Abstract
Hox genes are well known regulators of pattern formation (Capecchi, 1996; Krumlauf, 1994) and cell differentiation (Goff and Tabin, 1997; Papenbrock et al., 2000; Yueh et al., 1998) in the developing vertebrate skeleton. Although skeletal variations are not uncommon in humans (Hald et al., 1995), few mutations in human HOX genes have been described (Goodman and Scambler, 2001). If such mutations are compatible with life, there may be physiological modifiers for the manifestation of Hox gene-controlled phenotypes, masking underlying mutations. We here present evidence that the essential nutrient folate modulates genetically induced skeletal defects in Hoxd4 transgenic mice. We also show that chondrocytes require folate for growth and differentiation and that they express folate transport genes, providing evidence for a direct effect of folate on skeletal cells. To our knowledge, this is the first report of nutritional influence on Hox gene controlled phenotypes, and implicates gene-environment interactions as important modifiers of Hox gene function. Taken together, our results demonstrate a beneficial effect of folate on skeletal development that may also be relevant to disorders and variations of the human skeleton.
INTRODUCTION
Folate deficiency has long been recognized as an important contributor to susceptibility for birth defects (Lewis et al., 1998; Lucock, 2000) and epidemiological studies have demonstrated a beneficial effect of folate supplementation on prevention of birth defects (Berry and Li, 2002; Czeizel et al., 1999; Molloy and Scott, 2001; Ray et al., 2002). These studies have focused on neural tube defects and craniofacial abnormalities, due to their ease of detection at birth and visual ascertainment criteria (Berry and Li, 2002). With respect to skeletal development, evidence for the importance of folate metabolism comes from methotrexate-exposed rabbits (DeSesso and Goeringer, 1991) and skeletal involvement in the well-known aminopterin/methotrexate syndrome in humans (OMIM 600325). However, direct studies of the effect of folate on skeletal development have not been reported to date.
The development of most of the skeleton proceeds in an ordered succession of steps, known as endochondral bone formation, during which an initial cartilage model for skeletal structures is formed that is later replaced by bone. This process is regulated by systemic factors, such as the insulin-like growth factors and growth hormone (Robson et al., 2002), by the action of cell-type specific transcription factors, such as Sox-9/Sox-5/Sox-6 (Lefebvre et al., 2001), and also by local regulators of cartilage differentiation, such as fibroblast growth factors, hedgehog proteins, bone morphogenetic proteins and their respective receptors (Minina et al., 2002). The ultimate shape of future skeletal elements already becomes defined at the stage of cartilage formation, in the process known as pattern formation, and is mediated by the Hox transcription factors.
There is a large body of evidence that mutations in Hox genes, as well as overexpression and misexpression, affect patterning, growth and differentiation of skeletal elements in experimental animals. Yet, surprisingly few mutations in human HOX genes have been found to date. The limb phenotypes in patients with Hand-Foot-Genital Syndrome and Guttmacher Syndrome are caused by mutations in HOXA13 (Innis et al., 2002; Mortlock and Innis, 1997), and Syn/Polydactyly by mutations in HOXD13 (Akarsu et al., 1996; Muragaki et al., 1996). However, other Hox genes have not been implicated in skeletal abnormalities, and it was suggested that Hox gene mutations may be detrimental to survival even in heterozygous state and therefore infrequent in the population (Galis, 1999). However, if all mutations were reproductively unsuccessful, it becomes difficult to envision how evolutionary changes in the Hox gene system, and in animal body plans as patterned by Hox genes, could have been accomplished. Clearly, genetic variation does occur in human HOX gene loci (Dow et al., 1992; Faiella et al., 1998; Goodman et al., 1997; Goto et al., 1991; Ingram et al., 2000; Kolon et al., 1999; O'Brien et al., 1997). Furthermore, skeletal variations that resemble animal Hox phenotypes are frequent in asymptomatic humans (Hald et al., 1995), indicating that such variations are viable. A plausible explanation is that human HOX gene mutations may escape detection because the phenotypic manifestation of such mutations can be modulated by external factors, such as nutritional status. We here present evidence to support this hypothesis by showing that nutritional status can modulate a Hox gene controlled phenotype.
RESULTS
Cartilage defects in Hoxd4 transgenic mice
We have previously shown that the overexpression of Hoxc8 in transgenic mice impairs cartilage formation through a delay in chondrocyte maturation (Yueh et al., 1998). When a Hoxd4 transgene is expressed in exactly the same fashion as Hoxc8 (Figure 1), the Hoxd4 transgenic animals also exhibit profound cartilage defects: the cartilaginous portions of the ribs lack tensile strength, and the vertebral column is very flexible and unstable due to reduced or absent intervertebral cartilages (Figure 2). In the most severe cases, transgenic animals die from inability to inflate their ribcages during breathing. In addition, Hoxd4 transgenic animals are born with open eyes, a phenotype also observed in Hoxc8 transgenics. In Hoxc8 transgenic mice, the transgene is overexpressed in the normal expression domain of Hoxc8, which includes cartilage (Cormier and Kappen, manuscript in preparation). Hoxd4 is normally expressed in cartilage in more anterior structures, and in Hoxd4 transgenic mice, is now expressed ectopically within the Hoxc8 domain under control of Hoxc8 regulatory sequences. The similarities of defects in Hoxd4 and Hoxc8 transgenic mice (Yueh et al., 1998) show that both Hox genes - expressed under control of the same chondrocyte-specific enhancer - act in comparable fashion on developing cartilage. As for the Hoxc8 transgene, the severity of Hoxd4-induced cartilage defects is transgene-dosage-dependent (Yueh et al., 1998 and data not shown), consistent with a quantitative role of Hox transcription factors in cartilage development. The delay in chondrocyte maturation is evident during cartilage formation in Hoxd4 transgenic mice (Figure 2). In Hoxc8 transgenic mice, the delay is associated with decreased incorporation of BrdU, a marker for DNA synthesis (Cormier et al., 2003). In addition, expression studies indicated reduced expression of the Thymidylate Synthase gene in Hoxc8 transgenic chondrocytes (Talmadge and Kappen, unpublished), potentially implicating reduced DNA synthesis as the cause of delayed cartilage maturation. On this basis, we hypothesized that providing a key metabolite for DNA synthesis, the known mitogen folic acid (folate), might have a beneficial effect on skeletal development in these mice.
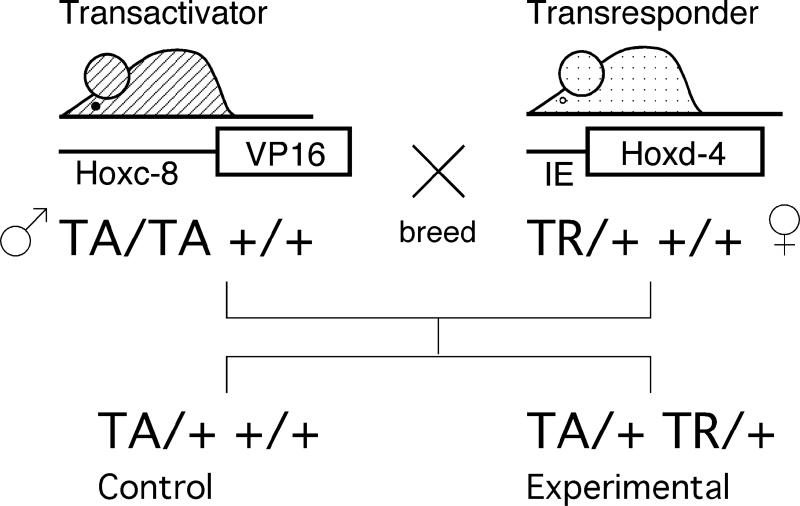
Figure 1. Generation of Hoxd4 transgene expressing mice in the VP16 binary transgenic system.
Two independent parental strains carry the transactivator (TA) and transresponder (TR) transgenes, respectively. Animals from both parental strains are normal; activation of the TR transgene is achieved only in simultaneous presence of the TA transgene. Experimental and control animals are generated within the same female, providing for comparable nutritional exposure of controls and Hoxd4 transgenic animals.
