Abstract
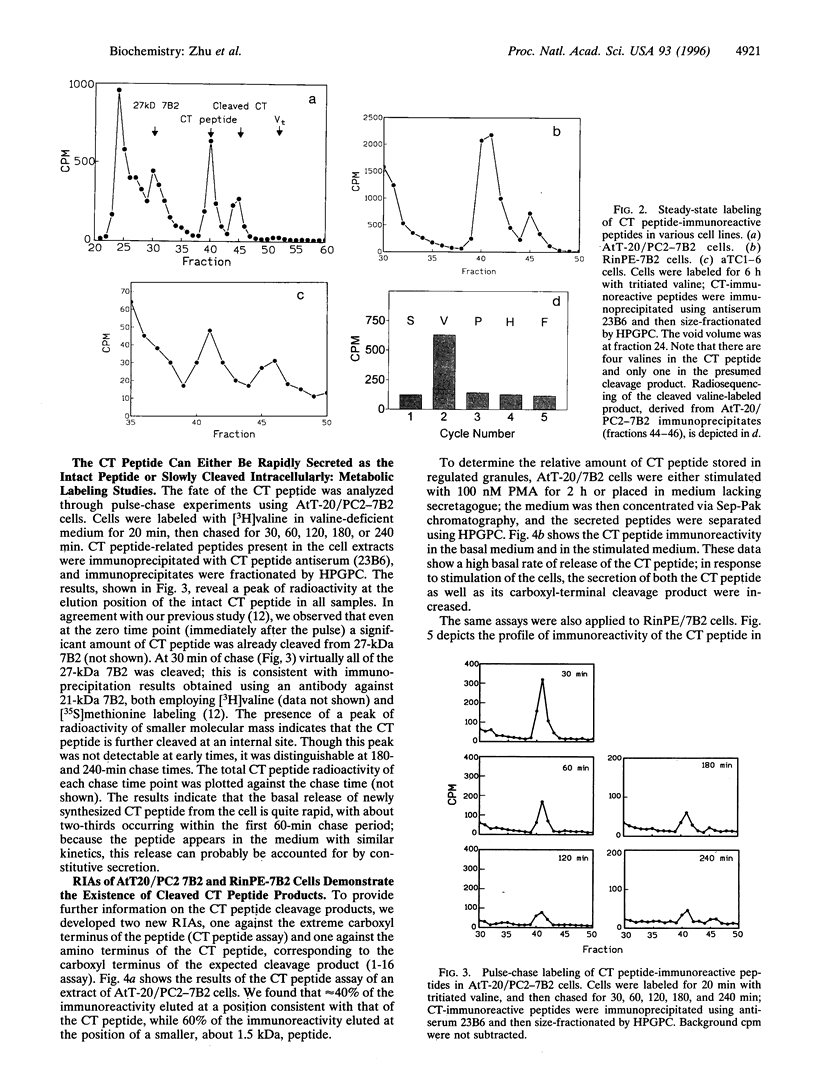
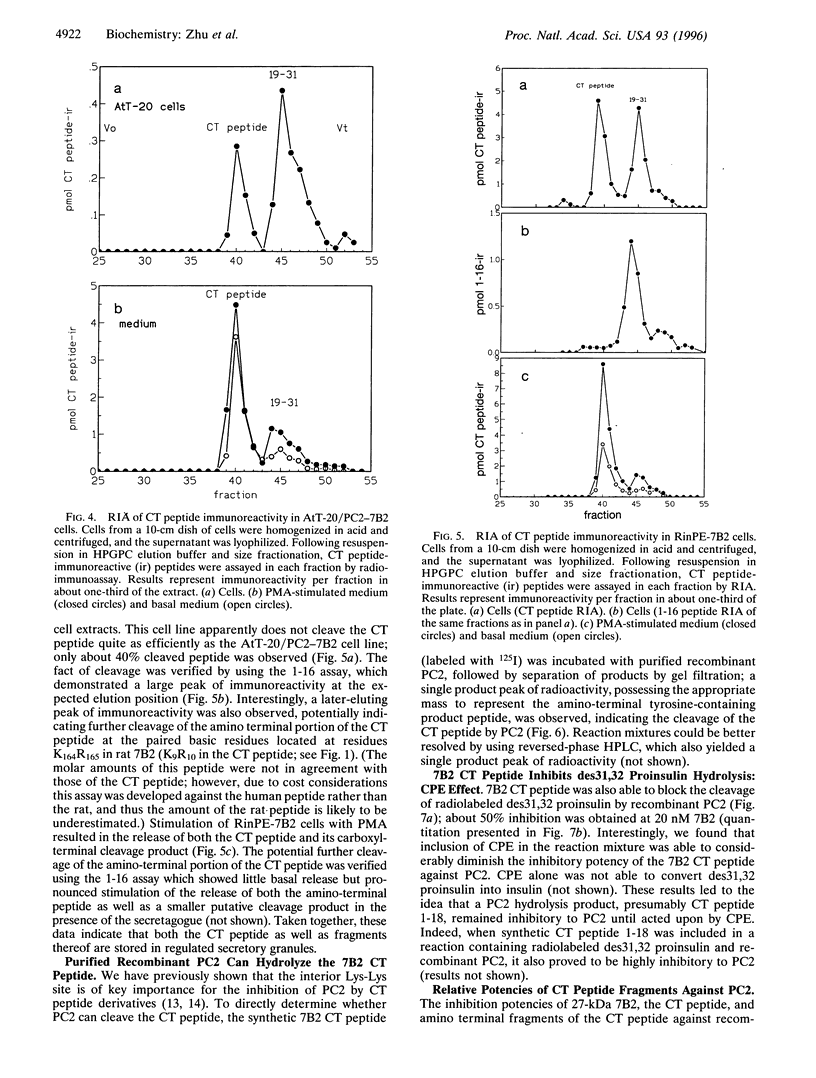
The neuroendocrine protein 7B2 contains two domains, a 21-kDa protein required for prohormone convertase 2 (PC2) maturation and a carboxyl-terminal (CT) peptide that inhibits PC2 at nanomolar concentrations. To determine how the inhibition of PC2 is terminated, we studied the metabolic fate of the 7B2 CT peptide in RinPE-7B2, AtT-20/PC2-7B2, and alphaTC1-6 cells. Extracts obtained from cells labeled for 6 h with [3H]valine were subjected to immunoprecipitation using an antibody raised against the extreme carboxyl terminus of r7B2, and immunoprecipitated peptides were separated by gel filtration. All three cell lines yielded two distinct peaks at about 3.5 kDa and 1.5 kDa, corresponding to the CT peptide and a smaller fragment consistent with cleavage at an interior Lys-Lys site. These results were corroborated using a newly developed RIA against the carboxyl terminus of the CT peptide which showed that the intact CT peptide represented only about half of the stored CT peptide immunoreactivity, with the remainder present as the 1.5-kDa peptide. Both peptides could be released upon phorbol 12-myristate 13-acetate stimulation. We investigated the possibility that PC2 itself could be responsible for this cleavage by performing in vitro experiments. When 125I-labeled CT peptide was incubated with purified recombinant PC2, a smaller peptide was generated. Analysis of CT peptide derivatives for their inhibitory potency revealed that CT peptide 1-18 (containing Lys-Lys at the carboxyl terminus) represented a potent inhibitor, but that peptide 1-16 was inactive. Inclusion of carboxypeptidase E (CPE) in the reaction greatly diminished the inhibitory potency of the CT peptide against PC2, in line with the notion that the CT peptide cleavage product is not inhibitory after the removal of terminal lysines by CPE. In summary, our data support the idea that PC2 cleaves the 7B2 CT peptide at its internal Lys-Lys site within secretory granules; deactivation of the cleavage product is then accomplished by CPE, thus providing an efficient mechanism for intracellular inactivation of the CT peptide.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benjannet S., Rondeau N., Day R., Chrétien M., Seidah N. G. PC1 and PC2 are proprotein convertases capable of cleaving proopiomelanocortin at distinct pairs of basic residues. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3564–3568. doi: 10.1073/pnas.88.9.