A. Introduction
The axial skeleton develops through a series of complex processes that require the coordinate regulation of many cellular and molecular events (Gilbert, 1994; Kaufman and Bard, 1999). During gastrulation of the vertebrate embryo, the formation of the primitive streak and notochord represent the first step in the formation of axial and paraxial mesoderm. The paraxial mesoderm is then segmented into somites, which in turn are further subdivided into rostral and caudal halves. The somites are the origin of sclerotomal cells that migrate to form condensations of mesenchymal cells that produce the cartilaginous ‘models’ for the skeletal elements. Through chondrification and ossification, prevertebrae and bones of the axial skeleton grow and achieve their final size and shape. Recently, experimental studies in chicken and genetic approaches in the mouse have contributed a wealth of molecular markers for different stages of axial skeleton development, and at the same time provided evidence for the critical functional role of various types of molecules in the formation of the axial skeleton. The results from these studies are now beginning to be integrated into a molecular framework of gene regulation and cell differentiation during skeletal development. It is expected that this knowledge will help to better understand the etiology of skeletal variations, and provide a scientifically informed basis for their evaluation as study parameters for potential reproductive toxicants.
Understanding the causes of variations in skeletal development is particularly important in light of the fact that skeletal variations are highly prevalent in the general population. Of 10922 asymptomatic young men whose axial skeleton was examined by X-ray, only 2.6 % resembled the textbook ideal and showed no pathological alterations of the axial skeleton (Hald et al., 1995). While 80% of axial abnormalities involved habit related mild scoliosis, the authors also reported more pronounced abnormalities in about 40–45% of subjects, including deformations of vertebral bodies and changes in vertebral identity. For many people, such abnormalities may have occupational implications, and may affect general health.
In the mouse, skeletal abnormalities often do not interfere with viability and reproductive capability. This allowed the isolation and maintenance of mutants with skeletal abnormalities, which either arose spontaneously or were induced during radiation and mutagenesis experiments (Lyon et al., 1996). On the basis of phenotypic and embryological analyses, mutations could be grouped into different classes reflecting the different steps of skeletal development affected (Grüneberg, 1963). Positional cloning techniques now permit the isolation of the genes underlying observed defects as successfully demonstrated for the cases of Brachyury (T; (Herrmann et al., 1990), short ear (se; (Kingsley et al., 1992) and brachypodism (bp; (Storm et al., 1994).
A complementary approach is to analyze the function of genes that are known to be expressed during formation of the axial skeleton. Many of these genes were thought to be required for normal skeletal development, based upon their similarity to developmental genes identified in mutant screens in Drosophila melanogaster (Nusslein-Volhard and Wieschaus, 1980). In the mouse, loss or gain of function mutants can be created through homologous recombination in embryonic stem cells and the subsequent production of mice completely derived from these cells (Joyner, 1993), and through transgenic approaches (Hogan et al., 1986). As the phenotypes of genetically manipulated mice resemble the malformations observed in a variety of human skeletal dysplasias, the mouse provides an excellent model system to understand the molecular and cellular pathways steps involved in human skeletal malformations.
Finally, not only genetic but also environmental factors influence skeletal development. Interfering with one of the many steps involved can give rise to malformations that resemble those caused by known genetic defects. Such resemblances suggest that similar molecular and cellular pathways are affected in both cases, and may help to identify the molecular basis for the actions of exogenous substances. The most prominent examples for the skeletal system are the Hox genes, whose expression is altered by retinoic acid. In this way, exposure to an environmental factor produces phenotypes similar to those caused by mutation or manipulation of Hox genes themselves (Kessel, 1992).
In this chapter, we will give a short overview of axial skeleton development in the mouse, focusing on selected morphological events. We will then review current knowledge on the molecular basis of skeletal development, including a discussion of genes that regulate growth and cell differentiation in skeletal elements. A section on selected environmental factors that interact with the complex genetic network controlling skeletal development will conclude this review.
B. Morphogenesis
Bone formation in a vertebrate embryo follows a specific program (Cancedda et al., 1995) and occurs in an ordered progression of steps: (i) Specification and formation of the mesoderm and its contribution to the body axes, here the anterior-posterior axis. (ii) Subdivsion of mesodermal derivatives and specification of sclerotomal cells. (iii) Migration of sclerotomal cells and condensation into prevertebrae. (iv) Differentiation of chondrogenic cells and formation of a cartilage model. (v) Maturation and growth of skeletal elements and endochondral ossification. Intramembraneous ossification is the process of bone formation found in the skull and part of the collar bone. Here, we focus our considerations on the formation of the vertebral column.
1. Formation of the Primitive Streak and the Notochord
The development of the axial skeleton is crucially linked to the formation of the primary body axis itself. At about day 6.5 postconception (primitive streak stage), epithelial cells from the primitive ectoderm begin to delaminate and migrate through the primitive streak to form mesoderm (reviewed in (Hogan et al., 1994). With the establishment of the primitive streak, the future anterior-posterior axis of the embryo is fixed. Concomitantly with the regression of the primitive streak, the notochord is developed, and in parallel, axial and lateral mesoderm is laid down. With exception of craniofacial structures (Couly et al., 1992; Couly and Le Douarin, 1985, 1987), the skeleton is derived from mesoderm (Christ and Ordahl, 1995; Christ and Wilting, 1992). Decisions that influence the fate of mesodermal cells are also made during and after gastrulation. At the end of this process, two essential structures for the development of the axial skeleton can be recognized: the notochord and the paraxial mesoderm. The notochord not only marks the anteroposterior axis of the embryo, it also acts as an organizer for adjacent embryonic tissues, both in the neural tube and in the mesoderm (Placzek et al., 1990; van Straaten et al., 1988). In the neuroepithelium, the notochord contributes to and induces the formation of the floor plate (Placzek et al., 1993; Placzek et al., 1990; Placzek et al., 1991), and is an important inducer of motor neurons (Tanabe and Jessell, 1996). The notochord also plays a key role during sclerotomal development (Brand-Saberi and Christ, 2000; Brand-Saberi et al., 1993; Koseki et al., 1993; Pourquie et al., 1993), from which the bones, cartilage, and connective tissue of the axial skeleton are derived (reviewed in (Brand-Saberi and Christ, 2000; Christ and Wilting, 1992).
2. Segmentation of the Paraxial Mesoderm into Somites
At the end of gastrulation, the mesoderm is organized into three main blocks, each with a different fate. These are the paraxial, the intermediate, and the lateral mesoderm. The axial skeleton derives entirely from the paraxial mesoderm (Christ and Ordahl, 1995; Christ and Wilting, 1992). Concomitant with its formation, the paraxial mesoderm is subdivided into blocks of epithelial tissue, the somites. The formation of somites proceeds in rostral-caudal direction by budding off at the rostral boundary of the caudally located unsegmented portion of the paraxial mesoderm. Somites consist of epithelial columnar cells that envelop a central cavity, the somitocoele, which is filled with mesenchymal cells in more mature somites (Huang et al., 1994). The outer side of the somite is surrounded by a basement membrane, and the somitic cells are polarized such that their apical sides face the somitocoele (Solursh et al., 1979). The molecular mechanisms underlying the segmentation of the paraxial mesoderm into somites are not yet understood. Segmentation of the paraxial mesoderm seems to be independent of the notochord and also occurs in an autonomous fashion during in vitro culture of paraxial mesoderm (Bellairs, 1979; Christ and Ordahl, 1995; Menkes and Sandor, 1977; Packard, 1978).
3. Differentiation of Somites into Dermomyotome and Sclerotome
Soon after their formation, somites start to differentiate. The principal fates of different parts of the somite have been examined using the chick quail chimera (Christ et al., 1972; Christ and Wilting, 1992; Huang et al., 2000; Huang et al., 1994; Jacob et al., 1975; Ordahl and Le Douarin, 1992). The dorsal half of a somite gives rise to the dermomyotome. This structure is the origin of the dermis of the back, and of all skeletal muscles. The ventral portion of the somite forms the sclerotomal precursor cells. This is accompanied by a delamination of the basement membrane, and a epithelial-mesenchymal transition of the ventral somite cells (Jacob et al., 1975). The fate of sclerotome cells depends on their relative position within the sclerotome (Verbout, 1985). Cells that are initially located ventromedially contribute to intervertebral disks and vertebral bodies. Lateral sclerotome cells participate in the formation of the ribs, the pedicles, and lamina and contribute to the connective tissue surrounding the dorsal root ganglia. The somitocoele cells become part of the posterior sclerotome and contribute to the intervertebral disks and the ribs. The molecular mechanisms underlying the commitment of somite-derived cells to the sclerotome are not well understood.
4. From Sclerotome to Mesenchymal Prevertebrae
Following de-epithelization, sclerotomal cells become migratory. The initially medially located cells migrate ventrally towards the notochord. In this process, the prior morphologically apparent metameric organization of these cells is lost. Instead, the perinotochordal tube is formed, consisting of loosely arranged mesenchymal cells with no apparent segmentation. Shortly thereafter, centers of condensations arise that will develop into intervertebral disks. The vertebral bodies will form from the loose intermediate zones between the condensations. In contrast to the medially derived sclerotome, lateral sclerotomal cells maintain their segmental organization. With respect to the original somite organization, the rostrally and caudally derived sclerotomes differ in cell density. In the caudal half, from which the pedicles, laminae, and ribs develop, the cell density is very high. It is significantly lower in the rostral half, which form the perineural connective tissues, also refered to as the “neurotome” (Blechschmidt, 1961), as it surrounds the spinal nerves and ventral part of the dorsal root ganglia (Verbout, 1985).
The precise relationship between the metameric organization of the somites and the secondary metameric pattern of the perinotochordal tube is the subject of a longstanding argument. The concept of a “resegmentation,” first proposed by (Remak, 1855), suggests that cells from a given somite contribute to two vertebrae, and that any given vertebra is composed from derivatives of two somites. Experimental evidence from chick quail transplantation studies and experimental manipulations of somite halves support this concept, with regard to the vertebral bodies, the ribs, and the laminae (Bagnall et al., 1988, 1989). Pedicles and intervertebral discs are thought to be derived from onIy a single somite (Christ and Wilting, 1992; Huang et al., 2000).