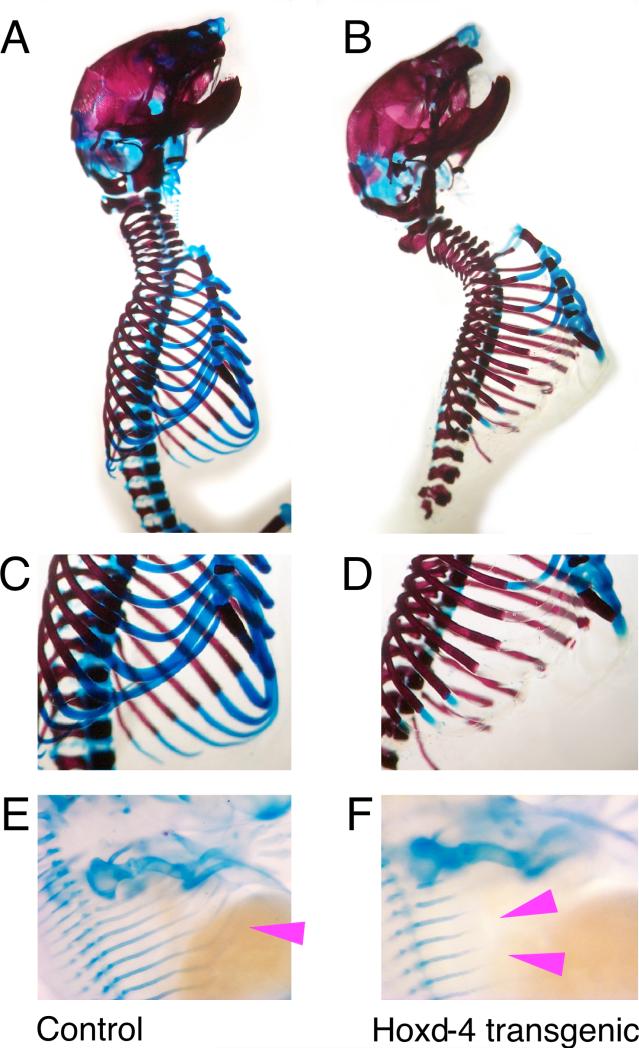
Figure 2. Cartilage defects in Hoxd4 transgenic mice.
Preparations of skeletons stained with Alizarin Red for bone and Alcian Blue for cartilage. Rib cages of (A,C,E) control and (B,D,F) Hoxd4 transgenic newborn mice (genotype TA/+ TR/+). (A,B) Staining for Alcian blue is absent or reduced in ribs (arrowhead) of the Hoxd4 transgenic animal (B,D). (C,D) Close-ups of rib cages caudal from the third rib in newborn skeletons. Embryos were isolated at gestational day 13.5 and stained for Alcian Blue. (E) Magnification of a control embryo showing the rib cartilage anlagen (arrowhead); their extension is reduced upon expression of the Hoxd4 transgene (F), indicating delayed cartilage formation and maturation (compare position of arrowheads). This embryo had the genotype TA/TA TR/+.
Folate supplementation reverses cartilage defects
We therefore supplemented folate to the diet of female mice that carry but do not express the Hoxd4 transgene (genotype +/+ TR/+, see Methods for details). When mated to homozygous transactivator males (TA/TA +/+), these females will generate Hoxd4 transgene-expressing (TA/+ TR/+) and control embryos (TA/+ +/+) in the same litter. Figure 3 shows that skeletons prepared from folate-supplemented Hoxd4 transgenic embryos exhibit restored Alcian Blue staining in rib and vertebral cartilage. The structural rigidity of skeletons was also improved. Cartilage staining was restored in up to 80% of progeny, a figure that is comparable to the rescue of neural tube defects by folate in Folbp1-deficient (92%), Cart-1-deficient (81%), Cited2-deficient and splotch mutant mice (Barbera et al., 2002; Fleming and Copp, 1998; Piedrahita et al., 1999; Zhao et al., 1996). Our results establish a protective effect of folate on skeletal development and provide evidence that nutritional status can modulate a genetically based skeletal phenotype.
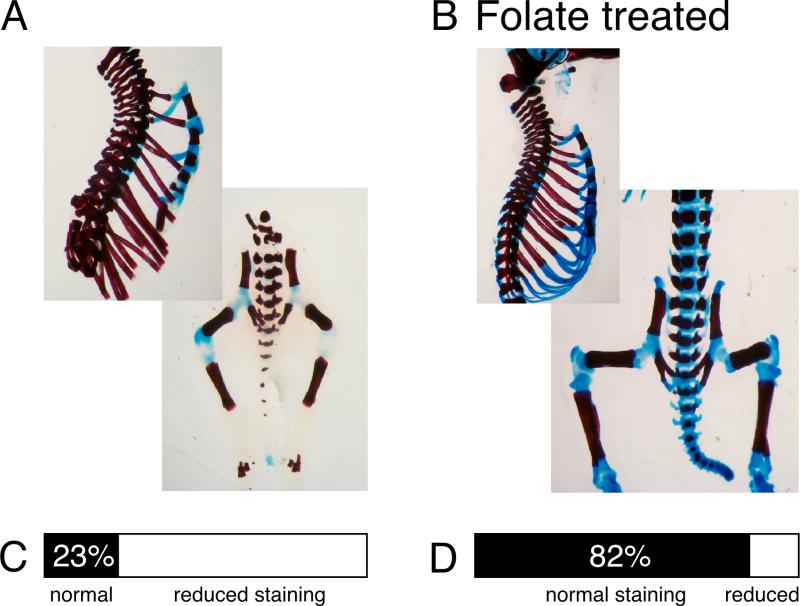
Figure 3. Folate supplementation restores Alcian Blue staining in transgenic cartilage.
(A) Skeleton of a Hoxd4 transgenic newborn mouse. (B) Skeleton of a Hoxd4 transgenic raised in a mother who received folate daily throughout the pregnancy at 25 mg/kg body weight. Fraction of skeletons with obvious staining defects in (C) unsupplemented (n=13) and (D) folate-supplemented (n=16) animals. Represented in (D) are results for animals supplemented with 25 mg folinic acid/kg body weight from gestational day 11.5 to 18.5, the group for which the best improvement in most skeletons was noted (p=0.0009). If results for all supplemented animals (n=89; summarizing all regimens) are compared to unsupplemented Hoxd4 transgenic animals (n=56; including all bedding conditions), the fraction of skeletons deficient in cartilage staining was reduced from 59% to 34% (p=0.0035). Alcian Blue staining in ribs and vertebral column is restored by folate supplementation, and rigidity of the skeleton is improved.
Differential effect of folate on different tissues
Interestingly, the open-eye-at-birth phenotype was not reversed, even with increasing doses of folate (data not shown). These data suggest differential susceptibility of different tissues - mesenchyme around the eye and cartilage, respectively - to the beneficial effects of folate. There are currently no data available to distinguish whether responsivity is related to differences in the action of Hoxd4 in either tissue, or by differences in folate availability or metabolism. Similarly, even at the highest dosage of folate supplementation, animals still died shortly after birth (Figure 4) from respiratory failure. Despite improved proteoglycan content in rib cartilage of supplemented Hoxd4 transgenics, the overall integrity of the rib cage and/or trachea may be still insufficient for survival (note the reduced Alcian Blue staining in the trachea of the folate-supplemented animal in Figure 3). Nonetheless, the higher incidence of normal Alcian Blue staining in offspring from supplemented females indicates that cartilage itself responds to folate supplementation.
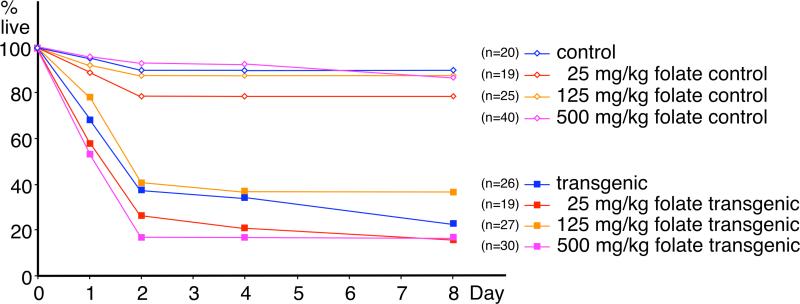
Figure 4. Effect of folate supplementation on survival of Hoxd4 transgenic mice.
The proportion of live mice declines rapidly after birth for Hoxd4 transgenic newborns (closed symbols), but not for controls (open symbols). The attrition rate for transgenic mice is independent of the dose of folinic acid (different colors), and there are no significant differences between the survival curves for the different transgenic groups (numbers of individuals in brackets), or between control groups.
