Abstract
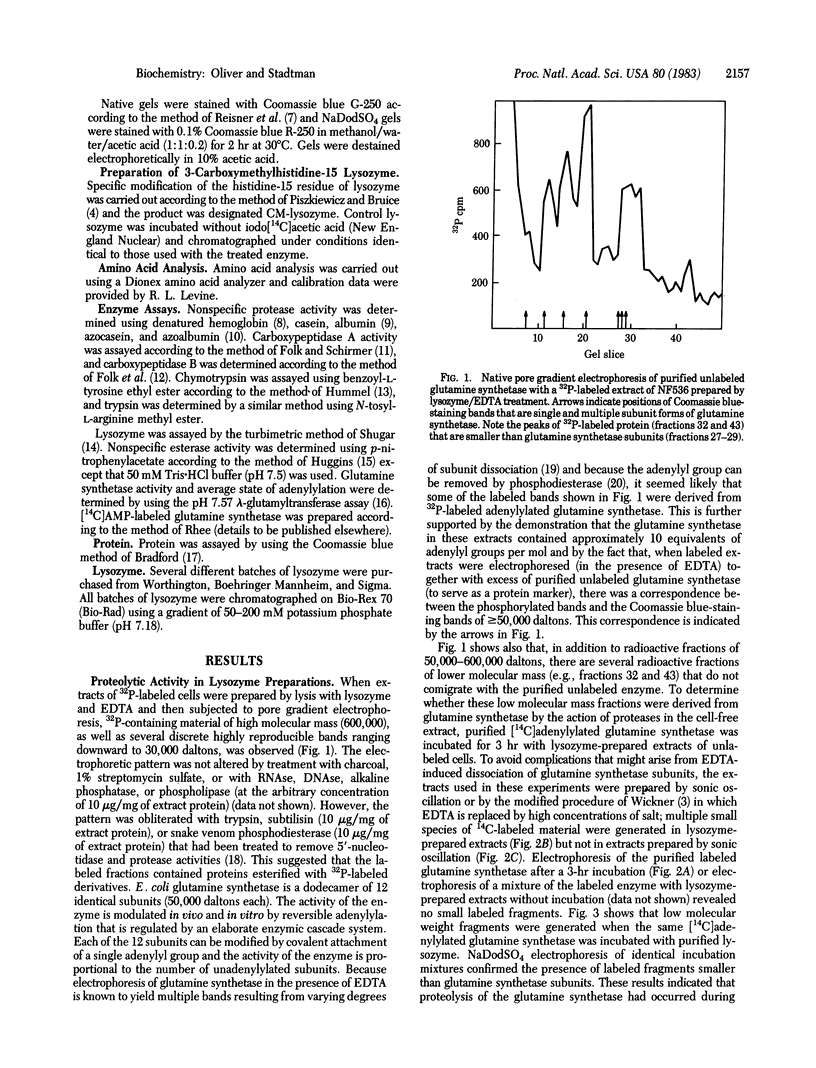
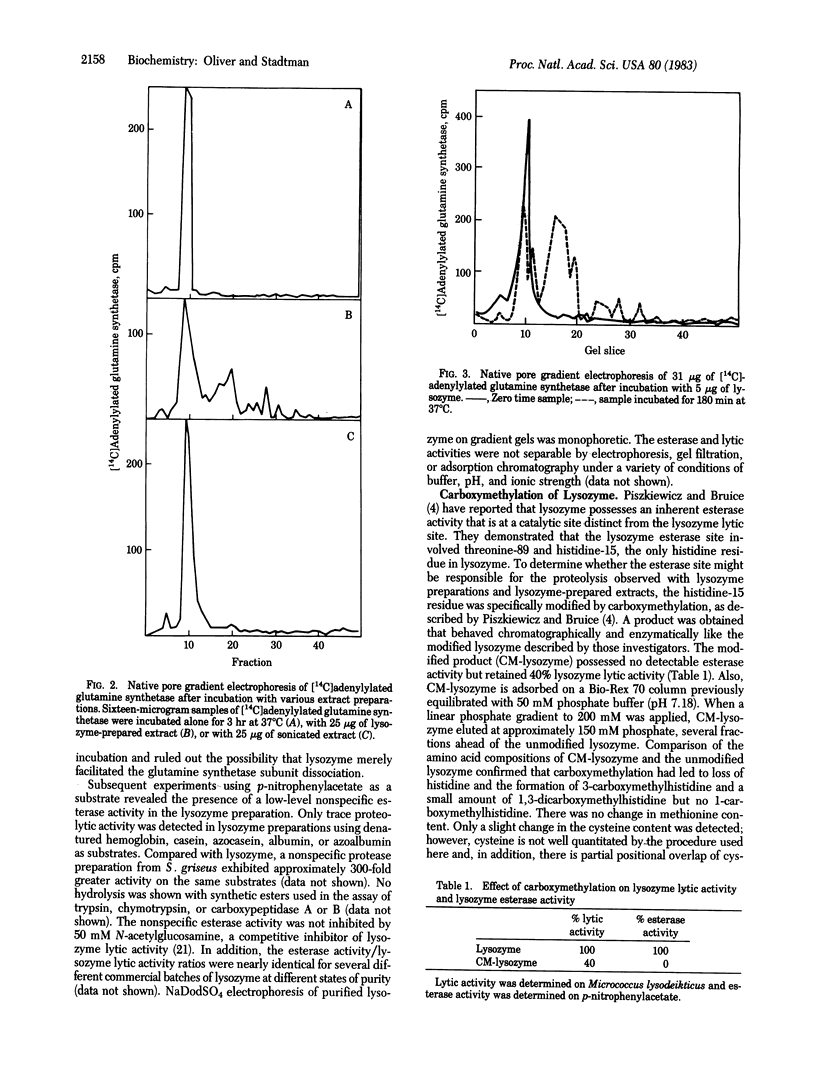
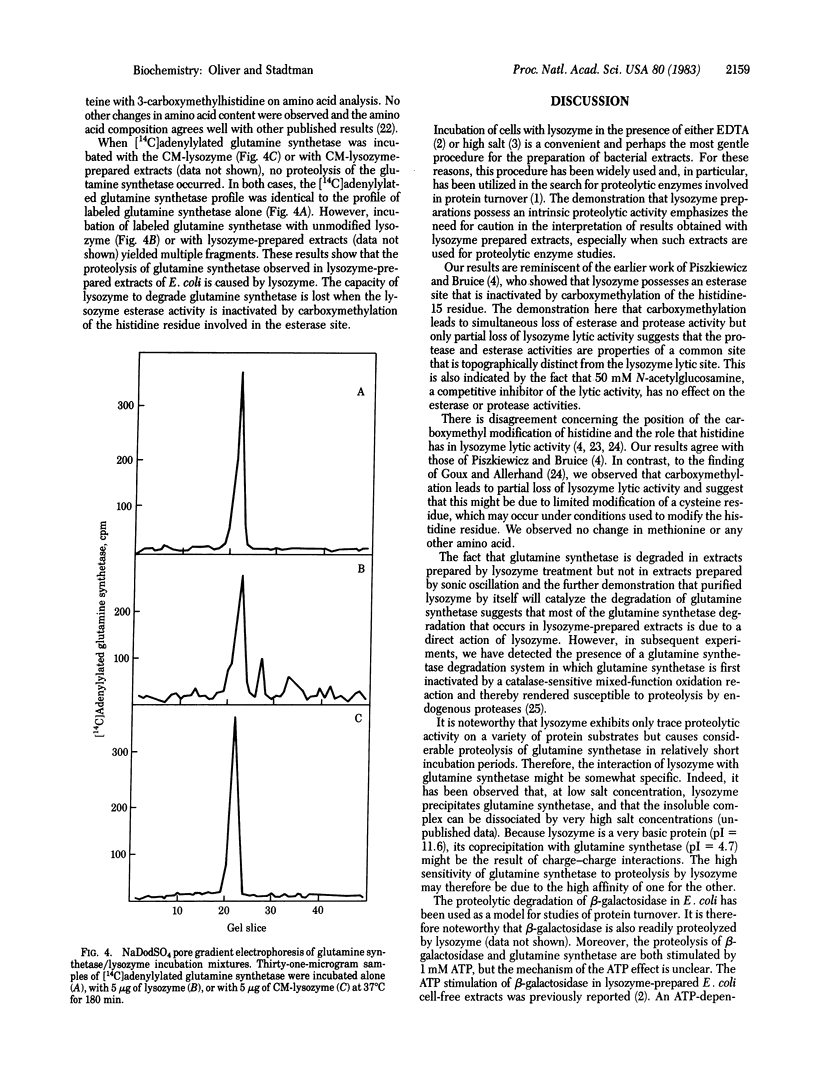
Polyacrylamide gel electrophoresis of cell-free extracts of Escherichia coli that had been grown in a medium containing 32Pi disclosed the presence of several 32P-labeled proteins. Comparison of the electrophoretic patterns obtained in the presence of carrier unlabeled purified E. coli glutamine synthetase before and after treatment with trypsin, subtilisin, or snake venom phosphodiesterase showed that most of the 32P was present in the adenylyl moieties of adenylylated glutamine synthetase. Low molecular weight 32P-labeled degradation products of glutamine synthetase were also observed in extracts prepared by treatment of cells with lysozyme but not in extracts prepared by sonic oscillation. The degradation of glutamine synthetase in lysozyme-prepared extracts is likely due to an intrinsic proteolytic activity of egg white lysozyme. Proteolysis probably occurs at the esterase site of lysozyme described by Piszkiewicz and Bruice [Piszkiewicz, D. & Bruice, T.C. (1968) Biochemistry 7, 3037-3047]. Selective carboxymethylation of lysozyme histidine-15 leads to simultaneous loss of esterase and protease activities but only to partial loss of lytic activity. In view of these findings, caution is needed in the interpretation of results obtained with extracts of cells prepared by lysozyme treatment, especially when such extracts are used to investigate the properties of