The molecular mechanisms that regulate sclerotome cell migration are not well understood, and neither is the basis of cell condensation. Notochord ablation and transplantation experiments in the chick and analyses of mouse notochord mutants have demonstrated that the notochord participates in the specification of ventral sclerotome derivatives (Brand-Saberi and Christ, 2000; Brand-Saberi et al., 1993; Dietrich et al., 1993; Goulding et al., 1993; Koseki et al., 1993; Pourquie et al., 1993). Ablation of the notochord leads to the loss of ventral sclerotome derivatives. In turn, transplantation of the notochord to an ectopic position next to unsegmented paraxial mesoderm induces ectopic sclerotome formation and ectopic cartilage production. Taken together, these studies provide evidence for a role of the notochord in specification, maintenance or survival of sclerotomal cells, or their commitment to the chondrocyte lineage.
It has recently been suggested (Burke and Nowicki, 2003) that sclerotomal precursors that migrate ventrally may find themselves in an environment different from that of dorsally migrating precursors, presumably due to a change in extracellular signals encountered after passing the lateral somitic frontier. The precise signals that would account for dorsal-ventral differences in the environment for migratory sclerotomal cells, however, have not been identified yet.
5. Chondrification of Prevertebrae
By around day 13 of mouse embryogenesis, the major components of the vertebral column have been laid down as mesenchymal condensations, also called blastema. At this time, chondrification of the vertebral column begins, proceeding in rostral to caudal direction. Chondrification starts from chondrification centers in the middle of vertebral bodies, within the costal processes, and from two eccentric positions within the pedicles (Patten, 1953; Thondury, 1985). The mesenchymal condensations forming the primordia for the lamina of the neural arches do not extend to the dorsal midline, leaving the neural arches open until gestational day 17 (Rugh, 1968). As the vertebral bodies chondrify, the diameter of the notochord is reduced at the sites of vertebral bodies, and somewhat enlarges at the sites of developing intervertebral disks (Christ and Wilting, 1992). The notochordal cells participate in the formation of the nucleus pulposus of the intervertebral disk (Smits and Lefebvre, 2003). Remnants of the notochord can still be found at birth, but usually disappear by adulthood (Rauber and Kopsch, 1987; Trout et al., 1982). The condensation and chondrification of skeletal elements culminates in the differentiation of chondrocytes, the cells that produce the extracellular matrix components of the cartilage, and in this way, form a cartilage model of the future axial skeleton.
6. Ossification of Prevertebrae
From day 14.5 of mouse embryogenesis, the elements of the skeleton begin the process of ossification (Rugh, 1968). In the axial skeleton, ossification follows the typical endochondral pattern, beginning with hypertrophy of the chondrocytes, mineralization of the cartilage matrix, and invasion of blood vessels, followed by cartilage erosion, invasion of osteoblasts and replacement of cartilage by bone material (Bloom and Fawcett, 1968). In each vertebra, three ossification centers appear, one in the center of the vertebral body and one in each pedicle (Patten, 1953). In addition, one ossification center develops in each costal process. Within the neural arches, a layer of perichondral bone is produced before cartilage erosion and endochondral ossification starts. In different regions of the vertebral column, the order of appearance of the ossification centers differs, and ossification starts at slightly different time points. Cervical neural arches ossify first, followed by the thoracic and upper lumbar vertebral bodies, the thoracic and lumbar neural arches, and finally the cervical and the lower lumbar vertebral bodies (Rugh, 1968). At birth, the three independently ossified parts of the vertebrae are still separated by cartilage. Cartilage is also present on both rostral and caudal surfaces of the vertebral bodies (Rauber and Kopsch, 1987), and in the ventral aspects of the ribs, which continue to grow after birth.
In summary, the process of skeletal development consists of successive events of cell migration and specification, patterning of tissues, migration and re-specification of cells, patterning of individual elements, further cell differentiation and maturation, and intermittent cell proliferation. It is obvious that the regulation of these processes occurs at various levels and has to be well coordinated over time and in a spatial context. Through molecular studies, the biochemical, cellular and mechanistic basis for skeletal development is becoming unraveled. In parallel, genetic manipulation approaches in the mouse now enable the development of in vivo systems for causative investigations into the regulatory and signaling pathways in skeletal development. The following paragraphs will include a selection of the most pertinent genetic approaches.
C. Genes Involved in Axial Skeleton Formation
Drawing upon the similarities of skeletal development to other developmental processes, a variety of molecules, and the genes encoding them, have been identified as essential for formation of the skeleton. Diffusible signaling molecules and growth factors, cell surface molecules as well as membrane-bound ligands and the corresponding receptors, all work to mediate intercellular interactions in the formation of the skeleton. The extracellular matrix can bind growth factors and modulate their action (reviewed in: (Adams and Watt, 1993). In addition, extracellular matrix molecules themselves serve as signals or ligands, triggering specific responses in cells attached to matrix (Juliano and Haskill, 1993). This is particularly important during cell migration. Direct cell-cell contact necessary for the integrity of tissues, and the modulation of these adhesive interactions require the differential expression of cell adhesion molecules, such as cadherins. Signals from the cell surface, as received via respective receptors, are then transduced via various pathways, involving protein kinases and phosphatases, to the nucleus. This eventually results in changes in gene expression through the action of specific transcription factors. The ultimate outcome of a series of cell differentiation steps is the terminally differentiated cell, which is characterized by production of specific collagens and proteoglycans (Ruoslahti and Yamaguchi, 1991). Indeed, on the way from mesodermal cells to the mature chondrocyte or osteoblast, cells continuously respond to instructive and permissive signals and, at the same time, execute autonomously determined programs. The intricate balance of these processes for the various cell types and phases in the developing skeleton are just beginning to be understood in molecular terms.
1. Gastrulation and Formation of Paraxial Mesoderm
As the vertebral column is entirely derived from mesoderm, genes involved in the formation and patterning of mesoderm during gastrulation frequently also affect the development of the axial skeleton, via the paraxial mesoderm. Defects in these genes, however, often lead to gross embryonic alterations and early embryonic lethality, making the analysis of their specific function for vertebral column development very difficult. Progress in understanding the genetic control of gastrulation in the mouse has been recently reviewed (Faust and Magnuson, 1993; Stern, 1992; Tam et al., 2000). While by no means comprehensive, we will focus here on selected genes that specifically affect paraxial mesoderm development, as paradigms of the types of evidence available from functional studies in the mouse.
1.a. Growth Factors, Signaling Molecules, and Their Receptors
Soluble/diffusible molecules involved in formation and patterning of mesoderm include growth factors from the WNT family (McMahon et al., 1992; Parr and McMahon, 1994), the fibroblast growth factor (FGF) family (Mason, 1994), and the transforming growth factor (TGFβ) superfamily (Kingsley, 1994b) and their respective receptors (Attisano et al., 1994; Givol and Yayon, 1992). Inactivation of the Wnt3a gene impairs development of dorsal, somitic mesoderm, and Ieads to a disrupted notochord, a lack of caudal somites, and a failure of tail bud formation. The end result is a truncation of the body axis at the level of the forelimbs (Augustine et al., 1993; Takada et al., 1994). In Wnt3 mutants, the primitive streak does not even form, and the node, as well as the mesoderm, is strikingly absent (Liu et al., 1999). Fgf genes are expressed in complex, partially overlapping patterns during embryogenesis. Loss of Fgf3 function causes malformations of caudal vertebrae and a dorsal curling of the tail, detectable by gestational day 11.5 (Mansour et al., 1993). More severe is the phenotype of mice with a targeted disruption of the FGF receptor gene, Fgfr1. Fgfr1 deficiency results in an expansion of axial mesoderm at the expense of paraxial mesoderm, and a subsequent lack of somitogenesis and death before embryonic day 9.5 (Deng et al., 1994; Yamaguchi et al., 1994). Several members of the TGFβ superfamily have also been implicated as being required during gastrulation. The TGFβ–related gene nodal has recently been shown to be affected in the retroviral insertion mouse mutation 413d (Zhou et al., 1993). Homozygous mutants are unable to form axial structures during gastrulation, including dorsal mesoderm and notochord. Bmp4-deficient mice also develop little if any mesoderm (Dunn et al., 1997; Winnier et al., 1995). Similarly, mutants for Bmp-receptor II, are arrested at the egg-cylinder stage and lacked mesoderm (Beppu et al., 2000). These examples illustrate that peptide growth factors play important roles during early axis development and mesoderm formation. The phenotypes of some of these mutants also indicate specific functions of each ligand-receptor system at temporally different junctions in the formation of the primary axis and the mesoderm. The interpretation and analysis of some of these mutants is further complicated by the presence and potential functional redundancy of other members of each growth factor family. In addition, the early embryonic lethality induced by targeted disruption often prevents a detailed analysis of growth factor (or receptor) function at later stages of skeletogenesis. Here, the use of tissue-specific and conditional technologies will help to distinguish different roles in different tissues and at distinct stages of development.