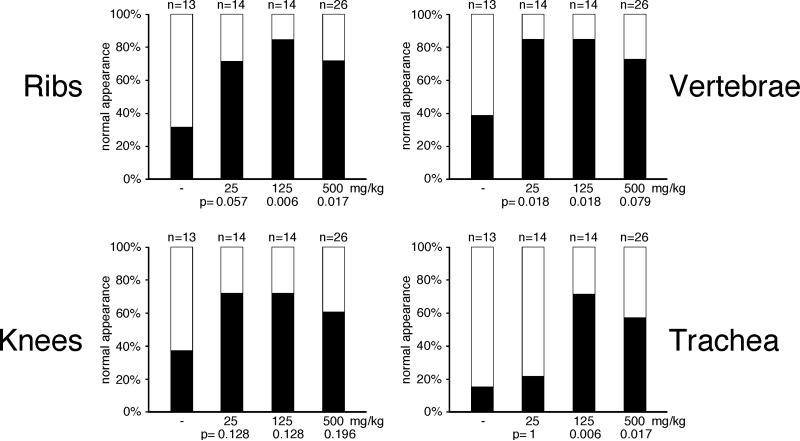
We also found differential responses to folate within different cartilage structures (Figure 5): Cartilage in ribs and vertebral column showed extensively restored staining and rigidity in a statistically significant number of animals (Fig. 5 a,b). The knee cartilage, however, did not respond significantly at any dose (Fig. 5c), and most animals had detectable staining in tracheal cartilage only at higher doses (Fig. 5d). The apparent reduced efficiency of supplementation at the highest dose (500mg/kg) could be interpreted to indicate possible detrimental effects of oversupply. However, no obvious negative effects were noted in non-Hoxd4 transgenic control offspring (which are reared in the same uterine environment, see Figure 1). Taken together, these results suggest that different skeletal structures either have a differential requirement for folate, or differential effects of Hoxd4 overexpression during the formation and maturation of different cartilage structures over time.
Figure 5. Differential effect of folate in different cartilage structures.
Restoration of Alcian Blue staining in different cartilage structures was assessed independently by two technicians for each skeleton without prior knowledge of genotype. Filled bars represent normal appearance of cartilage; open bars represent unstained or very weakly stained structure. All p-values are calculated relative to the untreated control. Rib (A) and vertebral (B) cartilages were restored by folate supplementation, while knee cartilage (C) did not respond in statistically significant fashion (compare p-values) to folate supplementation. Increased Alcian Blue staining in trachea (D) was only observed at higher doses of folate. Structural rigidity of the vertebral column was found in 23% of untreated controls, but restored in 92.9% of animals with folate supplementation of 25mg/kg (p=0.00034), in 85.7% with 125mg/kg (p=0.0018), and in 65.4% with the 500mg/kg dose (p=0.019). Vertebral column and ribs showed the best improvement of cartilage production and staining with folate supplementation.
Folate is beneficial in early skeletogenesis
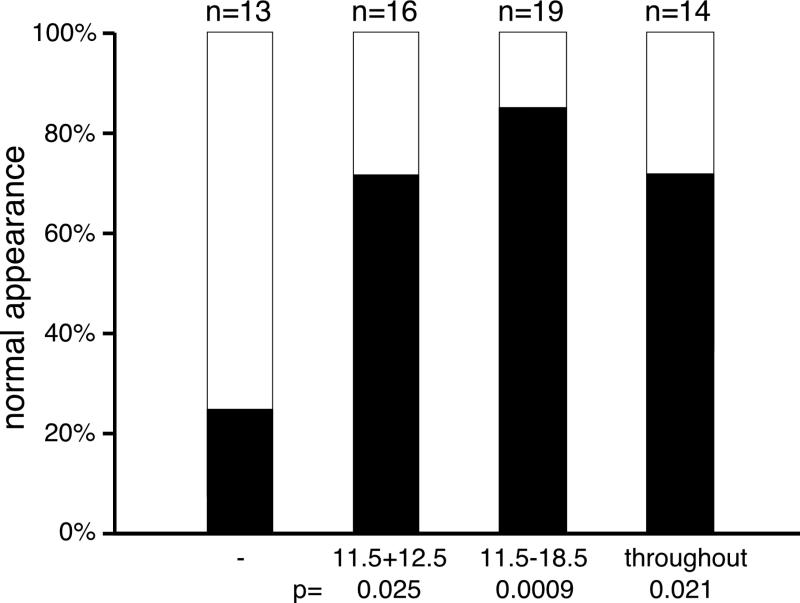
In order to establish whether there was a critical time window for the protective effect of folate on cartilage development in Hoxd4 transgenic mice, we restricted supplementation to the last half of the pregnancy (days 11.5 through 18.5), or gave folate only on embryonic days 11.5 and 12.5, just prior to overt cartilage formation (Figure 6). Even with only two days of supplementation, the skeletons of Hoxd4 transgenic mice were restored in their structural rigidity and in staining for mature cartilage. From these results, we conclude that Hoxd4 transgenic chondrocytes have a specific requirement for folate early in chondrogenesis. While we cannot exclude a continuing benefit derived from residual folate stored elsewhere in the body (Shane, 1995), our findings indicate that the effective time window for nutritional supplementation coincides with the early phase of cartilage formation.
Figure 6. Temporal window for cartilage rescue by folate supplementation.
Supplementation with folinic acid at 25mg/kg was performed either throughout the pregnancy (see Methods), or restricted the second half of the pregnancy, or gestational days 11.5 and 12.5. Restored cartilage in Hoxd4 transgenic skeletons was observed even with only two days of supplementation. Improvement for ribs and vertebrae was statistically significant (p=0.000025 for E11.5-18.5; p=0.0027 for E11.5/12.5); the longer supplementation lead to some improvement for knees (p=0.005 for E11.5-18.5; p=0.14 for E11.5/12.5) but little for trachea (p=0.062 for E11.5-18.5; p=0.66 for E11.5/12.5). Rigidity of the vertebral column was significantly restored with both treatment regimens (p=0.0009 for E11.5-18.5; p=0.00001 for E11.5/12.5). Supplementation of pregnancies with folate prior to overt skeletogenesis (E11.5/12.5) restores production and staining of normal cartilage in Hoxd4 transgenic mice.