proteolytic enzymes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Dolapchiev L. B., Sulkowski E., Laskowski M., Sr Purification of exonuclease (phosphodiesterase) from the venom of Crotalus adamanteus. Biochem Biophys Res Commun. 1974 Nov 6;61(1):273–281. doi: 10.1016/0006-291x(74)90563-4. [DOI] [PubMed] [Google Scholar]
- FOLK J. E., PIEZ K. A., CARROLL W. R., GLADNER J. A. Carboxy-peptidase B. 4. Purification and characterization of the porcine enzyme. J Biol Chem. 1960 Aug;235:2272–2277. [PubMed] [Google Scholar]
- FOLK J. E., SCHIRMER E. W. THE PORCINE PANCREATIC CARBOXYPEPTIDASE A SYSTEM. I. THREE FORMS OF THE ACTIVE ENZYME. J Biol Chem. 1963 Dec;238:3884–3894. [PubMed] [Google Scholar]
- Goldberg A. L., Swamy K. H., Chung C. H., Larimore F. S. Proteases in Escherichia coli. Methods Enzymol. 1981;80(Pt 100):680–702. doi: 10.1016/s0076-6879(81)80052-3. [DOI] [PubMed] [Google Scholar]
- Goux W. J., Allerhand A. Studies of chemically modified histidine residues of proteins by carbon 13 nuclear magnetic resonance spectroscopy. Reaction of hen egg white lysozyme with iodoacetate. J Biol Chem. 1979 Apr 10;254(7):2210–2213. [PubMed] [Google Scholar]
- HUMMEL B. C. A modified spectrophotometric determination of chymotrypsin, trypsin, and thrombin. Can J Biochem Physiol. 1959 Dec;37:1393–1399. [PubMed] [Google Scholar]
- Kaback H. R. The role of the phosphoenolpyruvate-phosphotransferase system in the transport of sugars by isolated membrane preparations of Escherichia coli. J Biol Chem. 1968 Jul 10;243(13):3711–3724. [PubMed] [Google Scholar]
