Abstract
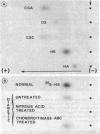
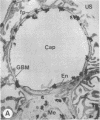
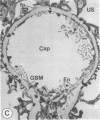
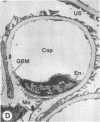
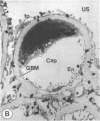
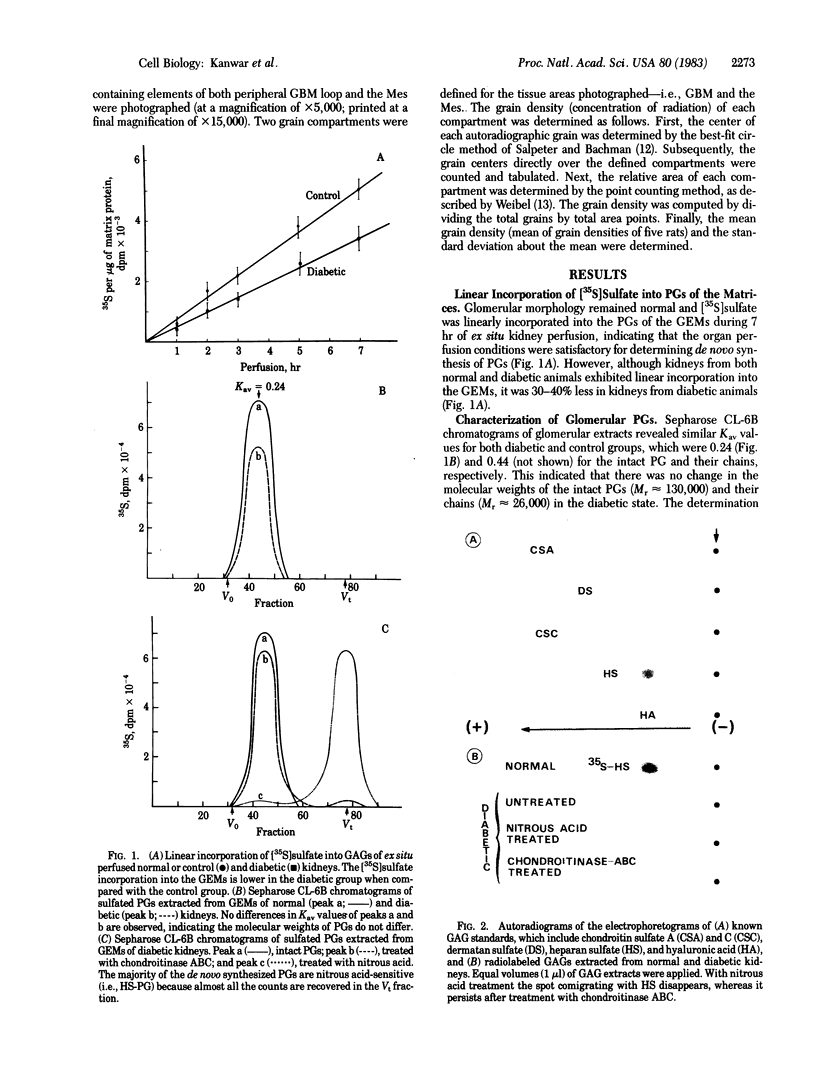
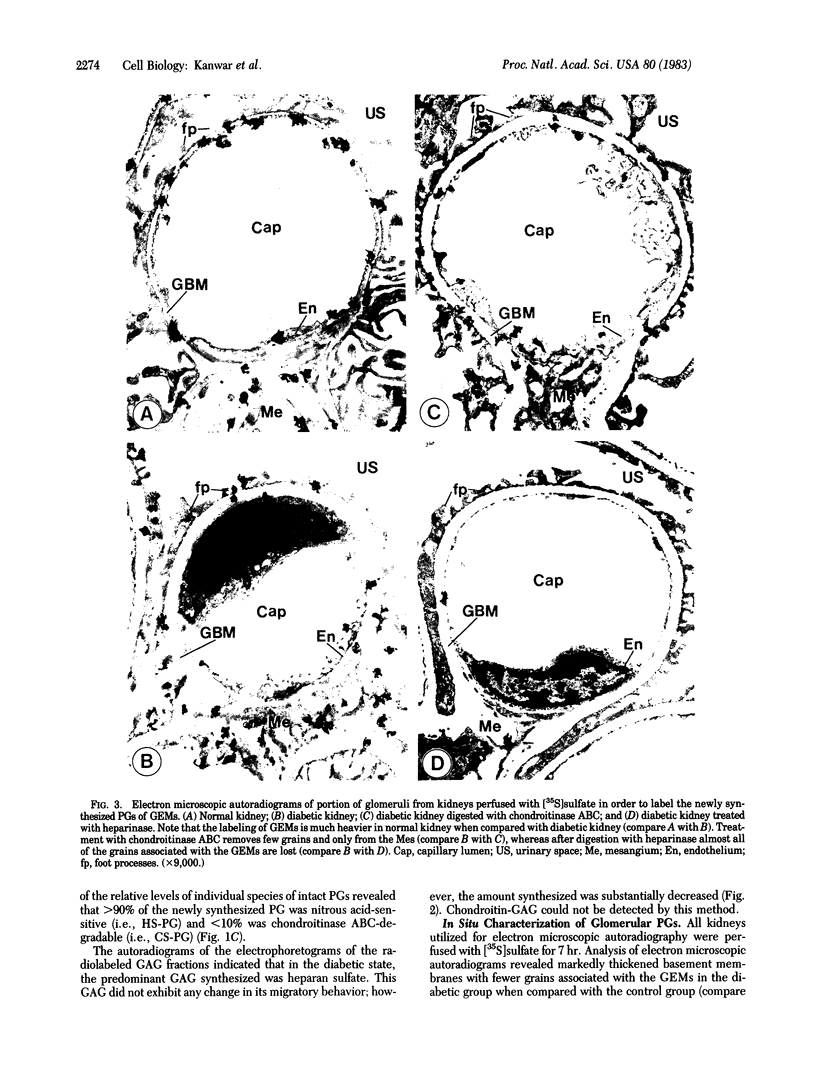
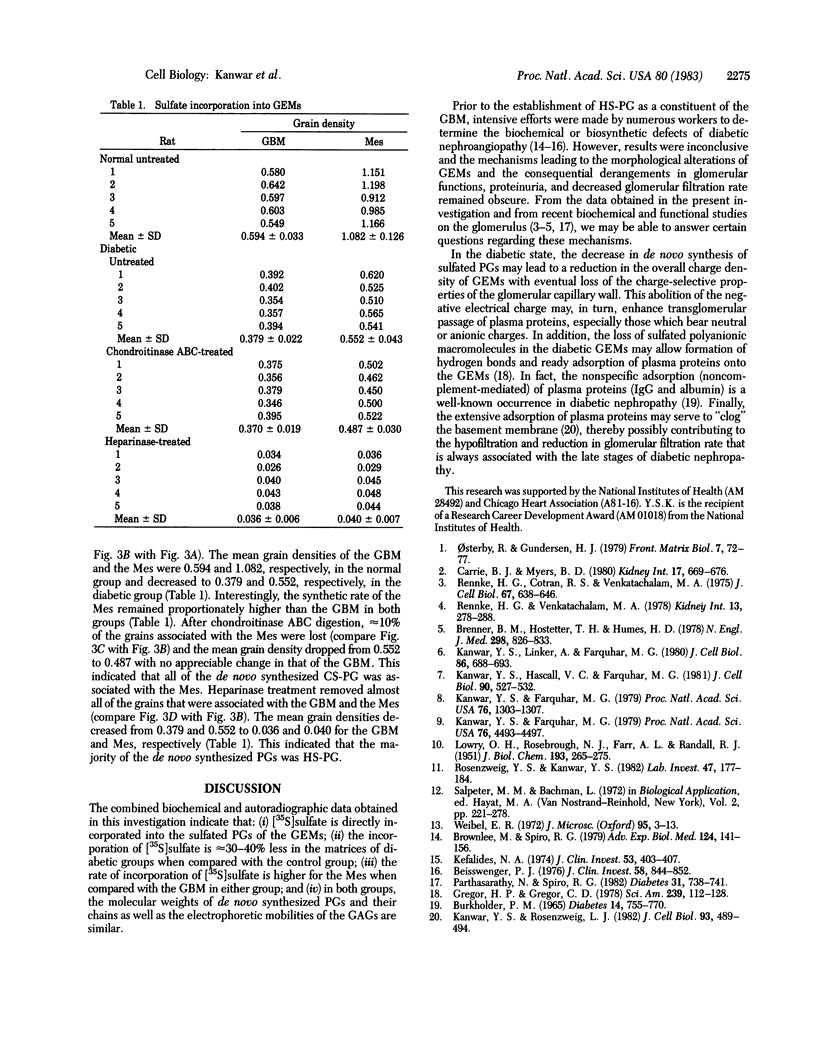
The experimental model of streptozotocin-induced diabetes in rats was utilized to determine the biosynthetic and biochemical alterations in the proteoglycans of the glomerular extracellular matrices (glomerular basement membrane and mesangial matrix) in diabetic nephropathy. Isolated kidneys from diabetic and control groups of animals were radiolabeled in an organ perfusion apparatus with [35S]sulfate of high specific activity (greater than 1,200 Ci/mmol; 1 Ci = 3.7 x 10(10) Bq) and processed for electron microscopic autoradiography, and the proteoglycans of the glomerular extracellular matrices were characterized. The results indicate that [35S]sulfate incorporation into glomerular extracellular matrices of diabetic animals was 30-40% less than that of the control group; however, no differences in the biochemical properties of the de novo synthesized proteoglycans from either group were observed. The relevance of the decreased de novo synthesis of sulfated proteoglycans of glomerular extracellular matrices is discussed in terms of increased glomerular permeability to plasma proteins and reduction in the glomerular filtration rate.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beisswenger P. J. Glomerular basement membrane: biosynthesis and chemical composition in the streptozotocin diabetic rat. J Clin Invest. 