1.b. Transcription Factors
The transcription factor Brachyury (T; (Herrmann and Kispert, 1994) is expressed in the primitive ectoderm, the adjacent mesoderm, and later on in the notochord. Consistent with this expression pattern, malformations in embryos homozygous for mutant T alleles include a blockade of elongation of the axis, in which the anterior portion of the notochord initially forms but does not persist as a coherent structure (Gluecksohn-Schoenheimer, 1944; Herrmann, 1991; Wilkinson et al., 1990). Furthermore, mutants do not develop structures posterior to the forelimb buds. T heterozygotes show loss of the tail and skeletal abnormalities, suggesting a quantitative requirement for the T gene product in development of the notochord and for formation of mesoderm, and this quantitative requirement is different at different axial levels (Stott et al., 1993). Several homeobox-containing transcription factors, among them goosecoid (Blum et al., 1992), Mox1 (Candia et al., 1992), Cdx2 (for review, see: (Epstein et al., 1997), Tlx2 (Tang et al., 1998) and Siamois (Lemaire et al., 1995), are also expressed during gastrulation, implying a potential role in mesoderm specification. However, loss of goosecoid function has no effect on mesoderm specification. Instead, goosecoid is required in those sites where it is expressed at later stages of development, including the appendicular skeleton, the shoulder and hip girdle, rib cage (Rivera-Perez et al., 1995) and craniofacial (Rivera-Perez et al., 1999) development. Tlx2 mutants exhibit defects in the formation of primitive streak and mesoderm (Tang et al., 1998). In the case of Cdx2 deficiency, germ-line mutant embryos die too early to allow a detailed analysis of mesoderm formation (Chawengsaksophak et al., 1997), but analysis of chimeras revealed that Cdx2 is involved in mesodermal axis elongation (Chawengsaksophak et al., 2004). Interestingly, mutation of the winged-helix transcription factor HNF3β revealed that mesoderm could be formed in the absence of the notochord, and that its regionalization along the anterior-posterior axis was normal (Ang and Rossant, 1994; Weinstein et al., 1994). However, somite organization was aberrant, indicating that the notochord provides a critical signal. Taken together, these results illustrate that various types of transcription factors participate in early mesoderm development at distinct stages. Some of these transcription factors, such as Cdx2 and goosecoid continue to play roles during later stages of skeletogenesis. In this regard, recent targeted mutation experiments in the mouse have revealed principally the earliest timepoints of functional requirement. Conditional technologies are now being used to dissect molecular pathways at different stages of skeletal development.
2. Segmentation of the Paraxial Mesoderm into Somites
The molecular mechanism underlying the formation of somites are just beginning to be defined (Rawls et al., 2000). Several models for somite formation have been advanced and are discussed by (Keynes and Stern, 1988) who propose a model in which segmentation is coupled to the cell cycle. Recently, (Conlon et al., 1995) suggested a new model for somitogenesis, which critically depends on the function of the transmembrane receptor Notch1.
2.a. Intercellular Signaling
Notch1 is a member of a family of proteins (Greenwald and Rubin, 1992); Reaume et al., 1992; Lardelli and Lendahl, 1993; (Lardelli et al., 1994; Lardelli and Lendahl, 1993; Reaume et al., 1992). These molecules are large transmembrane receptors with epidermal growth factor (EGF)-type repeats and Notch family-specific repeats at the extracellular side and tandem ankyrin repeats in the intracellular domain. Members of the Notch family in Drosophila have been shown to be essential for the correct implementation of cell fate decisions in a large number of developmental processes. This Notch signaling regulation occurs through interaction with ligands of the Delta family on the cell surface of adjacent cells (Greenwald and Rubin, 1992) In the mouse embryo, Notch1 is expressed in a somite-sized domain at the anterior end of the presomitic mesoderm and downregulated after somite formation (Reaume et al., 1992). This suggested a role of Notch1 in promoting cell aggregation prior to somite formation. Results from targeted disruption of Notch1 support this idea (Conlon et al., 1995; Swiatek et al., 1994). In Notch1-deficient embryos, somitogenesis is delayed and uncoordinated on contralateral sides. In contrast, specification of somitic cell sub-populations is apparently not disturbed. Notch1 expression is thought to be controlled by extrinsic factors. In the mouse, two other Notch genes have been identified (Lardelli et al., 1994; Lardelli and Lendahl, 1993). Notch2 is also expressed in the anterior presomitic mesoderm. Mutants in which the ankyrin repeats were deleted from the Notch2 gene die of excessive cell death by day 11.5 (Hamada et al., 1999). Up to this point, however, they show normal somitogenesis. The expression of Fgfr1 is strongly upregulated at the anterior end of the presomitic mesoderm, similar to Notch1, suggesting a possible interaction of growth factor mediated and intercellular signaling (Yamaguchi et al., 1992). After epithelialization, Fgfr1 expression becomes restricted to the rostral half of the newly formed somites. In Notch1-deficient mice, however, expression of Fgfr1 is unaffected, suggesting that Fgfr1 is apparently not downstream of the Notch1 signaling pathway (Conlon et al., 1995). On the other hand, mutants for Delta-like 3 exhibit malsegmentation of vertebrae (Bulman et al., 2000), indicating that Delta-like molecules interact with Notch in skeletal development (Beckers et al., 2000; Bettenhausen et al., 1995), and regulate its activity.
Intracellular Notch signaling activity is regulated by the presenilins (Donoviel et al., 1999), which are thought to function in proteolysis of Notch and other cell surface molecules (Selkoe, 2000). Furthermore, the activity of Notch signaling is mediated by the glycosyltranferase Fringe (Aulehla and Johnson, 1999; Barrantes et al., 1999; Moloney et al., 2000; Zhang and Gridley, 1998), further demonstrating the fine tuning of Notch signaling activity by biochemical mechanisms. Mice with a disruption of the lunatic fringe gene display defects in somite formation as well as in anterior posterior patterning of the somites (Zhang and Gridley, 1998). This was accompanied by a change of components of the Notch signaling pathway from metameric patterns of expression to more even expression in the presomitic mesoderm. These results establish fringe proteins as important regulators of Notch function and somitogenesis (Barrantes et al., 1999). Fringe molecules are expressed in a temporal pattern that is highly coordinated with the emergence of newly formed somites, suggesting that they regulate the formation of somites (Aulehla and Johnson, 1999; Barrantes et al., 1999). Recently, it has been shown that fringe participates in the temporal oscillation signal for somite formation (Aulehla and Johnson, 1999; Dale and Pourquie, 2000; Dubrulle and Pourquie, 2004; Holley et al., 2000; Kerszberg and Wolpert, 2000), and that coordinated regulation of fringe and Notch relative to the Wnt signaling pathway regulates proper temporal progression of somitogenesis (Dequeant et al., 2006). These results indicate that the evolutionarily conserved Notch signaling system (Greenwald and Rubin, 1992) is critical for somite formation.
2.b. Cell Adhesion
The formation of somites is obviously dependent on increased cell adhesion between the cells at the rostral end of the presomitic mesoderm as compared to cells located further caudally (Bellairs et al., 1978; Cheney and Lash, 1984; Lash et al., 1984). It has been shown that the expression of both the calcium-dependent adhesion molecule N-cadherin, and the neural cell adhesion molecule (NCAM), is upregulated just before somite formation (Hall and Miyake, 1992). NCAM expression continues throughout the somite (Duband et al., 1987; Hall and Miyake, 2000; Probstmeier et al., 1994), while N-cadherin is downregulated just before the epithelial-mesenchymal transition in the ventromedial part of the somite that gives rise to the sclerotome. Monoclonal antibodies directed against N-cadherin, but not against NCAM, result in a disaggregation of chick somites in vitro (Duband et al., 1987). In mice with a targeted disruption of the Ncam gene, however, defects of the vertebral column were not detected (Cremer et al., 1994). In contrast, N-cadherin mutants exhibited reduced somite size as well as defects in somite epithelialization. In addition to irregular shape, cellular cohesion within the somites was reduced, indicating a role for N-cadherin mediated cell adhesion in the formation of somites (Radice et al., 1997). In fact, the cyclical expression of genes in the presomitic mesoderm is critically dependent on cell-cell contact (Maroto et al., 2005).
A family of cell adhesion molecules that interact with extracellular matrix molecules are the integrins, which consist of alpha and beta subunits. In chick embryo cultures, interference with integrin signaling did not inhibit segmentation, but resulted in translocation of somites laterally (Drake and Little, 1991). However, the specific integrin subunits involved in this were not identified. Surprisingly little is known about the specific involvement of intergrins in vertebral development, despite a wealth of evidence that they participate in normal development of many systems (Brakebusch et al., 1997; Sheppard, 2000; DeArcangelis and Georges-Labouesse, 2000). The early embryonic death of β1-integrin deficient embryos (Fassler and Meyer, 1995) demonstrates its critical role for development. On the other hand, the presence of multiple subunit combinations with different specificities for extracellular ligands and with different signaling activities (Heino, 2000) confounds experimental analyses. The best demonstration of a role in somitogenesis comes from α5 subunit mutants. Integrinα5-deficient mice develop mesodermal defects, in particular in posterior somite formation, resulting in truncation of posterior tissues (Goh et al., 1997). While this apparently was not due to increased cell death, there was reduced expression of Pax1, indicating a reduction of skeletogenic cells. Other mesodermal markers, however were normally expressed, suggesting normal initial specification, while the maintenance of mesodermal derivatives and boundary formation was affected. While more extensive studies are needed, the importance of cell adhesion in somitogenesis is also illustrated by disruptions in the extracellular ligands of integrins (Chong and Jiang, 2005).
2.c. Extracellular Matrix Components
Fibronectin is a major component of the extracellular matrix and long been implicated in the epithelialization of somites (Cheney and Lash, 1984; Lash et al., 1984). For example, addition of fibronectin or a peptide corresponding to the cellular recognition site of fibronectin can stimulate aggregation of disaggregated paraxial mesoderm cells in vitro (Cheney and Lash, 1984; Lash et al., 1984). Targeted disruption of the fibronectin gene leads to mesodermal defects during gastrulation and embryonic lethality after day 9 (George et al., 1993). In embryos deficient of fibronectin, the notochord and somites never form. Because of the early and rather global defects in these mouse mutants; however, they are not informative with regard to the specific role of fibronectin for somite epithelialization. However, zebrafish genetic studies confirmed the requirement of fibronectin for epithelilization of newly formed somites and maintenace of somite boundaries (Koshida et al., 2005).
