at Case Western Reserve University.
This work was supported, in whole or in part, by National Institutes of Health Grants GM54072 (to P. R. C.) and R01-AI100560 and R01-AI063517 (to R. A. B.).
- OXA
- oxacillinase
- E:I
- enzyme:inhibitor
- NCF
- nitrocefin
- UVD
- ultraviolet difference
- MIC
- minimum inhibitory concentration.
REFERENCES
- 1. Ambler R. P. (1980) The structure of β-lactamases. Philos. Trans. R. Soc. Lond. B Biol. Sci. 289, 321–331 [DOI] [PubMed] [Google Scholar]
- 2. Sun T., Nukaga M., Mayama K., Braswell E. H., Knox J. R. (2003) Comparison of β-lactamases of classes A and D: 1.5-Å crystallographic structure of the class D OXA-1 oxacillinase. Protein Sci. 12, 82–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Poirel L., Naas T., Nordmann P. (2010) Diversity, epidemiology, and genetics of class D β-lactamases. Antimicrob. Agents Chemother. 54, 24–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lorimer G. H., Badger M. R., Andrews T. J. (1976) Activation of ribulose-1,5-bisphosphate carboxylase by carbon dioxide and magnesium ions—equilibria, kinetics, a suggested mechanism, and physiological implications. Biochemistry 15, 529–536 [DOI] [PubMed] [Google Scholar]
- 5. Jabri E., Carr M. B., Hausinger R. P., Karplus P. A. (1995). The crystal structure of urease from Klebsiella aerogenes. Science 268, 998–1004 [PubMed] [Google Scholar]
- 6. Shim H., Raushel F. M. (2000) Self-assembly of the binuclear metal center of phosphotriesterase. Biochemistry 39, 7357–7364 [DOI] [PubMed] [Google Scholar]
- 7. Meroueh S. O., Fisher J. F., Schlegel H. B., Mobashery S. (2005) Ab initio QM/MM study of class A β-lactamase acylation: dual participation of Glu166 and Lys73 in a concerted base promotion of Ser70. J. Am. Chem. Soc. 127, 15397–15407 [DOI] [PubMed] [Google Scholar]
- 8. Golemi D., Maveyraud L., Vakulenko S., Samama J. P., Mobashery S. (2001) Critical involvement of a carbamylated lysine in catalytic function of class D β-lactamases. Proc. Natl. Acad. Sci. U.S.A. 98, 14280–14285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Baurin S., Vercheval L., Bouillenne F., Falzone C., Brans A., Jacquamet L., Ferrer J. L., Sauvage E., Dehareng D., Frère J. M., Charlier P., Galleni M., Kerff F. (2009) Critical role of tryptophan 154 for the activity and stability of class D β-lactamases. Biochemistry 48, 11252–11263 [DOI] [PubMed] [Google Scholar]
- 10. Schneider K. D., Ortega C. J., Renck N. A., Bonomo R. A., Powers R. A., Leonard D. A. (2011) Structures of the class D carbapenemase OXA-24 from Acinetobacter baumannii in complex with doripenem. J. Mol. Biol. 406, 583–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Buchman J. S., Schneider K. D., Lloyd A. R., Pavlish S. L., Leonard D. A. (2012) Site-saturation mutagenesis of position V117 in OXA-1 β-lactamase: effect of side chain polarity on enzyme carboxylation and substrate turnover. Biochemistry 51, 3143–3150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leonard D. A., Bonomo R. A., Powers R. A. (2013) Class D β-lactamases: a reappraisal after five decades. Acc. Chem. Res. 46, 2407–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Leonard D. A., Hujer A. M., Smith B. A., Schneider K. D., Bethel C. R., Hujer K. M., Bonomo R. A. (2008) The role of OXA-1 β-lactamase Asp66 in the stabilization of the active-site carbamate group and in substrate turnover. Biochem. J. 410, 455–462 [DOI] [PubMed] [Google Scholar]
- 14. Bou G., Santillana E., Sheri A., Beceiro A., Sampson J. M., Kalp M., Bethel C. R., Distler A. M., Drawz S. M., Pagadala S. R., van den Akker F., Bonomo R. A., Romero A., Buynak J. D. (2010) Design, synthesis, and crystal structures of 6-alkylidene-2′-substituted penicillanic acid sulfones as potent inhibitors of Acinetobacter baumannii OXA-24 carbapenemase. J. Am. Chem. Soc. 132, 13320–13331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Naas T., Nordmann P. (1999) OXA-type β-lactamases. Curr. Pharm. Des. 5, 865–879 [PubMed] [Google Scholar]