- Kaempfer R. O., Magasanik B. Effect of infection with T-even phage on the inducible synthesis of beta-glactosidase in Escherichia coli. J Mol Biol. 1967 Aug 14;27(3):453–468. doi: 10.1016/0022-2836(67)90051-4. [DOI] [PubMed] [Google Scholar]
- Levine R. L., Oliver C. N., Fulks R. M., Stadtman E. R. Turnover of bacterial glutamine synthetase: oxidative inactivation precedes proteolysis. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2120–2124. doi: 10.1073/pnas.78.4.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis J., Kenrick K. G. Polyacrylamide gel electrophoresis in a continuous molecular sieve gradient. Anal Biochem. 1968 Oct 24;25(1):347–362. doi: 10.1016/0003-2697(68)90109-7. [DOI] [PubMed] [Google Scholar]
- Murakami K., Voellmy R., Goldberg A. L. Protein degradation is stimulated by ATP in extracts of Escherichia coli. J Biol Chem. 1979 Sep 10;254(17):8194–8200. [PubMed] [Google Scholar]
- Parsons S. M., Jao L., Dahlquist F. W., Borders C. L., Jr, Racs J., Groff T., Raftery M. A. The nature of amino acid side chains which are critical for the activity of lysozyme. Biochemistry. 1969 Feb;8(2):700–712. doi: 10.1021/bi00830a036. [DOI] [PubMed] [Google Scholar]
- Piszkiewicz D., Bruice T. C. The identification of histidine-15 as part of an esteratic site of hen's egg white lysozyme. Biochemistry. 1968 Sep;7(9):3037–3047. doi: 10.1021/bi00849a003. [DOI] [PubMed] [Google Scholar]