2. d. Transcription Factors
Recently, two members of the bHLH transcription factor family were identified, paraxis and scleraxis, that display interesting expression patterns during somite development (Burgess et al., 1995; Cserjesi et al., 1995). Before the onset of somitogenesis, paraxis is weakly expressed in part of the primitive mesoderm. Later it is highly expressed in the rostral part of the presomitic mesoderm, equivalent to approximately two somites in size. Expression of paraxis continues throughout the uncompartimentalized epithelial somite. After compartimentalization, paraxis expression is shut down soon after myotome formation, and gradually declines in the other somite derivatives (Burgess et al., 1995). In paraxis mutants, somite formation is disrupted because mesodermal cells are unable to form epithelia. However, the axial skeleton does form, albeit with abnormally patterned vertebrae. Expression of scleraxis, on the other hand, does not start until after compartmentalization of the somite (Cserjesi et al., 1995), and is most prominent within the lateral part of the sclerotome. Subsequently, scleraxis is expressed at high levels within mesenchymal precursors of the axial and appendicular skeleton and in cranial mesenchyme in advance of chondrogenesis and is downregulated when ossification is initiated, suggesting that it might regulate gene expression required for determination and differentiation of progenitor cells for cartilage and connective tissue. Unexpectedly, scleraxis mutants exhibited a failure to form mesoderm altogether, which turned out to be consistent with the earlier expression of the gene during gastrulation (Brown et al., 1999). In chimeras, scleraxis mutant cells never contributed to the sclerotome, indicating that scleraxis is required for specification of sclerotomal cells. Taken together, these results show how intricately the patterning of the somite is linked to mesoderm specification.
The temporal regulation of somite formation is also mediated by other transcription factors (Bessho and Kageyama, 2003). Degradiation of the bHLH transcription factor Hes7 has been shown to underlie cyclical gene expression in the presomitic mesoderm, through regulation of lunatic fringe expression (Cole et al., 2002). In addition, winged helix transcription factors Foxc1 and c2 play a role in somitogensis (Kume et al., 2001) by regulating the expression of the various bHLH transcription factors in presomitic mesoderm (Holley et al., 2002) and formation of sharp expression boundaries for molecules known to be involved in the Notch signaling pathway. Thus, the intricate temporal and spatial control of gene expression is crucial for proper specification and morphology of newly formed somites.
3. Patterning of Somites
3.a. Rostrocaudal Patterning: Rostral and Caudal Somite Halves
Even in the early epithelial somites, there exist differences between the rostral and caudal somite halves (Norris et al., 1989). Rotation experiments of the presomitic paraxial mesoderm in the chick indicated that this craniocaudal polarity of the somites is already determined before their segmentation (Keynes and Stern, 1988). The restricted expression of Fgfr1 to the rostral half of the newly formed epithelial somite has already been mentioned (Yamaguchi et al., 1992). Interestingly, in the chick embryo, Fgfr3 is expressed in a complementary pattern in the caudal half of the somite (Mahmood et al., 1995). Stern and Keynes (Stern and Keynes, 1987) showed that rostral and caudal sclerotomal cells are unable to mix, and thereby maintain their distinct segmental positions. Caudal sclerotome cells express T-cadherin, bind peanut agglutinin and secrete chondroitin-6-sulfate. In the rostral sclerotome half, butyryl cholinesterase and the extracellular matrix glycoprotein tenascin are expressed (Keynes and Stern, 1988). The relevance of localized tenascin expression, however, is unclear, as mice homozygous for a targeted disruption of the tenascin gene develop normally without displaying any malformations (Saga et al., 1992).
3.b. Dorsoventral Patterning: the ventral somite becomes sclerotome
Patterning of the somitic mesoderm in dorsal-ventral direction is important for the proper specification of skeletogenic cells, which derive from the ventral portions of the somite. In contrast to the anterior-posterior axis, in the presomitic mesoderm and newly segmented somites, a dorsoventral axis is not yet determined. Hence, cells of the early somite are not committed to a dermomyotomal or a sclerotomal lineage. Dorsoventral patterning of the somites is controlled by interactions with adjacent structures such as the notochord, the neural tube, and the surface ectoderm (reviewed by (Christ and Ordahl, 1995).
The diffusible molecule sonic hedgehog, (Shh), secreted from the notochord and floor plate of the neural tube, has been shown to mediate sclerotome induction (Fan et al., 1995; Fan and Tessier-Lavigne, 1994; Johnson et al., 1994). Similar to the Drosophila hedgehog protein, the vertebrate Shh is autocatalytically cleaved to yield two fragments (Fan et al., 1995; Porter et al., 1995; Roelink et al., 1995). Only the aminoterminal (ShhN) fragment has inducing activity for the sclerotome, and is thought to be responsible for both the short- and long-range inductive properties of the notochord, which seem to be mediated by different threshold concentrations of ShhN. A gene whose expression is induced by Shh is PaxI (Ebensperger et al., 1995), a prominent marker for a subgroup of skeletogenic cells. It has been proposed that Shh signaling directs cells to the proper sclerotomal lineage through control of expression of Sox9, a known chondrogenic gene (Akiyama et al., 2002; Bi et al., 2001; Zeng et al., 2002).
3.c. Transcription Factors of the Pax Gene Family
The inductive Shh signal leads to changes within the somite in the expression pattern of Pax genes, which are thought to be causally related to its dorsal ventral patterning (Fan et al., 1995; Wallin et al., 1994). Pax genes encode transcription factors characterized by the paired domain, a DNA-binding domain (Gruss and Walther, 1992). In mammals, nine Pax genes have been identified to date, of which four are known to be expressed during somite development. These include Pax1 (Deutsch et al., 1988; Wallin et al., 1994), Pax3 (Goulding et al., 1993; Vogan et al., 1993; Williams and Ordahl, 1994), Pax7 (Jostes et al., 1990), and Pax9 (Neubuser et al., 1995; Wallin et al., 1993). Pax3 is expressed throughout the unsegmented presomitic mesoderm. As a result of the interaction with the notochord, it is downregulated in the ventral half of the somite soon after epithelialization, but continues to be expressed in the dermomyotome (Goulding et al., 1994; Williams and Ordahl, 1994). In mice with a mutant Pax3 allele, rib formation is severely affected (Dickman et al., 1999). Concomitant with the downregulation of Pax3, Pax1 is induced in the ventral region of the epithelial somite and the mesenchymal core, and it continues to be expressed in the sclerotome after de-epithelialization of the somite (Ebensperger et al., 1995). Upon further differentiation of the sclerotome, Pax1 expression concentrates in the caudal half of the sclerotome and eventually in cells that give rise to the prospective intervertebral disk and in the perichondrium surrounding the vertebral bodies in the midaxial region. Pax1 is also expressed in the precursors of the developing pediculi and lamina of the neural arch, the transverse processes, and the proximal part of the ribs. For the most part, Pax1 is expressed in mesenchymal cells and downregulated upon terminal differentiation (Deutsch et al., 1988), suggesting a role for Pax1 in skeletogenic precursor cells.
Deficiency in Pax1 function leads to severe malformations of the vertebral column, as is dramatically documented by the phenotype of the three different alleles of undulated (un), which affect the Pax1 gene (Dietrich and Gruss, 1995; Koseki et al., 1993; Wallin et al., 1994). In the classical un mouse, a point mutation within the paired box replaces a highly conserved amino acid Gly with a Ser residue, strongly reducing its DNA-binding affinity (Balling et al., 1988). In undulated extensive (Unex), the last of five exons of the Pax1 gene, and in Undulated short tail (UnS), the complete Pax1 locus are deleted. Undulated mice have severe abnormalities both in the intervertebral disks as well as in the vertebral bodies. In these mice, the dorsal sclerotome derivatives, such as the laminae of the neural arches, are less affected. As for the UnS allele, no intervertebral disks can be found in homozygous UnS/UnS embryos. The remaining tissue in the perinotochordal region is still segmentally organized, suggesting that Pax1 might not be involved in segmentation or sclerotome induction per se but might be important for the specification of ventral sclerotome derivatives. un and u nex are recessive mutations, whereas UnS displays a semidominant phenotype. These results from classical mutants suggested that Pax1 may be haploinsufficient, and would lead to perinatal lethality in the homozygous state. Indeed, targeted mutation of Pax1 proves haploinsufficiency for some, but not all sclerotomal derivatives that express Pax1 (Wilm et al., 1998). This could be due to the ectopic activation of Nkx2.2 in the absence of Pax1 (Kokubu et al., 2003), or alternatively, another gene may functionally compensate for the loss of Pax1 in unaffected sclerotome. The closely related Pax gene, Pax9, is expressed in overlapping fashion within the somites and its derivatives (Neubuser et al., 1995), and may partially substitute for the loss of Pax1. Indeed, in the absence of Pax1, Pax9 expression is upregulated. In mice mutant for both Pax1 and Pax9, the derivatives of the medial somite are absent, such that vertebral bodies, proximal ribs and intervertebral disks are missing (Peters et al., 1999). The initial formation of somites and their anterior-posterior polarities is normal in double mutants, as is the specification of cells towards the chondrocyte lineage, as indicated by expression of Sox9 and Collagen II. These results, together with increased apoptosis in the affected sclerotomes, demonstrate that Pax1 and Pax9 are required for maintaining cell proliferation of sclerotomal cells (Peters et al., 1999).
4. Regionalization along the Anterior-Posterior Axis: Hox Genes
Although somites at different axial levels resemble each other morphologically and in the expression patterns of many genes, there are regional differences among them, which manifest themselves in the formation of the specific elements in different regions of the vertebral column. Transplantation experiments in the chick embryo suggested that the rib-forming potential is restricted to somites at the thoracic level (Jacob et al., 1975; Kieny et al., 1972). Transplantation of presomitic mesoderm from the prospective thoracic level to the cervical level resulted in the formation of cervical ribs, indicating that the presomitic mesoderm was already regionally determined before the somites were actually formed (Chevallier, 1975; Kieny et al., 1972).