- 16. Sun T., Nukaga M., Mayama K., Crichlow G. V., Kuzin A. P., Knox J. R. (2001) Crystallization and preliminary x-ray study of OXA-1, a class D β-lactamase. Acta Crystallogr. D Biol. Crystallogr. 57, 1912–1914 [DOI] [PubMed] [Google Scholar]
- 17. Poirel L., Marqué S., Héritier C., Segonds C., Chabanon G., Nordmann P. (2005) OXA-58, a novel class D β-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob Agents Chemother. 49, 202–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bou G., Oliver A., Martínez-Beltrán J. (2000) OXA-24, a novel class D β-lactamase with carbapenemase activity in an Acinetobacter baumannii clinical strain. Antimicrob. Agents Chemother. 44, 1556–1561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bergogne-Bérézin E., Towner K. J. (1996) Acinetobacter spp. as nosocomial pathogens: microbiological, clinical, and epidemiological features. Clin. Microbiol. Rev. 9, 148–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bethel C. R., Distler A. M., Ruszczycky M. W., Carey M. P., Carey P. R., Hujer A. M., Taracila M., Helfand M. S., Thomson J. M., Kalp M., Anderson V. E., Leonard D. A., Hujer K. M., Abe T., Venkatesan A. M., Mansour T. S., Bonomo R. A. (2008) Inhibition of OXA-1 β-lactamase by penems. Antimicrob. Agents Chemother. 52, 3135–3143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li J., Cross J. B., Vreven T., Meroueh S. O., Mobashery S., Schlegel H. B. (2005) Lysine carboxylation in proteins: OXA-10 β-lactamase. Proteins 61, 246–257 [DOI] [PubMed] [Google Scholar]
- 22. Héritier C., Poirel L., Lambert T., Nordmann P. (2005) Contribution of acquired carbapenem-hydrolyzing oxacillinases to carbapenem resistance in Acinetobacter baumannii. Antimicrob. Agents Chemother. 49, 3198–3202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hujer K. M., Hamza N. S., Hujer A. M., Perez F., Helfand M. S., Bethel C. R., Thomson J. M., Anderson V. E., Barlow M., Rice L. B., Tenover F. C., Bonomo R. A. (2005) Identification of a new allelic variant of the Acinetobacter baumannii cephalosporinase, ADC-7 β-lactamase: defining a unique family of class C enzymes. Antimicrob. Agents Chemother. 49, 2941–2948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Venkatesan A. M., Gu Y., Dos Santos O., Abe T., Agarwal A., Yang Y., Petersen P. J., Weiss W. J., Mansour T. S., Nukaga M., Hujer A. M., Bonomo R. A., Knox J. R. (2004) Structure-activity relationship of 6-methylidene penems bearing tricyclic heterocycles as broad-spectrum β-lactamase inhibitors: crystallographic structures show unexpected binding of 1,4-thiazepine intermediates. J. Med. Chem. 47, 6556–6568 [DOI] [PubMed] [Google Scholar]
- 25. National Committee on Clinical Laboratory Standards (2003) Performance Standards for Antimicrobial Disk Susceptibility Tests, Approved Standard, 8th Ed., NCCLS Document M2-A8, National Committee on Clinical Laboratory Standards, Wayne, PA [Google Scholar]
- 26. National Committee on Clinical Laboratory Standards (2005) Performance Standards for Antimicrobial Disk Susceptibility Testing, Fifteenth Informational Supplement, NCCLS Document M100-S15, National Committee on Clinical Laboratory Standards, Wayne, PA [Google Scholar]
- 27. Che T., Bonomo R. A., Shanmugam S., Bethel C. R., Pusztai-Carey M., Buynak J. D., Carey P. R. (2012) Carboxylation and decarboxylation of active site Lys 84 controls the activity of OXA-24 β-lactamase of Acinetobacter baumannii: Raman crystallographic and solution evidence. J. Am. Chem. Soc. 134, 11206–11215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Altose M. D., Zheng Y., Dong J., Palfey B. A., Carey P. R. (2001) Comparing protein-ligand interactions in solution and single crystals by Raman spectroscopy. Proc. Natl. Acad. Sci. U.S.A. 98, 3006–3011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dong J., Swift K., Matayoshi E., Nienaber V. L., Weitzberg M., Rockway T., Carey P. R. (2001) Probing inhibitors binding to human urokinase crystals by Raman microscopy: implications for compound screening. Biochemistry 40, 9751–9757 [DOI] [PubMed] [Google Scholar]