Folate transport protein expression in chondrocytes
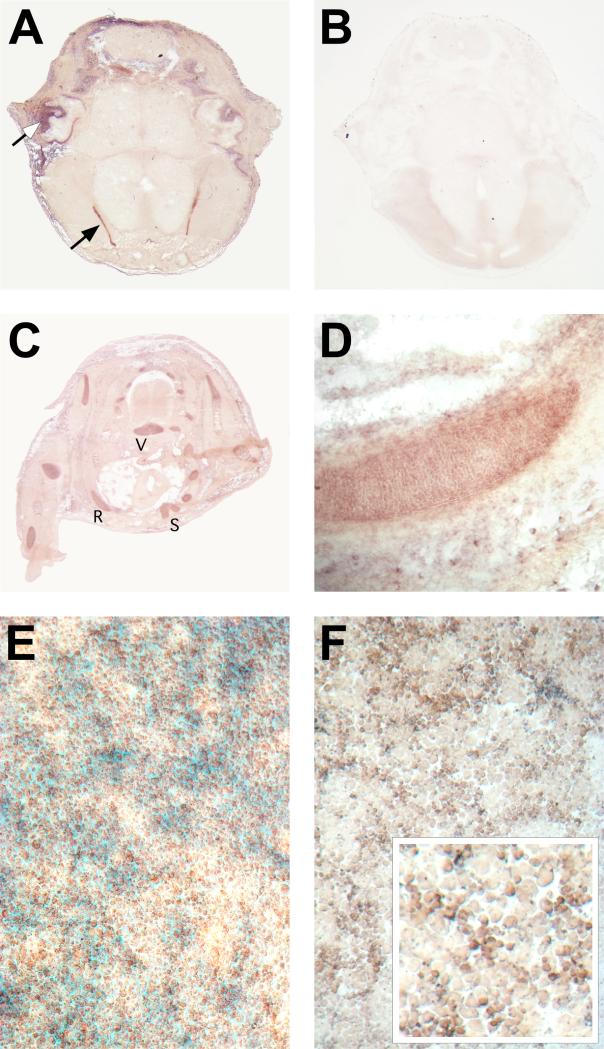
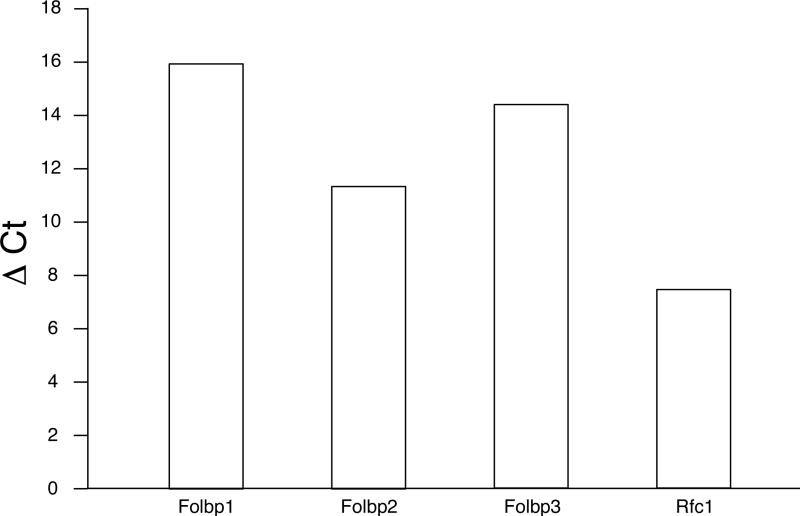
Utilization of folate requires uptake into cells, mediated by specific folate transport proteins (Antony, 1996), the GPI-anchored Folate receptors (Folate binding proteins in mouse) and the integral membrane protein Reduced folate carrier 1 (Rfc1). Evidence for the expression of these proteins in chondrocytes comes from our quantitative RT-PCR analyses and from in situ hybridization studies. None of the known folate receptor genes are expressed at an appreciable level in somitic mesoderm, while Rfc was found to be expressed widely in the developing embryo at all stages examined (Salbaum, unpublished). Folbp2 expression in chondrocytes is detectable by embryonic day 15.5 (Figure 7) whereas Folbp1 and Folbp3 are not detectable or expressed only at very low levels. When primary chondrocytes are placed into tissue culture, Folbp2 is expressed (Fig. 7f), and chondrocytes are positive for Folbp1 and Folbp3 after 4 days in culture (data not shown). The expression is specific to differentiating chondrocytes, since cells plated at low density - which leads to dedifferentiation into fibroblastoid cells - do not exhibit Folbp gene expression. By quantitative RT-PCR, Folbp2 is the major folate receptor in primary rib chondrocytes at birth and is co-expressed with Rfc1 (Figure 8). Thus, developing chondrocytes express known folate transport proteins and may require folate for proliferation and/or differentiation.
Figure 7. Expression of Folbp2 in developing cartilage.
In situ hybridization to sections from a mouse embryo at 15.5 days of development (A-D) and to cultured primary chondrocytes (F). A, Transverse section through the head reveals strong signal for Folbp2 in the choroid plexus (closed arrow) and also in developing cartilage (open arrow points to ear cartilage as an example). This signal is specific for the Folbp2 probe; a control section from the same embryo processed identically but without probe (B) does not reveal any AP activity. (C) Folbp2 expression is also found in limb and trunk cartilage, such as R: rib, S: sternum, and V: vertebral center. (D) Close-up with signal of rib cartilage. All sections are oriented with dorsal to the top. (E) Primary neonatal rib chondrocytes after 4 days in high-density culture. Cells were stained with Nuclear Fast Red to reveal nuclei, and with Alcian Blue to reveal production of extracellular cartilage matrix. (F) In situ hybridization for Folbp2 to chondrocytes cultured as shown in E. Folbp2 signal is evident in cultured chondrocytes with smaller, proliferating cells expressing Folbp2 at apparently higher levels than mature hypertrophic cells (inset).
Figure 8. Chondrocytes express genes encoding folate transport molecules.
Quantitative RT-PCR was performed in triplicate on pooled chondrocytes from normal FVB neonates. GAPDH (Glyceraldehydephosphate Dehydrogenase) expression was used as the standard, and results are expressed as the difference in threshold cycle number for detection of each gene relative to the threshold cycle number for GAPDH in the same sample (CtGENE - CtGAPDH = Δ Ct). Higher numbers indicate that more cycles are needed to detect expression, hence reflecting lower expression levels. The major folate transporters expressed in primary chondrocytes are Folbp2 and Rfc1.
Chondrocyte differentiation requires folate
The mechanistic basis for the ability of folate to prevent birth defects in humans (Czeizel, 1996) or mice (Fleming and Copp, 1998) is poorly understood. Folate has long been known to stimulate cell proliferation in lymphoid (Rogers and Lietman, 1977) and epithelial (McAuslan et al., 1979) cells, acting as a mitogen (James et al., 1993). We therefore tested whether folate has a direct effect also on chondrocyte proliferation. Primary chondrocytes were isolated from ribs of newborn FVB mice and cultured at high density (Cormier et al., 2003). Chondrocytes did not proliferate in the absence of folate (Figure 9), and there also was no differentiation to hypertrophy (data not shown). That this outcome is specifically mediated through folate metabolism is revealed by the fact that methotrexate, a well-known anti-folate (Calvert, 1999), inhibits chondrocyte proliferation and maturation in our culture system. These results demonstrate that chondrocytes require folate for proliferation and differentiation. Taking these findings together with the restored cartilage production in folate supplemented transgenic mice, we conclude that the beneficial effect of folate is likely exerted directly on the developing chondrocytes that express the Hoxd4 transgene.
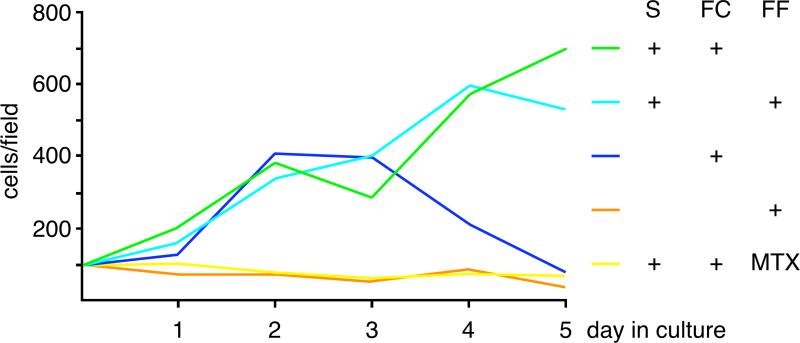
Figure 9. Chondrocytes require folate for growth and differentiation.
Primary rib chondrocytes from neonatal mice were placed at high density into culture in folate-containing (FC) or folate-free medium (FF), with or without serum (S). Green: folate and serum, turquoise: no folate but serum, purple: folate but no serum, orange: neither folate nor serum, red: folate and serum and methotrexate (MTX). Serum is able to provide folate (and other nutrients) to folate-depleted medium; without serum, cells in folate-containing medium initially divide, but are unable sustain proliferation (purple) or to differentiate to hypertrophy (not shown). Without folate (orange), or with inhibited folate metabolism (methotrexate, red), chondrocytes are unable to grow and differentiate.
DISCUSSION
Folate restores skeletal defects in Hox transgenic mice
Adequate folate supply is known to be critical for proper embryonic development (Czeizel, 1996) but detailed information on the role of folate metabolism in skeletal development is lacking. Complete dietary depletion for folate is difficult to achieve in animals, but maternal folate deficiency is linked to folate deficiency secondarily in the embryo (Fleming and Copp, 1998). This mechanism, however, is not likely to play a role in the Hoxd4 induced cartilage defects: Hoxd4 transgenic pregnant mothers do not express the Hoxd4 transgene and therefore are not expected to have any abnormalities in digestive tissues or metabolism. Indeed, the controls provide no indication that maternal metabolism is deficient in our experimental system: non-transgenic embryos, which are raised together with the Hoxd4 transgenics in the same uterine environment (see Figure 1), lack any discernable developmental defects. Systemic depletion of folate transport to the developing embryo has been achieved genetically by targeted disruption of Folbp1 (Piedrahita et al., 1999) and of Rfc1 (Zhao et al., 2001). Both deficiencies cause early embryonic death and are uninformative for skeletal development; tissue-specific deletion strategies will be needed to clarify the relevance of these transport mechanisms in cartilage formation. Indirect evidence from skeletal phenotypes caused by maternal exposure to methotrexate in rabbits and aminopterin/methotrexate syndrome in humans implicate folate availability in skeletal development. In Hox transgenic mice, chondrocytes may have a locally elevated folate requirement. Supplementation of folate increases availability of folate to those cells, and in this way overcomes the delayed maturation of Hoxd4 transgenic chondrocytes.