The most prominent gene family known to be crucially involved in patterning of skeletal elements in the axial skeleton is represented by the Hox genes (Krumlauf, 1994b). The transcriptional regulators encoded by the Hox genes are believed to be responsible for the establishment of positional information along the anterior-posterior body axis, as well as along the proximodistal and rostral-caudal axis of the limbs (Capecchi, 1996; Krumlauf, 1993, 1994a; McGinnis and Krumlauf, 1992; Morgan and Tabin, 1993). The 39 vertebrate Hox genes are organized in four clusters on different chromosomes (Kappen and Ruddle, 1993) and share a 180bp homeobox sequence that encodes the highly conserved DNA-binding homeodomain (Gehring et al., 1994).
The spatial and temporal patterns of Hox gene expression in the embryo are complex (Deschamps and Meijlink, 1992; Shashikant et al., 1991). Upon leaving the primitive streak, cells begin to consecutively express particular Hox genes, resulting in non-identical but overlapping domains of expression along the anterior-posterior axis. In this way, a so-called Hox-code (Kessel and Gruss, 1991a) is imparted onto different regions of the developing skeleton, suggesting the involvement of individual Hox genes or specific Hox gene combinations in specifying unique regions. Indeed, elements of the skeleton develop abnormally when Hox genes are misexpressed in transgenic mice (Charite et al., 1994; Jegalian and DeRobertis, 1992; Kessel et al., 1990; Lufkin et al., 1992; Pollock et al., 1992). Similarly, the disruption of Hox genes by targeted mutagenesis leads to alterations in the skeleton (Boulet and Capecchi, 1996; Boulet and Capecchi, 2002; Condie and Capecchi, 1993, 1994; Davis and Capecchi, 1994, 1996; Davis et al., 1995; Favier et al., 1995; Favier et al., 1996; Fromental-Ramain et al., 1996a; Fromental-Ramain et al., 1996b; Horan et al., 1995a; Horan et al., 1994; Kostic and Capecchi, 1994; LeMouellic et al., 1989; Ramirez-Solis et al., 1993; Rancourt et al., 1995; Rijli et al., 1994; Rijli et al., 1993; Rijli et al., 1995; Saegusa et al., 1996; Small and Potter, 1995; Suemori et al., 1995) Saegusa et al., 1996). Analogous to mutant phenotypes in the fruitfly Drosophila, the pattern abnormalities in vertebral shape and identity have generally been interpreted as homeotic transformations. By this it is meant that there is a conversion of one element or body region into the identity of another (McGinnis and Krumlauf, 1992; McGinnis and Kuziora, 1994). Such phenotypes are most often found in mice with a mutation of a single Hox gene, but also in mutants in which an entire Hox gene cluster was deleted (Medina-Martinez et al., 2000; Suemori and Noguchi, 2000). It is important to note that each mutation produces a distinct phenotype indicating a unique function for each Hox gene.
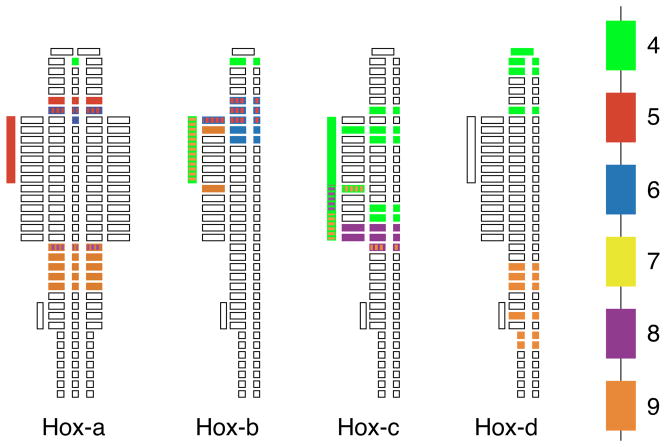
In Figure 1, the skeletal elements affected in homozygous single Hox gene mutants are schematically mapped onto the axial skeleton with reference to the cluster location of the corresponding gene. Here, we are focusing on alterations within the vertebral column that are predominantly caused by mutations of genes in paralogous groups 4 to 9. It is evident from these data that each Hox gene contributes to patterning of a specific region. However, paralogous genes do not always lead to alterations in the same elements as can be seen by the dispersed effects in group 9 Hox gene mutants. Thus, while the expression domains generally follow a serial pattern, the correlation between the relative position of a Hox gene and the axial level affected by its mutation is not as strict. This apparent discrepancy suggests that the function of Hox proteins is not strictly correlated to their earliest expression, but that later roles during growth of the skeleton may also influence the final shape of skeletal elements (see below). This may also explain those phenotypes that are not easily interpreted as homeotic transformations, such as sternal defects, rib fusions or dislocation of the shoulder girdle (as in the case of Hoxb5 mutants: (Rancourt et al., 1995). Interestingly, the phenotypes appear to cluster at the cervico-thoracic boundary, and the thoracic-lumbar boundary, while the middle of the cervical and thoracic regions seems less affected. This could be due to technical difficulties in distinguishing individual cervical and thoracic vertebrae. However, in Hox-transgenic mice, the alterations also most predominantly manifest at the cranial-cervical boundary (for Hoxb6: (Kaur et al., 1992); Hoxb7: (McLain et al., 1992); Hoxd4: (Lufkin et al., 1992), the cervico-thoracic boundary (for Hoxa4 and Hoxc8: (Sreenath et al., 1996); Hoxb8: (Charite et al., 1994), or the thoracic-lumbar boundary (for Hoxc6: (Jegalian and DeRobertis, 1992); Hoxc8: (Pollock et al., 1992). Taken together, these lines of evidence may suggest that the transition regions may be more labile in their specification, or that their specification can be influenced within a larger time window. The biological consequences seem to exhibit little regard for the specific Hox gene alteration, whether it is an addition or loss, or the presumed specificity of the protein. This has been interpreted to mean that the precise dosage of Hox gene expression in a body region is important for determining developmental outcome (Jegalian and DeRobertis, 1992; Pollock et al., 1992).
Figure 1. Skeletal Abnormalities in Hox gene Mutant Mice.
The regions of the skeleton affected in single Hox gene mutants were marked with respect to axial level and location of the respective genes on one of the Hoxa, Hoxb, Hoxc or Hoxd clusters. The template for the drawings was taken from (Rugh, 1968) and relevant references are found in the Legend to Fig. 1. The colors label the regions affected by genes in paralogous group 4 (green), group 5 (red), group 6 (blue), group 7 (yellow), group 8 (purple), group 9 (orange). Abnormalities in the sternum are indicated by bars to the left. Regions affected by more than one mutation contain several colors. No attempt was made to incorporate information on penetrance of an abnormality or the direction (loss or gain of character, rib bifurcation, sternal articulation, etc.). Except for more sacral and posterior lumbar regions, the developmental defects cluster in regions of morphological transitions, the cervico-thoracic and thoracic-lumbar transition as well as the transition between attached and floating ribs. Mid-cervical and mid-thoracic regions are relatively unaffected.
References for the mutants are: Hoxa4 (Horan et al., 1994; Kostic and Capecchi, 1994), Hoxa5 (Jeanotte et al., 1993), Hoxa6 (Kostic and Capecchi, 1994), Hoxa9 (Fromental-Ramain et al., 1996a), Hoxb4 (Ramirez-Solis et al., 1993), Hoxb5 (Rancourt et al., 1995), Hoxb6 (Rancourt et al., 1995); (Kappen, 2000), Hoxb9 (Chen and Capecchi, 1997), Hoxc4 (Boulet and Capecchi, 1996; Saegusa et al., 1996), Hoxc8 (LeMouellic et al., 1992), Hoxc9 (Suemori et al., 1995), Hoxd4 (Horan et al., 1994), Hoxd9 (Fromental-Ramain et al., 1996a).
Further insight comes from the deletion of larger domains of the Hox clusters that encompass multiple genes (Medina-Martinez et al., 2000; Suemori and Noguchi, 2000). With the deletion of multiple Hox genes in the Hoxb or Hoxc clusters, the extent of transformation of individual elements is unaffected, but the common region affected by transformation is extended along the axis. In this regard, whole cluster mutants display additive phenotypes of the single gene mutants. This indicates that the identity of skeletal elements may be determined by the dosage of Hox gene expression rather than by instruction from specific Hox proteins. This is also underscored by the phenotypes of mice in which mutations were superimposed that affect the same skeletal region. In compound mutant mice, often the size of an element rather than its identity is changed. Double mutants for Hoxa3 and Hoxb3 (Manley and Capecchi, 1997), or for Hoxa3 and Hoxd3 (Condie and Capecchi, 1994), lack the atlas, a phenotype not apparent in any of the known single gene mutants. Compound mutants for Hoxa4 and Hoxd4, and Hoxb4 and Hoxd4 (Horan et al., 1995a; Horan et al., 1995b) respectively, exhibit similar defects in the cervical region. Cooperativity between non-paralogous Hox genes has also been reported, such as for Hoxb5 and Hoxb6 in the thoracic region (Rancourt et al., 1995), for Hoxa11 and Hoxd11 in the lumbar region (Zakany et al., 1996), and for Hoxa10 and Hoxd11 in the sacral region (Favier et al., 1996) of the skeleton. There are also region-specific differences in functional redundancy: for example, Hoxa9 genetically cooperates with Hoxb9 in the thoracic region while it cooperates with Hoxd9 in the lumbrosacral region (Chen and Capecchi, 1997). These lines of evidence underscore the importance of Hox gene dosage for skeletal variations. How cells measure and functionally interpret the presence of multiple Hox transcription factors and how these proteins generate biological specificity is still unresolved. The collective results support the hypothesis of a dual role for the Hox proteins in the developing skeleton: patterning of elements as well as control of their growth (Capecchi, 1996; Kappen, 1998; Yueh et al., 1998)
While there is ample experimental evidence for a crucial function of Hox genes in skeletal patterning, it is still unclear how Hox transcription factors actually control patterning and growth. A simple model assumes that the collective local balance of Hox genes controls skeletogenic cell proliferation (Davis and Capecchi, 1994; Dolle et al., 1993; Duboule, 1995; Fromental-Ramain et al., 1996a; Fromental-Ramain et al., 1996b; Rancourt et al., 1995; Yueh et al., 1998). The control of rates of cell proliferation could be achieved either through transcriptional regulation, or through interactions with specific cell cycle proteins. In most cases, there is a decreasing size of skeletal elements in double and triple Hox mutants (Capecchi, 1996), indicating that increasing dosage of Hox genes is generally associated with increased growth potential. Thus, a model emerges where the locally effective concentration of Hox gene products regulates local proliferation rates of skeletogenic cells.