1976 Oct;58(4):844–852. doi: 10.1172/JCI108537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B. M., Hostetter T. H., Humes H. D. Molecular basis of proteinuria of glomerular origin. N Engl J Med. 1978 Apr 13;298(15):826–833. doi: 10.1056/NEJM197804132981507. [DOI] [PubMed] [Google Scholar]
- Brownlee M., Spiro R. G. Biochemistry of the basement membrane in diabetes mellitus. Adv Exp Med Biol. 1979;124:141–156. doi: 10.1007/978-1-4684-8508-0_8. [DOI] [PubMed] [Google Scholar]
- Burkholder P. M. Immunohistopathologic study of localized plasma proteins and fixation of guinea pig complement in renal lesions of diabetic glomerulosclerosis. Diabetes. 1965 Dec;14(12):755–770. doi: 10.2337/diab.14.12.755. [DOI] [PubMed] [Google Scholar]
- Carrie B. J., Myers B. D. Proteinuria and functional characteristics of the glomerular barrier in diabetic nephropathy. Kidney Int. 1980 May;17(5):669–676. doi: 10.1038/ki.1980.78. [DOI] [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Isolation of glycosaminoglycans (heparan sulfate) from glomerular basement membranes. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4493–4497. doi: 10.1073/pnas.76.9.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Farquhar M. G. Presence of heparan sulfate in the glomerular basement membrane. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1303–1307. doi: 10.1073/pnas.76.3.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Linker A., Farquhar M. G. Increased permeability of the glomerular basement membrane to ferritin after removal of glycosaminoglycans (heparan sulfate) by enzyme digestion. J Cell Biol. 1980 Aug;86(2):688–693. doi: 10.1083/jcb.86.