In flies transgenic for the Hox gene labial, expression of the gene for S-adenosylmethionine decarboxylase was found upregulated (Leemans et al., 2001); increased removal of the methyl-donor S-adenosylmethionine from the methylation pathway would be consistent with increased metabolic flow through the folate pathway. To date, we have not detected significant changes in the levels of mRNA expression of several folate pathway genes in Hoxd4 transgenic chondrocytes (Kruger, Talmadge and Kappen, unpublished data) leaving the mechanistic targets for folate supplementation in transgenic chondrocytes as of yet unidentified. We show that chondrocytes need folate specifically for proliferation and differentiation, and the levels of cellular folate metabolites are known to control the balance between DNA synthesis and methylation of DNA and proteins (Herbig et al., 2002). Altered DNA methylation (Bird, 2002) or histone methylation (Lachner and Jenuwein, 2002) may affect the activity of genes regulated by Hox transcription factors, but this remains speculation until such downstream targets - of either Hox genes or folate - have been identified in skeletal cells. Alternatively, folate may interact with the proposed function of Hox genes in cell cycle regulation (Duboule, 1995; Hu et al., 2001; Krosl and Sauvageau, 2000), which is also linked to histone methylation (Huang, 2002; Nielsen et al., 2001). Then, the mitogenic capacity of folate (McAuslan et al., 1979; Rogers and Lietman, 1977), via methylation of DNA or proteins or cell cycle regulation, could be responsible for restored cartilage development in our Hoxd4 transgenic mice.
Interestingly, we found that folate was also able to affect a Hox controlled skeletal phenotype in a loss-of-function paradigm. Mice with a targeted disruption of the Hoxb6 gene (Kappen, 2000) exhibit homeotic transformations of vertebra at the cervico-thoracic junction, encompassing incomplete development of the first pair of ribs (Kappen, manuscript submitted). Most notably, the rib heads are defective and do not articulate properly with the vertebral body. These articulation and rib head defects were reversible by folate supplementation in (manuscript in preparation). These findings complement the results reported here and establish a beneficial effect of folate on Hox gene controlled skeletal phenotypes in independent genetic paradigms.
Protective effect of folate on skeletogenesis
Folate has been shown to reduce the risk for neural tube defects and craniofacial defects in human populations (Berry and Li, 2002; Czeizel et al., 1999; Molloy and Scott, 2001; Ray et al., 2002). In genetic predisposition to developmental defects, folate was effective in rescue of neural tube defects in Folbp1-deficient, Cart-1-deficient, Cited2-deficient and splotch mutant mice (Barbera et al., 2002; Fleming and Copp, 1998; Piedrahita et al., 1999; Zhao et al., 1996). While not specifically analyzed by the authors, folate also restored cartilage development in the cranial region of Cart1 mutants, suggesting that it may be beneficial to precursor or effector cells in bones formed by intramembraneous ossification. However, the specific cell type targeted by folate supplementation in the above genetic models is not known. We show here that skeletal cells express folate transport genes, and we have evidence that they also express enzymes involved in folate metabolism (Kruger, Talmadge and Kappen, unpublished). Our combined results demonstrate that folate is beneficial during cartilage formation in Hoxb6 mutants (Kappen, manuscript in preparation), and for the developing chondrocytes in Hoxd4 transgenic mice. These mice, without folate supplementation, develop a phenotype similar to human chondrodysplasia. The protection by folate against skeletal defects in genetically predisposed mice encourages speculation that defects in human skeletal development may also be ameliorated by folate supplementation.
Taken together, our results establish folate as a nutritional modifier of Hox gene controlled phenotypes and suggest that other genetic predispositions to skeletal defects could also be modulated by folate. Skeletal variations are quite common even in clinically asymptomatic human subjects (Hald et al., 1995). Genetic factors that can be expected to play a role in such variations include the numerous transcription factors, growth factors and their receptors, as well as cell surface proteins and cell cycle regulators that have been implicated in control of skeletal development (Kappen et al., in press; Zelzer and Olsen, 2003). Insight into the relative contributions of local regulators, generalized factors, and nutrition to healthy bone formation and growth will come from a better understanding of their interactions at various stages of skeletal development.
Hox-controlled phenotypes are reversible
An important implication from our findings is that the action of Hox genes in cartilage formation and maturation can be reversed by folate. This indicates that overexpression of Hoxd4, and according to the cell culture evidence Hoxc8 (Cormier et al., 2003), induces reversible changes in chondrocytes rather than re-programming a cellular differentiation pathway. Prior work on the role of Hox genes in skeletal patterning (Condie and Capecchi, 1993; Kessel, 1992; Knezevic et al., 1997; Yokouchi et al., 1995) and in the hematopoietic system (Kappen, 2000; Lawrence et al., 1997; Perkins et al., 1990; Sauvageau et al., 1997; Shen et al., 1992) predicted that tissue-specific alterations in Hox gene expression lead to irreversible changes in cell fate and differentiation. Instead, the reversibility of cartilage defects in our Hoxd4 transgenic mice clearly indicates that Hoxd4 overexpression does not affect cell fate determination. Rather, the correlation of phenotype severity to gene dosage and the reversibility of the cartilage defects by folate argue for a quantitative regulatory role for Hoxd4 in cartilage development. Similarly, the ability of folate to restore rib articulation in Hoxb6 mutants provides evidence that loss of Hoxb6 function affects skeletal development in a quantitative manner. This conclusion is further supported by evidence that skeletal features controlled by Hoxb6 behave as a quantitative trait and can be genetically modified (Kappen, manuscript submitted). The results of our present study demonstrate that the cellular outcomes of Hox transcription factor function are subject to modulation by nutritional status, and establish gene-environment interactions as important factors for phenotype manifestation in the developing skeleton.
Nutritional status as a “buffering mechanism”
Folate can protect developing embryos against multiple teratogenic insults, including exposures to anticonvulsants (Trotz et al., 1987), the fungal toxin FumonisinB (Sadler et al., 2002), nitrous oxide (Keeling et al., 1986) and genetic predisposition (Barbera et al., 2002; Paros and Beck, 1999; Petter et al., 1977; Zhao et al., 1996) - as shown here for Hox gene expression. As each insult is presumably mediated through different molecular mechanisms, the beneficial effect of folate in different conditions can be hypothesized as follows: folate provides an optimal baseline for the response of cells to adverse stimuli, effectively raising the threshold for manifestation of developmental defects. Such a role of folate would be reminiscent of the role of Hsp90 as a capacitor of evolution. It has been shown that adequate expression of Hsp90 phenotypically masks variation in Drosophila and Arabidopsis (Queitsch et al., 2002; Rutherford and Lindquist, 1998), providing a “buffering mechanism” against phenotypic expression of underlying genetic variants (Rutherford, 2000). These variants express their traits only when the threshold for phenotype manifestation is altered by lowering effective Hsp90 levels. In our transgenic mice, optimal folate availability similarly results in phenotypic masking of underlying genetic defects. Our results thus advance the following hypothesis: nutritional status provides the buffer under which genetic variation in Hox genes can be accumulated, which becomes subject to selection upon change in the nutritional status and environment. Selection would only operate in the next generation: parents with the mutation that are newly exposed to altered conditions may themselves suffer from the adverse health effects of poor nutrition, but their own body plan would be unaffected. The developing progeny would experience altered nutritional status and altered Hox gene function, and this could potentially manifest in altered skeletal patterning or a new body plan.
METHODS
The creation, breeding and genotyping of transgenic strains have been described. All transgenic experiments were performed on the FVB inbred background using the VP16-based binary transgenic mouse system (Kappen, 1999). In this system, the Hoxc8 promoter directs expression of the viral VP16 transactivator to skeletogenic cells (Yueh et al., 1998). VP16 expression activates a Hox transgene linked to the VP16-responsive IE-promoter (this promoter is silent in mice in the absence of VP16 (Yaworsky and Kappen, 1999) as demonstrated by lack of defects in regions or tissues where the Hoxc8 promoter is not active). Hoxd4 transgenic animals were generated in crosses of fathers homozygous for the Hoxc8-VP16 transactivator transgene (TA/TA +/+) and mothers hemizygous for the IE-Hoxd4 transresponder transgene (+/+ TR/+). In this fashion, controls (TA/+ +/+) and Hoxd4 transgenic animals (TA/+ TR/+) were generated within the same litter (Figure 1) and exposed to the same maternal nutritional environment. Embryos were isolated at gestational day 13.5 and stained as whole-mount specimen for Alcian Blue as described (Jegalian and DeRobertis, 1992). The Hoxd4 transgenic embryo in Fig.2f has the TA/TA TR/+ genotype, generated in a cross of one of the few surviving compound hemizygous (TA/+ TR/+) females to a homozygous transactivator (TA/TA +/+) male.