The overwhelming evidence from mouse studies implicates the Hox genes as important contributors to human skeletal variations. However, direct corresponding mutations between the mouse and humans have only been discovered for a few Hox genes. In humans, hypodactyly and synpolydactyly are associated with mutations in HOXA13, and hand-foot-genital syndrome is associated with mutation in the HOXD13 gene, respectively (Goodman et al., 1997; Mortlock and Innis, 1997; Mortlock et al., 1996; Muragaki et al., 1996). Recently, novel mutations in both genes have been found associated with previously undescribed limb abnormalities and with Guttmacher syndrome (Debeer et al., 2002; Frisen et al., 2003; Innis et al., 2002; Johnson et al., 2003; Kan et al., 2003; Utsch et al., 2002). These discoveries were aided by the insight from mouse mutants (Zakany and Duboule, 1996). It is therefore possible that additional Hox genes might be mutated in other human skeletal syndromes as well as in syndromes that do not manifest skeletal changes. In this regard, it is interesting that allelic variants of HOXA1 and HOXB1 appear to be correlated with susceptibility to autism spectrum disorders (Ingram et al., 2000; Tischfield et al., 2005). In addition, null-mutations in HOX genes may be deleterious in humans, as they have been shown to cause infertility, reproductive failure, embryonic lethality, as well as perinatal and postnatal lethality in selected cases in mice (Branford et al., 2000; Jeanotte et al., 1993; Ramirez-Solis et al., 1993; Satokata et al., 1995). A much more likely scenario is one in which subtle or transient alterations in Hox gene expression and regulation may cause variations in skeletal patterning during development. This may involve not only mutations in the coding regions but also regulatory mutations, which would be difficult to detect. In either case, the molecular and cellular pathways through which Hox transcription factors control skeletal development are already operative at the level of the individual somite, and continue to be maintained through migration of skeletogenic cells as well as in their condensations to form the first manifestations of individual skeletal elements. On the level of individual cells, the role of Hox proteins may be mediated through molecules that regulate the progression of skeletogenic cells through various stages of differentiation, or through products that define the final phenotypes of differentiated cells.
5. Local Control of Bone Shape During Embryogenesis and Growth
As sclerotomal cells migrate from the somites, they maintain their positional information and contribute it to the patterning of condensations. The formation of skeletal condensations has been amply reviewed (Hall, 1987, 2000; Hall and Miyake, 1995; Hall and Miyake, 2000). Interestingly, much the same molecules are thought to be involved in chondrogenic cell condensation that are involved at earlier (see above) and later (see below) stages of skeletal development. However, it is as yet unknown how information about patterning and cell specification is carried and maintained through the condensation process into the process of chondrogenesis.
In the axial skeleton, the final formation of skeletal elements takes place in the form of endochondral bone formation. This involves the commitment of mesenchymal precursor cells to the chondrogenic lineage, initiation of chondrocyte differentiation, maturation, and the transition of chondrocytes into their fully differentiated state, into hypertrophy. At each stage of this pathway, chondrocytes express a specific profile of genes, the best studied of which are the components of the cartilage extracellular matrix, the collagens. In addition to systemic control by growth factors and nutrients, the process is thought to be regulated locally in each skeletal element in order to provide its final unique shape and size. Disruptions of this process are known to cause abnormalities in cartilage and subsequent bone formation (Mundlos and Olsen, 1997a; 1997b).
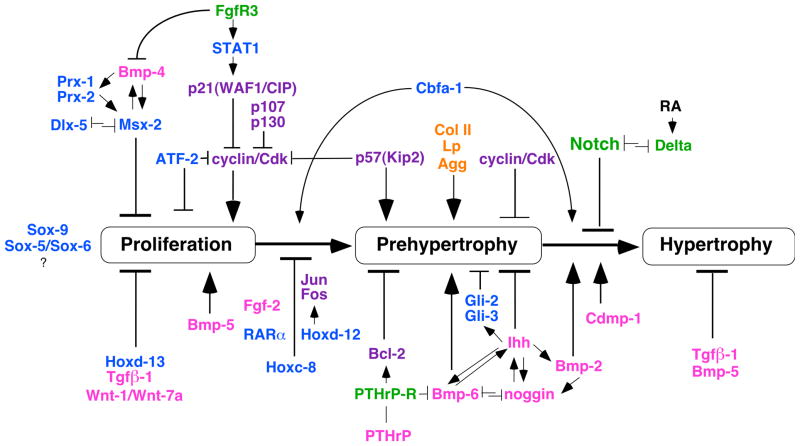
A whole variety of molecules have been implicated in local control of bone shape and growth, and their effects on skeletal development are being widely investigated using mouse models. The differentiation and maturation of chondrocytes in bones undergoing endochondral ossification (such as the vertebrae and ribs in the axial skeleton), is regulated by a various types of molecules. Figure 2 places these molecules into the pathway of chondrocyte differentiation according to their actions in mouse models that were generated by transgenic or knockout technologies.
Figure 2. Regulators of chondrocyte proliferation and maturation.
Transcription factors (blue), cell cycle regulators (purple), cell surface receptors (green), soluble mediators and growth factors (pink), and structural molecules (orange) have all been shown to affect chondrocyte maturation. Sox9 and additional factors are needed to induce the chondrocyte phenotype initially (for references, see text). The effects of other regulators are depicted as interpreted from phenotypes observed in in vivo overexpression or disruption paradigms (see text for details). The receptors for BMPs (Wozney and Rosen, 1998a) signal through Smad proteins (Massague, 1998) at various stages of chondrocyte differentiation and were not listed to reduce complexity of the graph.
The progression from the proliferative to the hypertrophic stage, also addressed as chondrocyte maturation (Hickok et al., 1998), is characterized by an ordered sequence of the expression of structural molecules, such as collagen II, aggrecan, link protein and collagen X (Cancedda et al., 1995). While these extracellular matrix components do not directly regulate chondrocyte behavior, disturbances nevertheless have profound effects on the structural integrity of cartilage (Aszodi et al., 1998; Garofalo et al., 1993; Kwan et al., 1997; Li et al., 1995a; Mundlos et al., 1996; Wai et al., 1998; Watanabe and Yamada, 1999; Yang et al., 1997). Indeed, collagen gene mutations feature prominently as causes for human chondrodysplasias (Francomano et al., 1996; Mundlos and Olsen, 1997b).
The initial commitment of skeletogenic cells to the chondrocyte lineage requires Sox9 (Bi et al., 1999; Lefebvre and de Crombrugghe, 1998). However, Sox9 alone is not sufficient for induction of the chondrocyte differentiation program (Cancedda et al., 1995; Lefebvre et al., 1998; Zhou et al., 1998), thus other yet unidentified factors, are most likely necessary. These may include genes such as Sox5 and Sox6 (de Crombrugghe et al., 2000). Once cells have committed to the chondrogenic lineage, several diffusible molecules regulate their proliferation and progression to hypertrophy. The best characterized of these include bone morphogenetic proteins (BMPs; (Hogan, 1996; Wozney and Rosen, 1998a), and TGFβ1 (Serra et al., 1997b; Wang et al., 1999), fibroblast growth factors (FGFs; (DeLuca and Baron, 1999), Parathyroid Hormone related Peptide (PTHrP; (Amizuka et al., 1996; Chung et al., 1998; Kronenberg et al., 1998; Lanske et al., 1996), and Indian hedgehog (Ihh; (Vortkamp, 1997). Consequently, mutations in the receptors for these ligands are involved in skeletal abnormalities, such as constitutive activation of FGFR1, -2, and -3 in human achondroplasia and thanatophoric dysplasia (Francomano et al., 1996; Naski et al., 1996; Nerlich et al., 1996; Wilkin et al., 1998), and PTH/PTHrP receptor mutations in Jansen (Schipani et al., 1997) and Blomstrand chondrodysplasia (Karaplis et al., 1998). Furthermore, one of the downstream effectors of the Ihh receptor pathway, the Zinc-finger transcription factor Gli3, is involved in Greig’s poly/syndactyly (Wild et al., 1997). Once cells have reached the hypertrophic stage, they can inhibit their neighbors from further progressing through the Notch/Delta cell surface receptor system (Crowe et al., 1999). Indeed, there appear to be several negative feedback loops (directly or indirectly) involved in regulating the pathway at specific points, such as FGFs at the level of the proliferating chondrocyte (Naski et al., 1998), PTHrP at the level of the prehypertrophic chondrocyte (Kronenberg et al., 1997; Lanske et al., 1996; Weir et al., 1996) and Ihh at the level of hypertrophic cells (Chung et al., 1998; Kameda et al., 1999; Pathi et al., 1999; Vortkamp et al., 1996). Positive regulators include Cdmp1, Bmp2 and Bmp6, which have been shown to influence growth and maturation of chondrocytes (Enomoto-Iwamoto et al., 1998; Gimsrud et al., 1999; Kawakami et al., 1996; Tsumaki et al., 1999), while Bmp4 and Bmp7 appear to act negatively (Katagiri et al., 1998a; Marazzi et al., 1997). As for earlier developmental processes, the actions of BMPs can be antagonized by Noggin (Brunet et al., 1998). Experimentally, cellular regulators of growth, proliferation and apoptosis (Beier et al., 1999) have also been implicated in chondrocyte maturation, such as bcl2 (Amling et al., 1997), the p107 and p130 cell cycle regulators (Cobrinik et al., 1996), STAT1 (Su et al., 1997), p21(WAF/CIP2; (Chin et al., 1996) and p57(Kip2; (Yan et al., 1997; Zhang et al., 1997c), which interacts with Proliferating Cell Nuclear Antigen, (Watanabe et al., 1998). These presumably regulate chondrocyte cell cycle progression through modulation of activity of cyclins/cdk kinases, as well as the exit from the cell cycle in the progression to hypertrophy. Further, the transcription factors AP1 (jun/fos: (Kameda et al., 1997; Wang et al., 1992; Watanabe et al., 1997), ATF2 (Reimold et al., 1996), Cbfa1 (Ducy and Karsenty, 1998; Inada et al., 1999; Kim et al., 1999; Komori et al., 1997), RARα (Cash et al., 1997; Yamaguchi et al., 1998), Gli2 and Gli3 (Mo et al., 1997), Prx1 and Prx2 (Lu et al., 1999), Dlx5 (Ferrari et al., 1995), Msx2 (Ferrari et al., 1998; Marazzi et al., 1997; Zhang et al., 1997b) and Hox transcription factors (see below) influence the propensity of chondrocytes to differentiate and mature.