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjannet S., Rondeau N., Paquet L., Boudreault A., Lazure C., Chrétien M., Seidah N. G. Comparative biosynthesis, covalent post-translational modifications and efficiency of prosegment cleavage of the prohormone convertases PC1 and PC2: glycosylation, sulphation and identification of the intracellular site of prosegment cleavage of PC1 and PC2. Biochem J. 1993 Sep 15;294(Pt 3):735–743. doi: 10.1042/bj2940735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjannet S., Savaria D., Chrétien M., Seidah N. G. 7B2 is a specific intracellular binding protein of the prohormone convertase PC2. J Neurochem. 1995 May;64(5):2303–2311. doi: 10.1046/j.1471-4159.1995.64052303.x. [DOI] [PubMed] [Google Scholar]
- Braks J. A., Martens G. J. 7B2 is a neuroendocrine chaperone that transiently interacts with prohormone convertase PC2 in the secretory pathway. Cell. 1994 Jul 29;78(2):263–273. doi: 10.1016/0092-8674(94)90296-8. [DOI] [PubMed] [Google Scholar]
- Bump N. J., Hackett M., Hugunin M., Seshagiri S., Brady K., Chen P., Ferenz C., Franklin S., Ghayur T., Li P. Inhibition of ICE family proteases by baculovirus antiapoptotic protein p35. Science. 1995 Sep 29;269(5232):1885–1888. doi: 10.1126/science.7569933. [DOI] [PubMed] [Google Scholar]
- Efrat S., Linde S., Kofod H., Spector D., Delannoy M., Grant S., Hanahan D., Baekkeskov S. Beta-cell lines derived from transgenic mice expressing a hybrid insulin gene-oncogene. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9037–9041. doi: 10.1073/pnas.85.23.9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricker L. D., Das B., Klein R. S., Greene D., Jung Y. K. Regulation of carboxypeptidase E (enkephalin convertase). NIDA Res Monogr. 1991;111:171–187. [PubMed] [Google Scholar]
- Hsi K. L., Seidah N. G., De Serres G., Chrétien M. Isolation and NH2-terminal sequence of a novel porcine anterior pituitary polypeptide. Homology to proinsulin, secretin and Rous sarcoma virus transforming protein TVFV60. FEBS Lett. 1982 Oct 18;147(2):261–266. doi: 10.1016/0014-5793(82)81055-7. [DOI] [PubMed] [Google Scholar]
- Hutton J. C. Subtilisin-like proteinases involved in the activation of proproteins of the eukaryotic secretory pathway. Curr Opin Cell Biol. 1990 Dec;2(6):1131–1142. doi: 10.1016/0955-0674(90)90167-d. [DOI] [PubMed] [Google Scholar]
- Iguchi H., Chan J. S., Seidah N. G., Chrétien M. Tissue distribution and molecular forms of a novel pituitary protein in the rat. Neuroendocrinology. 1984 Nov;39(5):453–458. doi: 10.1159/000124020. [DOI] [PubMed] [Google Scholar]
- Johanning K., Mathis J. P., Lindberg I. Role of PC2 in proenkephalin processing: antisense and overexpression studies. J Neurochem. 1996 Mar;66(3):898–907. doi: 10.1046/j.1471-4159.1996.66030898.x. [DOI] [PubMed] [Google Scholar]