Folate supplementation was done by gavage administration of folinic acid, the biologically active and more stable form of folate (Piedrahita et al., 1999). Each female received 25mg/kg of body weight (or other dose as indicated) of folinic acid one week prior to mating and every day after a copulation plug was detected. The day of presence of the plug was counted as day 0.5 of development. Females on a restricted supplementation regimen did not receive any dose prior to gestational day 11.5. All transgenic females scheduled for participation in folate supplementation experiments were reared and maintained on non-foodstuff bedding (Irradiated Isopads, Harlan, Indianapolis, IN). To control for effects of bedding conditions on phenotype, some Hoxd4 transgenic animals were maintained on conventional irradiated bedding (Bed-O'cobs Corncob bedding and litter, Maumee, OH), but these were not included in folate supplementation experiments. All animals were housed in individually vented microisolator cages with ad libitum access to food (Harlan-Teklad diet LM-485, folic acid content is 6.70mg/kg chow) and water.
Skeletal preparations from newborn animals were done as described before (Yueh et al., 1998). The carcasses were deskinned and eviscerated, fixed in 95% ethanol for 5 days, then stained with 0.015% Alcian Blue for one day and rinsed in 95% ethanol for two days. Alcian Blue detects sulfated proteoglycans, which constitute a major component of the skeletal cartilages. Subsequently, samples were cleared in 1% KOH and counterstained for three hours with 0.005% Alizarin Red, which stains ossified skeletal structures (bone). Skeletons were further cleaned with 2%KOH for 3-6 hours, and then incubated in the following volume ratios of 2% KOH and glycerol: 80:20, 60:40, 40:60 and 20:80. Specimen were observed under a Leica MZ6 stereomicroscope, and photographed with a Kodak MDS290 digital camera. Scoring was done for Alcian Blue staining (presence with high intensity in more than 50% of the structure, absence in more than 50% of the structure or very weak staining), and for skeletal rigidity as assessed by integrity of the skeleton after repeated transfer between vessels and at 4 weeks after preparation (transgenic skeletons typically break apart upon transfer and later disintegrate into pieces due to loose cartilage; supplemented skeletons maintain structure even with repeated transfers and in long-term storage). All data were collected independently by three technicians without prior knowledge of genotype and confirmed by the principal investigator. Statistical evaluation of results was done by 2-tailed Fisher's exact test (http://www.matforsk.no/ola).
Primary chondrocytes were isolated for cell culture using a modification of the method of Lefebvre et al. (Lefebvre et al., 1994), and cells were seeded on 35 mm dishes coated with 0.1% Gelatin at a density of 3×105 cells/dish. Cells were counted daily from triplicates of cultures set up with the following conditions: The base medium was either a 1:1 DMEM/F12 mixture (Invitrogen, folate concentration of 6 μM) supplemented with insulin, transferrin and selenium (Mello and Tuan, 1999), or Folate-free DMEM (Biosource, Camarillo, CA) with the same additives. In serum-containing cultures, Fetal Calf Serum (Gibco/Invitrogen) was added to 10% final volume; methotrexate, a potent inhibitor of folate metabolism, was added at a concentration of 0.5 μM.
Quantitative RT-PCR analyses were performed exactly as described before (Cormier et al., 2003).
In situ hybridizations were carried out as described before (Salbaum and Kappen, 2000) using digoxygenin labeled probes and an Alkaline Phosphatase-based detection system (Boehringer). Sections processes without probe showed no residual AP activity under these conditions. The Folbp probes were derived from full-length cDNAs, and the Rfc probe came from IMAGE clone IC572932 which contains a 1 kb fragment.
ACKNOWLEDGEMENTS
Diane Costanzo provided substantial experimental support for this project, of which Dr. Claudia Kappen is the Principal Investigator. Dr. J. Michael Salbaum performed in situ hybridizations, Dr. Maria Alice Mello did the tissue culture experiments, and Dr. Richard Finnell advised on folate supplementation. The quantitative PCR assays were expertly performed by Catherine Talmadge. We thank Tarra Wiggins, Matthew Northam and Amy Johnson for technical help, and Dr. Jörg Rahnenführer for advice with statistics. This work was funded in parts by grants from the Arthritis Foundation, the Aircast Foundation, The Philip Morris External Research Program, and the NIH (R21-DE14523).
REFERENCES
- Akarsu AN, Stoilov I, Yilmaz E, Sayli BS, Sarfarazi M. Genomic structure of HOXD13 gene: a nine polyalanine duplication causes synpolydactyly in two unrelated families. Hum Mol Genet. 1996;5:945–952. doi: 10.1093/hmg/5.7.945. [DOI] [PubMed] [Google Scholar]
- Antony AC. Folate Receptors. Annu Rev Nutr. 1996;16:501–521. doi: 10.1146/annurev.nu.16.070196.002441. [DOI] [PubMed] [Google Scholar]
- Barbera JP, Rodriguez TA, Greene ND, Weninger WJ, Simeone A, Copp AJ, Beddington RS, Dunwoodie S. Folic acid prevents exencephaly in Cited2 deficient mice. Hum Mol Genet. 2002;11:283–293. doi: 10.1093/hmg/11.3.283. [DOI] [PubMed] [Google Scholar]
- Berry RJ, Li Z. Folic acid alone prevents neural tube defects: evidence from the China study. Epidemiology. 2002;13:114–116. doi: 10.1097/00001648-200201000-00021. [DOI] [PubMed] [Google Scholar]
- Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- Calvert H. An overview of folate metabolism: features relevant to the action and toxicities of antifolate anticancer agents. Semin Oncol. 1999;26:3–10. [PubMed] [Google Scholar]
- Capecchi MR. Function of Homeobox Genes in Skeletal Development. Ann NY Acad Sci. 1996;785:34–37. doi: 10.1111/j.1749-6632.1996.tb56241.x. [DOI] [PubMed] [Google Scholar]
- Condie BG, Capecchi MR. Mice homozygous for a targeted disruption of hoxd-3 (hox-4.1) exhibit anterior transformations of the first and second cervical vertebrae, the atlas and the axis. Development. 1993;119:579–595. doi: 10.1242/dev.119.3.579. [DOI] [PubMed] [Google Scholar]
- Cormier S, Mello MA, Kappen C. Normal proliferation and differentiation of Hoxc-8 transgenic chondrocytes in vitro. BMC Dev Biol. 2003;3:4. doi: 10.1186/1471-213X-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeizel AE. Folic acid and prevention of birth defects. Jama. 1996;275:1635–1636. [PubMed] [Google Scholar]
- Czeizel AE, Timar L, Sarkozi A. Dose-dependent effect of folic acid on the prevention of orofacial clefts. Pediatrics. 1999;104:e66. doi: 10.1542/peds.104.6.e66. [DOI] [PubMed] [Google Scholar]
- DeSesso JM, Goeringer GC. Amelioration by leucovorin of methotrexate developmental toxicity in rabbits. Teratology. 1991;43:201–215. doi: 10.1002/tera.1420430304. [DOI] [PubMed] [Google Scholar]
- Dow E, Ferguson-Smith AC, Caplin B, Williamson R. Dinucleotide repeat polymorphism at the HOX 2B locus. Hum Mol Genet. 1992;1:218. doi: 10.1093/hmg/1.3.218. [DOI] [PubMed] [Google Scholar]
- Duboule D. Vertebrate Hox-genes and proliferation: an alternative pathway to homeosis? Curr Opin Genet Dev. 1995;5:525–528. doi: 10.1016/0959-437x(95)90058-o. [DOI] [PubMed] [Google Scholar]