It is important here to note that the contribution of signaling systems to particular skeletal structures varies (Koziel et al., 2005), which provides a mechanism by which the diversity of bones in all shapes and sizes can be achieved. Secondly, it is evident that the molecules that regulate chondrocyte differentiation are also involved in prior phases of skeletal development. Examples are the Notch/Delta signaling system, which plays a role early in somitogenesis and again later in progression to hypertrophy; the BMP and hedgehog families of signaling molecules that antagonize each other in setting up dorsal-ventral polarity in developing somites, and again antagonize each other in regulating the progression to hypertrophy; and finally, the Hox genes, that pattern skeletal elements prior to the condensation phase, and then continue to participate in regulating the progression of chondrocytes along their differentiation pathway (for details, see below). These examples only serve to illustrate that during different phases of skeletal development, fundamental molecular mechanisms are being re-utilized. In this way, uncommitted progenitor cells, committed precursors, and terminally differentiating cells, may all use components of the same molecular machinery and signaling pathways, yet temporal regulation and different combinations of these pathways may provide specificity at any given stage. Indeed, some of the molecules involved in chondrocyte proliferation are also involved in their differentiation. These include Cbfa1, cyclins and their kinases (Beier et al., 1999), Bmp5, and Fgfr3 (Iwata et al., 2000). Together with the fact that different regions of the developing skeleton are differentially sensitive to perturbations in any of these pathways, quantitative and combinatorial variations may enable a limited number of key molecules to control multiple differentiation pathways, and the individual outcomes in the formation of all the different elements of the axial skeleton.
5.a. Bone Morphogenetic Proteins
Secreted factors locally influence the shape of individual skeletal elements by regulating mesenchymal condensation and coordinating growth (reviewed in (Erlebacher et al., 1995). The bone morphogenetic proteins (BMPs), secreted proteins belonging to the TGFβ superfamily (except for Bmp1), have been implicated in this task (Reddi, 1992, 1994). The BMPs were originally identified as the factors responsible for the induction of ectopic cartilage and bone at nonskeletal sites (Kingsley, 1994a; Reddi, 1994); and references therein). The temporal and spatial expression patterns of BMPs suggest that they are involved in multiple aspects of development, including endochondral bone formation, with coordinate expression of different members of the BMP family involved in similar processes (Kingsley, 1994b; Wozney and Rosen, 1998b).
The role of BMPs for the correct formation of mesenchymal condensation is highlighted by the mutation of the Bmp5 gene in the mouse skeletal mutant short ear (se; (Kingsley et al., 1992). In se mice, the size, shape, and number of many small bone and cartilage elements, as well as some soft tissues, are affected. The observed defects include reduction of the size of the external ear, altered size and shape of the sternum, loss of one pair of ribs and of two ventral processes unique to the sixth cervical vertebrae, and reduced ability to repair rib fractures (King et al., 1994; Kingsley et al., 1992). Interestingly, the long bones of the appendicular skeleton are not affected in se mice, whereas these are the same bones that are reduced in size in the brachypodism (bp) mouse mutant. The abnormalities observed in bp mice were recently attributed to mutations in the growth and differentiation factor5 (Gdf5) gene (Storm et al., 1994). The GDFs are also members of the TGFβ family, and are closely related to the BMPs (Kingsley, 1994a). In both se and bp, the abnormalities were traced back to altered size or shape already apparent in the mesenchymal condensation for the respective skeletal elements. The expression of Gdf5 during skeletogenesis is restricted to the structures affected in bp, the precartilaginous condensation and the perichondrium of the appendicular long bones (Storm et al., 1994). In contrast, Bmp5 transcripts are not restricted to only the affected skeletal elements but are, for example, present in all vertebral bodies, neural arches, and ribs. Bmp5 is also expressed in mesenchymal tissues already prior to the formation of obvious condensations, continues to be expressed in the condensed mesenchyme, and becomes restricted to the perichondrium upon chondrification. This suggests that growth factors of the BMP family may act in both an autocrine and a paracrine fashion. Furthermore, the restricted phenotype despite wider expression in the case of Bmp5, indicates that different skeletal elements are differentially sensitive to the loss of Bmp5. This may be the result of a compensatory function of other BMP-family members (Katagiri et al., 1998b) For example, Bmp6deficient mice show a delay in ossification due to an effect on hypertrophic cells that is restricted to the sternal cartilage (Solloway et al., 1998). These defects are slightly exacerbated by concomitant absence of Bmp5; however, the restriction to the sternum likely reflects the compensatory action of Bmp2. In Bmp7 homozygous mutants, there is cartilage underdevelopment with the consequences of fused ribs and vertebrae, and reduced neural arches (Jena et al., 1997). The vertebral defects are restricted to the lumbar and sacral region, again, pointing towards functional overlap by other BMP family members. Consistent with this idea, double heterozygotes for mutant Bmp4 and Bmp7 alleles also exhibit abnormalities in restricted areas of the skeleton, namely the limbs and the rib cage (Katagiri et al., 1998b). Taken together, these data show that BMPs play a quantitative and qualitative role for skeletal development in specific spatial domains.
Mice with targeted mutations of the BMP receptor II gene exhibit severe developmental defects, attesting to the importance of BMP signaling in development. However, the defects affect early gastrulation stage embryos, preventing a detailed analysis of their role for bone formation, specifically (Beppu et al., 2000). Bmp-receptor 1b-deficient mice exhibit defects in the appendicular skeleton (Yi et al., 2000), again suggesting functional overlap with other Bmp receptors in the developing skeleton (Rosen et al., 1996). As the downstream signaling pathways for various BMPs are being identified and converge onto the Smad family of proteins, the interesting question becomes how specificity of BMP signaling is achieved in different regions and cell types of the developing skeleton (Goumans and Mummery, 2000).
5.b. Fibroblast Growth Factors and Their Receptors
In bones that develop via endochondral ossification, this process is coordinated with both the longitudinal growth at the epiphyseal growth plate, and the radial growth of the periosteum. Incomplete coordination of these processes can result in achondroplasia, the most frequent cause of human dwarfism. In achondroplastic patients, overgrowth of the periosteum relative to the epiphyses results in short, wide bones (Erlebacher et al., 1995). Recently, mutations in the transmembrane region of the FGF receptor3 (FGFR3) were identified as the cause for this dominantly inherited disease (Shiang et al., 1994). In achondroplastic patients, only endochondral bone formation is affected, consistent with FGFR3 being exclusively expressed in chondrocytes. Other dominantly inherited human skeletal disorders are also associated with mutations in FGF receptors. Different mutations in FGFR2 are responsible for the malformations observed in patients with Crouzon and Jackson-Weiss syndromes (Jabs et al., 1994), and mutations in FGFR1 were identified as the cause of Pfeiffer’s syndrome (Muenke et al., 1994), although FGFR2 mutations have also been described in sporadic Pfeiffer Syndrome patients (Rutland et al., 1995). In all three syndromes, premature closure of the skull sutures results in craniosynostosis, which in the latter two syndromes is accompanied by limb defects. Interestingly, the axial skeleton is largely unaffected. The difference in phenotypes in these skeletal dysplasias, and the dominant inheritance patterns suggest that either the mutations in the FGF receptor genes are gain of function mutations, or that the mutated receptors can function in a dominant negative fashion. This is consistent with the failure to observe any malformations in mice heterozygous for a targeted disruption of the Fgfr1 gene (Deng et al., 1994; Yamaguchi et al., 1994). In mice with transgenic expression of Fgfr3 specifically directed to chondrocytes, Fgfr3 downregulated the expression of ihh and Bmp4 in growth plate chondrocytes, suggesting that Fgfr3 is a negative regulator upstream of the Ihh pathway (Naski et al., 1998). Studies on mice with a targeted disruption of Fgfr3 reveal that this gene is critically important not only in regulating chondrocyte proliferation, but also differentiation of these cells (Iwata et al., 2000). Taken together, these data suggest that specific FGFs regulate growth rates of different parts of the skeleton (Coffin et al., 1995) as well as of different zones in individual bones. This may involve autocrine and paracrine signaling. The local action of BMPs and FGFs and other systemic factors, such as insulin-like growth factor Igf1 (Baker et al., 1993), is then integrated to various signaling pathways (see above) that control overall skeletal growth through positive or negative feedback (Ornitz, 2005).
