Abstract
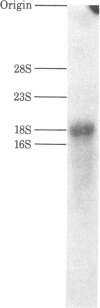
A mixture of tetradecamer oligodeoxyribonucleotides complementary to the codons specifying the carboxyl-terminal sequence, Ile-His-Pro-Phe-His, of angiotensin was chemically synthesized as two pools and used for the isolation of a cDNA clone specific for angiotensinogen from a cDNA bank of rat liver mRNA sequences. The two pools (oligo 1 and oligo 2), each containing 24 oligodeoxyribonucleotides, were first used as primers to initiate reverse transcription of rat liver mRNA. One of the pools (oligo 1) was found to prime a specific 32P-labeled cDNA of approximately 160 nucleotides that contained the anticoding sequence corresponding exactly to the amino acid sequence of rat angiotensin. This cDNA, in turn, was used to rescreen cDNA clones that were isolated by initially selecting the rat liver cDNA bank by hybridization with the oligo 1 mixture. One clone thus obtained, designated pRag16, was subjected to nucleotide sequence analysis and verified to contain a nearly full-length cDNA sequence coding for rat angiotensinogen precursor. The deduced amino acid sequence indicates that the precursor molecular consists of angiotensinogen of 453 amino acid residues and a putative signal peptide of 24 amino acid residues. The predicted molecular weight and amino acid composition of angiotensinogen agree well with those determined by using the purified protein. An angiotensin moiety is located at the amino-terminal part of angiotensinogen, preceded directly by the signal peptide and followed by a large carboxyl-terminal sequence that contains two internally homologous sequences and three potential glycosylation sites.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal K. L., Brunstedt J., Noyes B. E. A general method for detection and characterization of an mRNA using an oligonucleotide probe. J Biol Chem. 1981 Jan 25;256(2):1023–1028. [PubMed] [Google Scholar]
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouhnik J., Clauser E., Strosberg D., Frenoy J. P., Menard J., Corvol P. Rat angiotensinogen and des(angiotensin I)angiotensinogen: purification, characterization, and partial sequencing. Biochemistry. 1981 Nov 24;20(24):7010–7015. doi: 10.1021/bi00527a036. [DOI] [PubMed] [Google Scholar]
- Brownstein M. J., Russell J. T., Gainer H. Synthesis, transport, and release of posterior pituitary hormones. Science. 1980 Jan 25;207(4429):373–378. doi: 10.1126/science.6153132. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Land H., Schütz G., Schmale H., Richter D. Nucleotide sequence of cloned cDNA encoding bovine arginine vasopressin-neurophysin II precursor. Nature. 1982 Jan 28;295(5847):299–303. doi: 10.1038/295299a0. [DOI] [PubMed] [Google Scholar]
- Marshall R. D. The nature and metabolism of the carbohydrate-peptide linkages of glycoproteins. Biochem Soc Symp. 1974;(40):17–26. [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison D. A. Transformation and preservation of competent bacterial cells by freezing. Methods Enzymol. 1979;68:326–331. doi: 10.1016/0076-6879(79)68023-0. [DOI] [PubMed] [Google Scholar]
- Nawa H., Kitamura N., Hirose T., Asai M., Inayama S., Nakanishi S. Primary structures of bovine liver low molecular weight kininogen precursors and their two mRNAs. Proc Natl Acad Sci U S A. 1983 Jan;80(1):90–94. doi: 10.1073/pnas.80.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Ramsay D. J. The brain renin angiotensin system: a re-evaluation. Neuroscience. 1979;4(3):313–321. doi: 10.1016/0306-4522(79)90096-4. [DOI] [PubMed] [Google Scholar]
- Reid I. A., Morris B. J., Ganong W. F. The renin-angiotensin system. Annu Rev Physiol. 1978;40:377–410. doi: 10.1146/annurev.ph.40.030178.002113. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Skeggs L. T., Dorer F. E., Levine M., Lentz K. E., Kahn J. R. The biochemistry of the renin-angiotensin system. Adv Exp Med Biol. 1980;130:1–27. doi: 10.1007/978-1-4615-9173-3_1. [DOI] [PubMed] [Google Scholar]
- Tewksbury D. A., Dart R. A., Travis J. The amino terminal amino acid sequence of human angiotensinogen. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1311–1315. doi: 10.1016/0006-291x(81)90762-2. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Shaffer J., Murphy R. F., Bonner J., Hirose T., Itakura K. Hybridization of synthetic oligodeoxyribonucleotides to phi chi 174 DNA: the effect of single base pair mismatch. Nucleic Acids Res. 1979 Aug 10;6(11):3543–3557. doi: 10.1093/nar/6.11.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigand K., Warnze H., Falge C. Synthesis of angiotensinogen by isolated rat liver cells and its regulation in comparison to serum albumin. Biochem Biophys Res Commun. 1977 Mar 7;75(1):102–110. doi: 10.1016/0006-291x(77)91295-5. [DOI] [PubMed] [Google Scholar]