- Reisner A. H., Nemes P., Bucholtz C. The use of Coomassie Brilliant Blue G250 perchloric acid solution for staining in electrophoresis and isoelectric focusing on polyacrylamide gels. Anal Biochem. 1975 Apr;64(2):509–516. doi: 10.1016/0003-2697(75)90461-3. [DOI] [PubMed] [Google Scholar]
- SHUGAR D. The measurement of lysozyme activity and the ultra-violet inactivation of lysozyme. Biochim Biophys Acta. 1952 Mar;8(3):302–309. doi: 10.1016/0006-3002(52)90045-0. [DOI] [PubMed] [Google Scholar]
- Shapiro B. M., Ginsburg A. Effects of specific divalent cations on some physical and chemical properties of glutamine synthetase from Escherichia coli. Taut and relaxed enzyme forms. Biochemistry. 1968 Jun;7(6):2153–2167. doi: 10.1021/bi00846a018. [DOI] [PubMed] [Google Scholar]
- Stadtman E. R., Smyrniotis P. Z., Davis J. N., Wittenberger M. E. Enzymic procedures for determining the average state of adenylylation of Escherichia coli glutamine synthetase. Anal Biochem. 1979 May;95(1):275–285. doi: 10.1016/0003-2697(79)90217-3. [DOI] [PubMed] [Google Scholar]
- Tronick S. R., Ciardi J. E., Stadtman E. R. Comparative biochemical and immunological studies of bacterial glutamine synthetases. J Bacteriol. 1973 Sep;115(3):858–868. doi: 10.1128/jb.115.3.858-868.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickner W., Brutlag D., Schekman R., Kornberg A. RNA synthesis initiates in vitro conversion of M13 DNA to its replicative form. Proc Natl Acad Sci U S A. 1972 Apr;69(4):965–969. doi: 10.1073/pnas.69.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]