- 30. Heidari Torkabadi H., Che T., Shou J., Shanmugam S., Crowder M. W., Bonomo R. A., Pusztai-Carey M., Carey P. R. (2013) Raman spectra of interchanging β-lactamase inhibitor intermediates on the millisecond time scale. J. Am. Chem. Soc. 135, 2895–2898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carey P. R. (2006) Raman crystallography and other biochemical applications of Raman microscopy. Annu. Rev. Phys. Chem. 57, 527–554 [DOI] [PubMed] [Google Scholar]
- 32. Drawz S. M., Bonomo R. A. (2010) Three decades of β-lactamase inhibitors. Clin. Microbiol. Rev. 23, 160–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Frisch M. J., Trucks G. W., Schlegel H. B., Scuseria G. E., Robb M. A., Cheeseman J. R., Montgomery J. A., Jr., Vreven T., Kudin K. N., Burant J. C., Millam J. M., Iyengar S. S., Tomasi J., Barone V., Mennucci B., Cossi M., Scalmani G., Rega N., Petersson G. A., Nakatsuji H., Hada M., Ehara M., Toyota K., Fukuda R., Hasegawa J., Ishida M., Nakajima T., Honda Y., Kitao O., Nakai H., Klene M., Li X., Knox J. E., Hratchian H. P., Cross J. B., Bakken V., Adamo C., Jaramillo J., Gomperts R., Stratmann R. E., Yazyev O., Austin A. J., Cammi R., Pomelli C., Ochterski J. W., Ayala P. Y., Morokuma K., Voth G. A., Salvador P., Dannenberg J. J., Zakrzewski V. G., Dapprich S., Daniels A. D., Strain M. C., Farkas O., Malick D. K., Rabuck A. D., Raghavachari K., Foresman J. B., Ortiz J. V., Cui Q., Baboul A. G., Clifford S., Cioslowski J., Stefanov B. B., Liu G., Liashenko A., Piskorz P., Komaromi I., Martin R. L., Fox D. J., Keith T., Al-Laham M. A., Peng C. Y., Nanayakkara A., Challacombe M., Gill P. M. W., Johnson B., Chen W., Wong M. W., Gonzalez C., Pople J. A. (2004) Gaussian 03, Revision C.02, Gaussian, Inc., Wallingford, CT [Google Scholar]
- 34. Kim K., Jordan K. D. (1994) Comparison of density functional and Mp2 calculations on the water monomer and dimer. J. Phys. Chem. 98, 10089–10094 [Google Scholar]
- 35. Stephens P. J., Devlin F. J., Chabalowski C. F., Frisch M. J. (1994) Ab initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 98, 11623–11627 [Google Scholar]
- 36. Pattanaik P., Bethel C. R., Hujer A. M., Hujer K. M., Distler A. M., Taracila M., Anderson V. E., Fritsche T. R., Jones R. N., Pagadala S. R., van den Akker F., Buynak J. D., Bonomo R. A. (2009) Strategic design of an effective β-lactamase inhibitor: LN-1-255, a 6-alkylidene-2′-substituted penicillin sulfone. J. Biol. Chem. 284, 945–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bush K., Macalintal C., Rasmussen B. A., Lee V. J., Yang Y. (1993) Kinetic interactions of tazobactam with β-lactamases from all major structural classes. Antimicrob. Agents Chemother. 37, 851–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sulton D., Pagan-Rodriguez D., Zhou X., Liu Y., Hujer A. M., Bethel C. R., Helfand M. S., Thomson J. M., Anderson V. E., Buynak J. D., Ng L. M., Bonomo R. A. (2005) Clavulanic acid inactivation of SHV-1 and the inhibitor-resistant S130G SHV-1 β-lactamase. Insights into the mechanism of inhibition. J. Biol. Chem. 280, 35528–35536 [DOI] [PubMed] [Google Scholar]
- 39. Charnas R. L., Fisher J., Knowles J. R. (1978) Chemical studies on the inactivation of Escherichia coli RTEM β-lactamase by clavulanic acid. Biochemistry 17, 2185–2189 [DOI] [PubMed] [Google Scholar]
- 40. Broom N. J. P., Farmer T. H., Osborne N. F., Tyler J. W. (1992) Studies on the mechanism of action of (5R)-(Z)-6-(1-methyl-1,2,3-triazol-4-ylmethylene)penem-3-carboxylic acid (Brl-42715), a potent inhibitor of bacterial β-lactamase. J. Chem. Soc. Chem. Comm. 1663–1664 [Google Scholar]
- 41. Helfand M. S., Totir M. A., Carey M. P., Hujer A. M., Bonomo R. A., Carey P. R. (2003) Following the reactions of mechanism-based inhibitors with β-lactamase by Raman crystallography. Biochemistry 42, 13386–13392 [DOI] [PubMed] [Google Scholar]
- 42. MacClement B. A., Carriere R. G., Phelps D. J., Carey P. R. (1981) Evidence for two acyl group conformations in some furylacryloyl- and thienylacryloylchymotrypsins: resonance Raman studies of enzyme-substrate intermediates at pH 3.0. Biochemistry 20, 3438–3447 [DOI] [PubMed] [Google Scholar]