- Laskowski M., Jr, Kato I. Protein inhibitors of proteinases. Annu Rev Biochem. 1980;49:593–626. doi: 10.1146/annurev.bi.49.070180.003113. [DOI] [PubMed] [Google Scholar]
- Lindberg I., Lincoln B., Rhodes C. J. Fluorometric assay of a calcium-dependent, paired-basic processing endopeptidase present in insulinoma granules. Biochem Biophys Res Commun. 1992 Feb 28;183(1):1–7. doi: 10.1016/0006-291x(92)91599-l. [DOI] [PubMed] [Google Scholar]
- Lindberg I., van den Hurk W. H., Bui C., Batie C. J. Enzymatic characterization of immunopurified prohormone convertase 2: potent inhibition by a 7B2 peptide fragment. Biochemistry. 1995 Apr 25;34(16):5486–5493. doi: 10.1021/bi00016a020. [DOI] [PubMed] [Google Scholar]
- Martens G. J., Braks J. A., Eib D. W., Zhou Y., Lindberg I. The neuroendocrine polypeptide 7B2 is an endogenous inhibitor of prohormone convertase PC2. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):5784–5787. doi: 10.1073/pnas.91.13.5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis J. P., Lindberg I. Posttranslational processing of proenkephalin in AtT-20 cells: evidence for cleavage at a Lys-Lys site. Endocrinology. 1992 Nov;131(5):2287–2296. doi: 10.1210/endo.131.5.1425427. [DOI] [PubMed] [Google Scholar]
- Matthews G., Shennan K. I., Seal A. J., Taylor N. A., Colman A., Docherty K. Autocatalytic maturation of the prohormone convertase PC2. J Biol Chem. 1994 Jan 7;269(1):588–592. [PubMed] [Google Scholar]
- Naggert J. K., Fricker L. D., Varlamov O., Nishina P. M., Rouille Y., Steiner D. F., Carroll R. J., Paigen B. J., Leiter E. H. Hyperproinsulinaemia in obese fat/fat mice associated with a carboxypeptidase E mutation which reduces enzyme activity. Nat Genet. 1995 Jun;10(2):135–142. doi: 10.1038/ng0695-135. [DOI] [PubMed] [Google Scholar]
- Paquet L., Bergeron F., Boudreault A., Seidah N. G., Chrétien M., Mbikay M., Lazure C. The neuroendocrine precursor 7B2 is a sulfated protein proteolytically processed by a ubiquitous furin-like convertase. J Biol Chem. 1994 Jul 29;269(30):19279–19285. [PubMed] [Google Scholar]
- Paquet L., Rondeau N., Seidah N. G., Lazure C., Chrétien M., Mbikay M. Immunological identification and sequence characterization of a peptide derived from the processing of neuroendocrine protein 7B2. FEBS Lett. 1991 Dec 2;294(1-2):23–26. doi: 10.1016/0014-5793(91)81334-5. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Hsi K. L., De Serres G., Rochemont J., Hamelin J., Antakly T., Cantin M., Chrétien M. Isolation and NH2-terminal sequence of a highly conserved human and porcine pituitary protein belonging to a new superfamily. Immunocytochemical localization in pars distalis and pars nervosa of the pituitary and in the supraoptic nucleus of the hypothalamus. Arch Biochem Biophys. 1983 Sep;225(2):525–534. doi: 10.1016/0003-9861(83)90063-2. [DOI] [PubMed] [Google Scholar]