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwar Y. S., Rosenzweig L. J. Clogging of the glomerular basement membrane. J Cell Biol. 1982 May;93(2):489–494. doi: 10.1083/jcb.93.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kefalides N. A. Biochemical properties of human glomerular basement membrane in normal and diabetic kidneys. J Clin Invest. 1974 Feb;53(2):403–407. doi: 10.1172/JCI107573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Parthasarathy N., Spiro R. G. Effect of diabetes on the glycosaminoglycan component of the human glomerular basement membrane. Diabetes. 1982 Aug;31(8 Pt 1):738–741. doi: 10.2337/diab.31.8.738. [DOI] [PubMed] [Google Scholar]
- Rennke H. G., Cotran R. S., Venkatachalam M. A. Role of molecular charge in glomerular permeability. Tracer studies with cationized ferritins. J Cell Biol. 1975 Dec;67(3):638–646. doi: 10.1083/jcb.67.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennke H. G., Patel Y., Venkatachalam M. A. Glomerular filtration of proteins: clearance of anionic, neutral, and cationic horseradish peroxidase in the rat. Kidney Int. 1978 Apr;13(4):278–288. doi: 10.1038/ki.1978.41. [DOI] [PubMed] [Google Scholar]
- Rosenzweig L. J., Kanwar Y. S. Removal of sulfated (heparan sulfate) or nonsulfated (hyaluronic acid) glycosaminoglycans results in increased permeability of the glomerular basement membrane to 125I-bovine serum albumin. Lab Invest. 1982 Aug;47(2):177–184. [PubMed] [Google Scholar]
- Weibel E. R. The value of stereology in analysing structure and function of cells and organs. J Microsc. 1972 Feb;95(1):3–13. doi: 10.1111/j.1365-2818.1972.tb03707.x. [DOI] [PubMed] [Google Scholar]