- 43. Ke W., Pattanaik P., Bethel C. R., Sheri A., Buynak J. D., Bonomo R. A., van den Akker F. (2012) Structures of SHV-1 β-lactamase with penem and penam sulfone inhibitors that form cyclic intermediates stabilized by carbonyl conjugation. PLoS One 7, e49035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kalp M., Totir M. A., Buynak J. D., Carey P. R. (2009) Different intermediate populations formed by tazobactam, sulbactam, and clavulanate reacting with SHV-1 β-lactamases: Raman crystallographic evidence. J. Am. Chem. Soc. 131, 2338–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schneider K. D., Bethel C. R., Distler A. M., Hujer A. M., Bonomo R. A., Leonard D. A. (2009) Mutation of the active site carboxy-lysine (K70) of OXA-1 β-lactamase results in a deacylation-deficient enzyme. Biochemistry 48, 6136–6145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Golemi-Kotra D., Cha J. Y., Meroueh S. O., Vakulenko S. B., Mobashery S. (2003) Resistance to β-lactam antibiotics and its mediation by the sensor domain of the transmembrane BlaR signaling pathway in Staphylococcus aureus. J. Biol. Chem. 278, 18419–18425 [DOI] [PubMed] [Google Scholar]
- 47. Birck C., Cha J. Y., Cross J., Schulze-Briese C., Meroueh S. O., Schlegel H. B., Mobashery S., Samama J. P. (2004) X-ray crystal structure of the acylated β-lactam sensor domain of BlaR1 from Staphylococcus aureus and the mechanism of receptor activation for signal transduction. J. Am. Chem. Soc. 126, 13945–13947 [DOI] [PubMed] [Google Scholar]
- 48. Borbulevych O., Kumarasiri M., Wilson B., Llarrull L. I., Lee M., Hesek D., Shi Q., Peng J., Baker B. M., Mobashery S. (2011) Lysine Nζ-decarboxylation switch and activation of the β-lactam sensor domain of BlaR1 protein of methicillin-resistant Staphylococcus aureus. J. Biol. Chem. 286, 31466–31472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Thumanu K., Cha J., Fisher J. F., Perrins R., Mobashery S., Wharton C. (2006) Discrete steps in sensing of β-lactam antibiotics by the BlaR1 protein of the methicillin-resistant Staphylococcus aureus bacterium. Proc. Natl. Acad. Sci. U.S.A. 103, 10630–10635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Llarrull L. I., Toth M., Champion M. M., Mobashery S. (2011) Activation of BlaR1 protein of methicillin-resistant Staphylococcus aureus, its proteolytic processing, and recovery from induction of resistance. J. Biol. Chem. 286, 38148–38158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cha J., Mobashery S. (2007) Lysine Nζ-decarboxylation in the BlaR1 protein from Staphylococcus aureus at the root of its function as an antibiotic sensor. J. Am. Chem. Soc. 129, 3834–3835 [DOI] [PubMed] [Google Scholar]
- 52. Fuda C. C., Fisher J. F., Mobashery S. (2005) β-Lactam resistance in Staphylococcus aureus: the adaptive resistance of a plastic genome. Cell. Mol. Life Sci. 62, 2617–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Toney M. D., Kirsch J. F. (1989) Direct Brønsted analysis of the restoration of activity to a mutant enzyme by exogenous amines. Science 243, 1485–1488 [DOI] [PubMed] [Google Scholar]
- 54. Wilke M. S., Hills T. L., Zhang H. Z., Chambers H. F., Strynadka N. C. (2004) Crystal structures of the apo and penicillin-acylated forms of the BlaR1 β-lactam sensor of Staphylococcus aureus. J. Biol. Chem. 279, 47278–47287 [DOI] [PubMed] [Google Scholar]
- 55. Kerff F., Charlier P., Colombo M. L., Sauvage E., Brans A., Frère J. M., Joris B., Fonzé E. (2003) Crystal structure of the sensor domain of the BlaR penicillin receptor from Bacillus licheniformis. Biochemistry 42, 12835–12843 [DOI] [PubMed] [Google Scholar]
- 56. Tien M., Berlett B. S., Levine R. L., Chock P. B., Stadtman E. R. (1999) Peroxynitrite-mediated modification of proteins at physiological carbon dioxide concentration: pH dependence of carbonyl formation, tyrosine nitration, and methionine oxidation. Proc. Natl. Acad. Sci. U.S.A. 96, 7809–7814 [DOI] [PMC free article] [PubMed] [Google Scholar]