- Faiella A, Zortea M, Barbaria E, Albani F, Capra V, Cama A, Boncinelli E. A genetic polymorphism in the human HOXB1 homeobox gene implying a 9bp tandem repeat in the amino-terminal coding region. Mutations in brief no. 200. Online. Hum Mutat. 1998;12:363. [PubMed] [Google Scholar]
- Fleming A, Copp AJ. Embryonic folate metabolism and mouse neural tube defects. Science. 1998;280:2107–2109. doi: 10.1126/science.280.5372.2107. [DOI] [PubMed] [Google Scholar]
- Galis F. Why do almost all mammals have seven cervical vertebrae? Developmental constraints, Hox genes, and cancer. J Exp Zool. 1999;285:19–26. [PubMed] [Google Scholar]
- Goff DJ, Tabin CJ. Analysis of Hoxd-13 and Hoxd-11 misexprssion in chick limb buds reveals that Hox genes affect both bone condensation and growth. Development. 1997;124:627–636. doi: 10.1242/dev.124.3.627. [DOI] [PubMed] [Google Scholar]
- Goodman FR, Mundlos S, Muragaki Y, Donnai D, Giovannucci-Uzielli ML, Lapi E, Majewski F, McGaughran J, McKeown C, Reardon W, Upton J, Winter RM, Olsen BR, Scambler PJ. Synpolydactyly phenotypes correlate with size of expansions in HOXD13 polyalanine tract. Proc Natl Acad Sci USA. 1997;94:7458–7463. doi: 10.1073/pnas.94.14.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman FR, Scambler PJ. Human HOX gene mutations. Clin Genet. 2001;59:1–11. doi: 10.1034/j.1399-0004.2001.590101.x. [DOI] [PubMed] [Google Scholar]
- Goto J, Figlewicz DA, Lutchman M, Ruddle F, Rouleau GA. A PvuII RFLP at the HOX 1.4 homeobox locus (HOX1D). Nucl Acids Res. 1991;19:3755. doi: 10.1093/nar/19.13.3755-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald HJ, Danz B, Schwab R, Burmeister K, Bahren W. [Radiographically demonstrable spinal changes in asymptomatic young men]. Rofo Fortschr Geb Röntgenstr Neuen Bildgeb Verfahr. 1995;163:4–8. doi: 10.1055/s-2007-1015936. [DOI] [PubMed] [Google Scholar]
- Herbig K, Chiang EP, Lee LR, Hills J, Shane B, Stover PJ. Cytoplasmic serine hydroxymethyltransferase mediates competition between folate-dependent deoxyribonucleotide and S-adenosylmethionine biosyntheses. J Biol Chem. 2002;277:38381–38389. doi: 10.1074/jbc.M205000200. [DOI] [PubMed] [Google Scholar]
- Hu G, Lee H, Price SM, Shen MM, Abate-Shen C. Msx homeobox genes inhibit differentiation through upregulation of cyclin D1. Development. 2001;128:2373–2384. doi: 10.1242/dev.128.12.2373. [DOI] [PubMed] [Google Scholar]
- Huang S. Histone methyltransferases, diet nutrients and tumour suppressors. Nat Rev Cancer. 2002;2:469–476. doi: 10.1038/nrc819. [DOI] [PubMed] [Google Scholar]
- Ingram JL, Stodgell CJ, Hyman SL, Figlewicz DA, Weitkamp LR, Rodier PM. Discovery of allelic variants of HOXA1 and HOXB1: genetic susceptibility to autism spectrum disorders. Teratology. 2000;62:393–405. doi: 10.1002/1096-9926(200012)62:6<393::AID-TERA6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Innis JW, Goodman FR, Bacchelli C, Williams TM, Mortlock DP, Sateesh P, Scambler PJ, McKinnon W, Guttmacher AE. A HOXA13 allele with a missense mutation in the homeobox and a dinucleotide deletion in the promoter underlies Guttmacher syndrome. Hum Mutat. 2002;19:573–574. doi: 10.1002/humu.9036. [DOI] [PubMed] [Google Scholar]
- James SJ, Miller BJ, Cross DR, McGarrity LJ, Morris SM. The essentiality of folate for the maintenance of deoxynucleotide precursor pools, DNA synthesis, and cell cycle progression in PHA-stimulated lymphocytes. Environ Health Perspect 101 Suppl. 1993;5:173–178. doi: 10.1289/ehp.93101s5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegalian BG, DeRobertis EM. Homeotic transformations in the mouse induced by overexpression of a human Hox3.3 transgene. Cell. 1992;71:901–910. doi: 10.1016/0092-8674(92)90387-r. [DOI] [PubMed] [Google Scholar]
- Kappen C. The VP16-dependent binary system for inducible gene expression in transgenic mice. In: Accili D, editor. Genetic manipulation of receptor expression and function. John Wiley & Sons; 1999. pp. 69–92. [Google Scholar]
- Kappen C. Disruption of the homeobox gene Hoxb-6 results in increased numbers of early erythrocyte progenitors. Am J. Hematol. 2000;65:111–118. doi: 10.1002/1096-8652(200010)65:2<111::aid-ajh4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Kappen C, Neubüser A, Balling R, Finnell RH. Daston M, editor. Molecular basis for skeletal variation: insights from developmental genetic studies in mice. Review. Huamn Skeletal Variations. doi: 10.1002/bdrb.20136. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling PA, Rocke DA, Nunn JF, Monk SJ, Lumb MJ, Halsey MJ. Folinic acid protection against nitrous oxide teratogenicity in the rat. Br J Anaesth. 1986;58:528–534. doi: 10.1093/bja/58.5.528. [DOI] [PubMed] [Google Scholar]
- Kessel M. Respecification of vertebral identities by retinoic acid. Development. 1992;115:487–501. doi: 10.1242/dev.115.2.487. [DOI] [PubMed] [Google Scholar]
- Knezevic V, DeSanto R, Schughart K, Huffstadt U, Chiang C, Mahon KA, Mackem S. Hoxd-12 differentially affects preaxial and postaxial chondrogenic branches in the limb and regulates Sonic hedgehog in a positive feedback loop. Development. 1997;124:4523–4536. doi: 10.1242/dev.124.22.4523. [DOI] [PubMed] [Google Scholar]
- Kolon TF, Wiener JS, Lewitton M, Roth DR, Gonzales ET, Jr., Lamb DJ. Analysis of homeobox gene HOXA10 mutations in cryptorchidism. J Urol. 1999;161:275–280. [PubMed] [Google Scholar]
- Krosl J, Sauvageau G. AP-1 complex is effector of Hox-induced cellular proliferation and transformation. Oncogene. 2000;19:5134–5141. doi: 10.1038/sj.onc.1203897. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Hox Genes in Vertebrate Development. Cell. 1994;78:191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Lachner M, Jenuwein T. The many faces of histone lysine methylation. Curr Opin Cell Biol. 2002;14:286–298. doi: 10.1016/s0955-0674(02)00335-6. [DOI] [PubMed] [Google Scholar]
- Lawrence HJ, Helgason CD, Sauvageau G, Fong S, Izon DJ, Humphries RK, Largman C. Mice bearing a targeted interruption of the homeobox gene HOXA9 have defects in myeloid, erythroid, and lymphoid hematopoiesis. Blood. 1997;89:1922–1930. [PubMed] [Google Scholar]
- Leemans R, Loop T, Egger B, He H, Kammermeier L, Hartmann B, Certa U, Reichert H, Hirth F. Identification of candidate downstream genes for the homeodomain transcription factor Labial in Drosophila through oligonucleotide-array transcript imaging. Genome Biol. 2001;2:RESEARCH0015. doi: 10.1186/gb-2001-2-5-research0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre V, Behringer RR, de Crombrugghe B. L-Sox5, Sox6 and Sox9 control essential steps of the chondrocyte differentiation pathway. Osteoarth Cart. 2001;9(Suppl A):S69–75. doi: 10.1053/joca.2001.0447. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Garofalo S, Zhou G, Metsaranta M, Vuorio E, De Crombrugghe B. Characterization of primary cultures of chondrocytes from type II collagen/beta-galactosidase transgenic mice. Matrix Biol. 1994;14:329–335. doi: 10.1016/0945-053x(94)90199-6. [DOI] [PubMed] [Google Scholar]
- Lewis DP, Van Dyke DC, Stumbo PJ, Berg MJ. Drug and environmental factors associated with adverse pregnancy outcomes. Part I: Antiepileptic drugs, contraceptives, smoking, and folate. Ann Pharmacother. 1998;32:802–817. doi: 10.1345/aph.17297. [DOI] [PubMed] [Google Scholar]