- Shennan K. I., Taylor N. A., Jermany J. L., Matthews G., Docherty K. Differences in pH optima and calcium requirements for maturation of the prohormone convertases PC2 and PC3 indicates different intracellular locations for these events. J Biol Chem. 1995 Jan 20;270(3):1402–1407. doi: 10.1074/jbc.270.3.1402. [DOI] [PubMed] [Google Scholar]
- Sigafoos J., Chestnut W. G., Merrill B. M., Taylor L. C., Diliberto E. J., Jr, Viveros O. H. Identification of a 7B2-derived tridecapeptide from bovine adrenal medulla chromaffin vesicles. Cell Mol Neurobiol. 1993 Jun;13(3):271–278. doi: 10.1007/BF00733755. [DOI] [PubMed] [Google Scholar]
- Spangelo B. L., Gorospe W. C. Role of the cytokines in the neuroendocrine-immune system axis. Front Neuroendocrinol. 1995 Jan;16(1):1–22. doi: 10.1006/frne.1995.1001. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Smeekens S. P., Ohagi S., Chan S. J. The new enzymology of precursor processing endoproteases. J Biol Chem. 1992 Nov 25;267(33):23435–23438. [PubMed] [Google Scholar]
- Thomas L., Leduc R., Thorne B. A., Smeekens S. P., Steiner D. F., Thomas G. Kex2-like endoproteases PC2 and PC3 accurately cleave a model prohormone in mammalian cells: evidence for a common core of neuroendocrine processing enzymes. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5297–5301. doi: 10.1073/pnas.88.12.5297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldbieser G. C., Aimi J., Dixon J. E. Cloning and characterization of the rat complementary deoxyribonucleic acid and gene encoding the neuroendocrine peptide 7B2. Endocrinology. 1991 Jun;128(6):3228–3236. doi: 10.1210/endo-128-6-3228. [DOI] [PubMed] [Google Scholar]
- Zhou A., Mains R. E. Endoproteolytic processing of proopiomelanocortin and prohormone convertases 1 and 2 in neuroendocrine cells overexpressing prohormone convertases 1 or 2. J Biol Chem. 1994 Jul 1;269(26):17440–17447. [PubMed] [Google Scholar]
- Zhu X., Lindberg I. 7B2 facilitates the maturation of proPC2 in neuroendocrine cells and is required for the expression of enzymatic activity. J Cell Biol. 1995 Jun;129(6):1641–1650. doi: 10.1083/jcb.129.6.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Horssen A. M., van den Hurk W. H., Bailyes E. M., Hutton J. C., Martens G. J., Lindberg I. Identification of the region within the neuroendocrine polypeptide 7B2 responsible for the inhibition of prohormone convertase PC2. J Biol Chem. 1995 Jun 16;270(24):14292–14296. doi: 10.1074/jbc.270.24.14292. [DOI] [PubMed] [Google Scholar]