- Lucock M. Folic acid: nutritional biochemistry, molecular biology, and role in disease processes. Mol Genet Metab. 2000;71:121–138. doi: 10.1006/mgme.2000.3027. [DOI] [PubMed] [Google Scholar]
- McAuslan BR, Reilly W, Hannan GN. Stimulation of endothelia cell proliferation by precursors of thymidylate. J Cell Physiol. 1979;100:87–93. doi: 10.1002/jcp.1041000109. [DOI] [PubMed] [Google Scholar]
- Mello MA, Tuan RS. High density micromass cultures of embryonic limb bud mesenchymal cells: an in vitro model of endochondral skeletal development. In Vitro Cell Dev Biol Anim. 1999;35:262–269. doi: 10.1007/s11626-999-0070-0. [DOI] [PubMed] [Google Scholar]
- Minina E, Kreschel C, Naski MC, Ornitz DM, Vortkamp A. Interaction of FGF, Ihh/Pthlh, and BMP signaling integrates chondrocyte proliferation and hypertrophic differentiation. Dev Cell. 2002;3:439–449. doi: 10.1016/s1534-5807(02)00261-7. [DOI] [PubMed] [Google Scholar]
- Molloy AM, Scott JM. Folates and prevention of disease. Public Health Nutr. 2001;4:601–609. doi: 10.1079/phn2001144. [DOI] [PubMed] [Google Scholar]
- Mortlock DP, Innis JW. Mutation of HOXA13 in hand-foot-genital syndrome. Nature Genet. 1997;15:179–180. doi: 10.1038/ng0297-179. [DOI] [PubMed] [Google Scholar]
- Muragaki Y, Mundlos S, Upton J, Olsen BR. Altered growth and branching pattern in synpolydactyly caused by mutations in HOXD13. Science. 1996;272:548–515. doi: 10.1126/science.272.5261.548. [DOI] [PubMed] [Google Scholar]
- Nielsen SJ, Schneider R, Bauer UM, Bannister AJ, Morrison A, O'Carroll D, Firestein R, Cleary M, Jenuwein T, Herrera RE, Kouzarides T. Rb targets histone H3 methylation and HP1 to promoters. Nature. 2001;412:561–565. doi: 10.1038/35087620. [DOI] [PubMed] [Google Scholar]
- O'Brien S, Even DA, Murray JC. Complex trinucleotide repeat polymorphism in the HOX B6 gene. Hum Mutat. 1997;9:280–281. doi: 10.1002/(SICI)1098-1004(1997)9:3<280::AID-HUMU12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Papenbrock T, Visconti RP, Awgulewitsch A. Loss of fibula in mice overexpressing Hoxc11. Mech Dev. 2000;92:113–123. doi: 10.1016/s0925-4773(99)00344-5. [DOI] [PubMed] [Google Scholar]
- Paros A, Beck SL. Folinic acid reduces cleft lip [CL(P)] in A/WySn mice. Teratology. 1999;60:344–347. doi: 10.1002/(SICI)1096-9926(199912)60:6<344::AID-TERA6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Perkins A, Konsuwan K, Visvader J, Adams JM, Cory S. Homeobox gene expression plus autocrine growth factor production elicits myeloid leukemia. Proc Natl Acad Sci USA. 1990;87:8398–8402. doi: 10.1073/pnas.87.21.8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petter C, Bourbon J, Maltier JP, Jost A. Simultaneous prevention of blood abnormalities and hereditary congenital amputations in a brachydactylous rabbit stock. Teratology. 1977;15:149–157. doi: 10.1002/tera.1420150204. [DOI] [PubMed] [Google Scholar]
- Piedrahita JA, Oemata B, Bennett GD, van Waes J, Kamen BA, Richardson J, Lacey SW, Anderson RG, Finnell RH. Mice lacking the folate acid-binding protein Folbp1 are defective in early embryonic development. Nat Genet. 1999;23:228–232. doi: 10.1038/13861. [DOI] [PubMed] [Google Scholar]
- Queitsch C, Sangster TA, Lindquist S. Hsp90 as a capacitor of phenotypic variation. Nature. 2002;417:618–624. doi: 10.1038/nature749. [DOI] [PubMed] [Google Scholar]
- Ray JG, Meier C, Vermeulen MJ, Boss S, Wyatt PR, Cole DE. Association of neural tube defects and folic acid food fortification in Canada. Lancet. 2002;360:2047–2048. doi: 10.1016/S0140-6736(02)11994-5. [DOI] [PubMed] [Google Scholar]
- Robson H, Siebler T, Shalet SM, Williams GR. Interactions between GH, IGF-I, glucocorticoids, and thyroid hormones during skeletal growth. Pediatr Res. 2002;52:137–147. doi: 10.1203/00006450-200208000-00003. [DOI] [PubMed] [Google Scholar]
- Rogers HJ, Lietman PS. Effect of folate and folinate on 3H-thymidine incorporation by transforming human lymphocytes in vitro. Experientia. 1977;33:671–672. doi: 10.1007/BF01946567. [DOI] [PubMed] [Google Scholar]
- Rutherford SL. From genotype to phenotype: buffering mechanisms and the storage of genetic information. Bioessays. 2000;22:1095–1105. doi: 10.1002/1521-1878(200012)22:12<1095::AID-BIES7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Rutherford SL, Lindquist S. Hsp90 as a capacitor for morphological evolution. Nature. 1998;396:336–342. doi: 10.1038/24550. [DOI] [PubMed] [Google Scholar]
- Sadler TW, Merrill AH, Stevens VL, Sullards MC, Wang E, Wang P. Prevention of fumonisin B1-induced neural tube defects by folic acid. Teratology. 2002;66:169–176. doi: 10.1002/tera.10089. [DOI] [PubMed] [Google Scholar]
- Salbaum JM, Kappen C. Cloning and Expression of Nope, a new Mouse Gene of the Immunoglobulin Superfamily related to Guidance Receptors. Genomics. 2000;64:15–23. doi: 10.1006/geno.2000.6114. [DOI] [PubMed] [Google Scholar]
- Sauvageau G, Thorsteinsdottir U, Hough MR, Hugo P, Lawrence HJ, Largman C, Humphries RK. Overexpression of HOXB3 in hematopoietic cells causes defective lymphoid development and progressive myeloproliferation. Immunity. 1997;6:13–22. doi: 10.1016/s1074-7613(00)80238-1. [DOI] [PubMed] [Google Scholar]
- Shane B. Folate Chemistry and Metabolism. In: Bailey L, editor. Folate in Health and Disease. Marcel Dekker; New York: 1995. pp. 1–22. [Google Scholar]
- Shen WF, Detmer K, Mathews CH, Hack FM, Morgan DA, Largman C, Lawrence HJ. Modulation of homeobox gene expression alters the phenotype of human hematopoietic cell lines. EMBO J. 1992;11:983–989. doi: 10.1002/j.1460-2075.1992.tb05137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotz M, Wegner C, Nau H. Valproic acid-induced neural tube defects: reduction by folinic acid in the mouse. Life Sci. 1987;41:103–110. doi: 10.1016/0024-3205(87)90562-5. [DOI] [PubMed] [Google Scholar]
- Yaworsky PJ, Kappen C. Heterogeneity of neural progenitor cells revealed by enhancers in the nestin gene. Dev Biol. 1999;205:309–321. doi: 10.1006/dbio.1998.9035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokouchi Y, Nakazato S, Yamamoto M, Goto Y, Kameda T, Iba H, Kuroiwa A. Misexpression of Hoxa-13 induces cartilage homeotic transformation and changes cell adhesiveness in chick limb buds. Genes Dev. 1995;9:2509–2522. doi: 10.1101/gad.9.20.2509. [DOI] [PubMed] [Google Scholar]
- Yueh YG, Gardner DP, Kappen C. Evidence for regulation of cartilage differentiation by the homeobox gene Hoxc-8. Proc Natl Acad Sci USA. 1998;95:9956–9961. doi: 10.1073/pnas.95.17.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelzer E, Olsen BR. The genetic basis for skeletal diseases. Nature. 2003;423:343–348. doi: 10.1038/nature01659. [DOI] [PubMed] [Google Scholar]
- Zhao Q, Behringer RR, de Crombrugghe B. Prenatal folic acid treatment suppresses acrania and meroanencephaly in mice mutant for the Cart1 homeobox gene. Nat Genet. 1996;13:275–283. doi: 10.1038/ng0796-275. [DOI] [PubMed] [Google Scholar]
- Zhao R, Russell RG, Wang Y, Liu L, Gao F, Kneitz B, Edelmann W, Goldman ID. Rescue of embryonic lethality in reduced folate carrier-deficient mice by maternal folic acid supplementation reveals early neonatal failure of hematopoietic organs. J Biol Chem. 2001;276:10224–10228. doi: 10.1074/jbc.c000905200. [DOI] [PubMed] [Google Scholar]