5.c. Parathyroid Hormone-Related Peptide and Ihh
Recently, a negative feedback loop has been described that involves the signaling molecule indian hedgehog (Ihh; (Vortkamp et al., 1996) and parathyroid hormone-related peptide, PTHrP (Lanske et al., 1996; Lanske and Kronenberg, 1998). Targeted inactivation of the PTHrP gene (Karaplis et al., 1994) leads to reduced proliferation of epiphyseal growth plate chondrocytes and premature maturation, resulting in a greatly increased rate of endochondral ossification and an almost completely ossified skeleton at the time of birth. PTHrP does not seem to be involved in the initial steps of mesenchymal condensation and differentiation into chondrocytes, consistent with the expression of the PTHrP receptor (PTHrPR) that occurs only after cells have become proliferating chondrocytes (Karperien et al., 1994). Upon chondrocyte hypertrophy, PTHrPR expression is downregulated, indicating that PTHrP and its receptor are involved in the regulation of the rate of chondrocyte maturation (Karaplis and Kronenberg, 1996). The phenotype observed in the PTHrP deficient mice is reminiscent of the symptoms observed in several human osteochondrodysplasia syndromes. Indeed, in patients with Jansen metaphyseal chondrodysplasia and Blomstrand lethal chondrodysplasia, mutations in the PTHrP receptor have been found (Karperien et al., 1999; Schipani et al., 1995; Schipani et al., 1999).
Within the developing bones, PTHrP production is stimulated by Indian hedgehog (Ihh). Ihh is produced by chondrocytes shortly before and upon reaching hypertrophy (Vortkamp et al., 1996). It thought that, in this way, hypertrophic cells signal back to regulate the rate of progression of chondrocytes to hypertrophy through PTHrP (Lanske et al., 1996; Vortkamp, 1997). This signaling feedback is likely mediated through indirect mechanisms as the receptor for Ihh, patched, is expressed in the perichondrium. In Ihh-deficient mice, a reduction in chondrocyte proliferation and maturation of chondrocytes at inappropriate sites has been reported. The addition of PTHrP can rescue the differentiation defects of Ihh-deficient chondrocytes, but it does not recover normal chondrocyte proliferation. Thus, in addition to regulating PTHrP, Ihh is acts as a growth factor for proliferating chondrocytes. Interestingly, there are also defects in osteoblast development in endochondral bones (St-Jacques et al., 1999) implicating Ihh signaling in the differentiation of osteoblasts. Thus, Ihh functions in endochondral bone formation are both PTHrP dependent and independent (Karp et al., 2000). Recently, connections between Ihh- and BMP-signaling have been reported (Bitgood and McMahon, 1995; Pathi et al., 1999); again, these interactions document that an intricate balance of positive and negative feedback mechanisms regulate the chondrocyte differentiation pathway (Juppner, 2000; Perrimon and McMahon, 1999). Clearly, the temporal regulation of growth factor activities (Kobayashi et al., 2002) adds further complexity to the regulation of chondrocyte maturation.
5.d. Homeobox genes in chondrogenesis
While well documented for early stages of development (Deschamps and Meijlink, 1992), the expression of Hox genes at later stages of skeletogenesis has not been systematically analyzed. For isolated cases, expression in chondrocytes has been inferred from the activity of LacZ reporter genes placed into the Hoxc8 (LeMouellic et al., 1992) or Hoxd13 loci (Zakany and Duboule, 1996) by homologous recombination. We have shown by in situ hybridization that Hoxc8 is expressed specifically in condensing skeletogenic cells and chondrocyte precursor cells (Yueh et al., 1998). It continues to be expressed in proliferating chondrocytes, but is downregulated upon progression to hypertrophy. Thus, higher levels of Hoxc8 expression correlate with proliferation of cells; conversely, lower levels of Hoxc8 expression are associated with the differentiated state.
Functional evidence for a role of Hox proteins in chondrocyte differentiation comes from several experiments. It has been demonstrated that ectopic expression of Hoxb8 in the developing chick limbs can induce Shh (Charite et al., 1994), as does ectopic expression of Hoxd12 (Knezevic et al., 1997). The consequences of these altered patterns of gene expression are abnormal limb bone growth. In ectopic expression experiments using Hoxd13 and Hoxd11, Goff and Tabin (1997) found the predominant effects on the late stages of skeletogenesis, in the regulation of cell division in proliferative chondrocytes in the chick limb. Also, upon BMP-induced ectopic cartilage formation in vivo, Hox gene expression is altered (Iimura et al., 1994), which is consistent with the stimulation of chondrocyte proliferation.
For the axial skeleton, the role of Hoxc8 in chondrocyte proliferation was demonstrated by Yueh et al. (Yueh et al., 1998). Using a binary transgenic mouse model to reproducibly achieve overexpression of Hoxc8 under control of its own promoter, significant cartilage defects were observed. In Hoxc8 transgenic mice, the cartilaginous regions of the ribs showed reduced alcian blue staining and reduced structural rigidity. In addition, the vertebral bodies were malformed, particularly in lumbar and sacral regions, the neural arches did not extend dorsally, while the intervertebral cartilage was missing. Thus, while the condensations for the skeletal elements were formed normally, their differentiation was significantly altered. Histological analyses showed an enrichment of immature chondrocytes at the expense of prehypertrophic and hypertrophic cells. The numbers of proliferating cells, expressing PCNA, were significantly elevated, indicating a role for Hoxc8 in controlling chondrocyte proliferation (Yueh et al., 1998). Taking advantage of the binary transgenic mouse system, we were able to correlate the severity of cartilage phenotypes with the gene dosage of Hoxc8 overexpression. From these results, the model emerged that increasing Hoxc8 levels promote cell proliferation, while decreasing levels are associated with cell differentiation. Hoxd4 transgenic mice generated by the same transgenic technology exhibit very similar phenotypes (Kappen et al., 2004), confirming a role for Hox transcription factors in regulation of chondrogenesis by way of dosage-dependent control. This model is in excellent agreement with conclusions from compound Hox mutants (see above) in which skeletal elements were reduced in growth upon successive reduction of Hox wildtype allele dosage (Carpenter et al., 1993; Condie and Capecchi, 1993). Thus, despite similar effects on cell proliferation at early and late stages in skeletogenesis (Kappen, 1998), the functional outcomes for skeletal development upon perturbation of Hox gene expression may be quite different: early interference will affect skeletal patterning, interference at later stages will affect cell proliferation and differentiation. Collectively, these results support the notion that skeletogenic and chondrogenic cells respond differentially to changes in Hox gene expression according to their developmental and differentiation stage. This implies that the temporal regulation of Hox gene expression during skeletogenesis is critically important for the both the formation and differentiation of cartilage (Kappen, 1998). The precise molecular targets of the Hox transcription factors that mediate the different biological outcomes are still largely unknown. Upon Hoxc8 overexpression, the expression of the mRNA encoding collagen type II is elevated (Yueh et al., 1998), suggesting genes of the differentiated chondrocyte as potential targets.
5.e. Collagens and the Extracellular Matrix
Collagens are the major proteins in bone and cartilage matrices. They form a large family of at least 25 members, some of which are expressed at specific places and stages during endochondral ossification (reviewed by (van der Rest and Garrone, 1991). Collagen type II is the main collagen component of cartilage, while collagens IX and XI are two minor cartilage collagens. Collagen X is specifically expressed in hypertrophic cartilage (Reichenberger et al., 1991), while type I collagen is the most abundant protein in bone (Byers and Steiner, 1992; Francomano et al., 1996). Procollagen monomers associate to form trimers with triple-helical structure, which aggregate to form larger structures such as fibrils or networks, depending on the collagen type. Mutations in procollagens frequently lead to a dominant negative effect, by disrupting the structural integrity of fibers or by inhibiting the function of wildtype subunits. Indeed, mutant collagen genes induce skeletal malformations in transgenic mice (de Crombrugghe et al., 1995; Garofalo et al., 1993) demonstrating that they can be causative for bone abnormalities (Barbieri et al., 2003; Hiltunen et al., 1994). Numerous mutations in human collagen genes have been detected, and these are frequently associated with skeletal malformations (reviewed by: Vandenberg, 1993(Olsen, 1995; Vandenberg, 1993). Mutations in collagen type I cause osteogenesis imperfecta (reviewed by: (Byers and Steiner, 1992; Willing et al., 1993), and collagen type II mutations cause different chondrodysplasias (reviewed by (Vikkula et al., 1994) Recently, mutations in mouse and human collagen XI have been identified as the molecular basis underlying chondrodysplasia in a mouse mutant strain (cho mice), and in recessive and autosomal dominant human osteochondrodysplasias (Li et al., 1995b; Vikkula et al., 1994). Collagen X gene mutations are responsible for spondylometaphyseal and metaphyseal dyplasias in humans, in which the hypertrophic cartilage of the growth plates is compressed and bone formation is reduced. Expression of a mutated type X collagen in transgenic mice leads to a similar phenotype (reviewed in: (Jacenko et al., 1994). Upon targeted disruption of the Collagen X gene, the skeletal defects observed in mutant mice resemble those in Schmid metaphyseal chondrodysplasia, in particular, the reduction of the growth plate and atypical distribution of matrix components (Kwan et al., 1997).
Recently, the mutation was identified that is responsible for diastrophic dysplasia, an autosomal recessive osteochondrodysplasia characterized by dwarfism, spinal deformation, and joint abnormalities. In this disorder, a novel sulfate transporter is disrupted, leading to undersulfation of proteoglycans such as chondroitin sulfate (Hastbacka et al., 1994). While these results clearly demonstrate that the extracellular matrix is required as the major component of cartilage and bone, they also implicate the extracellular matrix as a signal for the regulation of chondrocyte differentiation (Cancedda et al., 1995).
6. Regulation of Maintenance and Remodeling in Cartilage and Bone: Osteopetrosis, Osteoporosis, and Osteoarthritis
Disorders that affect the maintenance of the fully developed skeleton may not seem directly relevant in the context of skeletal variation. However, skeletal variations can be aggravated by problems with the proper maintenance of cartilage and bone structure. Here, we include a few examples to illustrate recent advances in understanding the molecular basis for disorders and defects in bone and cartilage of the mature skeleton.
For the development of the final bone structure and for its maintenance, the continued deposition of new bone matrix by osteoblasts is important. This is balanced with the resorption of old bone matrix by osteoclasts, which are derived from the hematopoietic cell lineage. If bone resorption is impaired, the bone marrow space normally present in the long bones is completely filled by dense homogenous trabeculation, whereas the density of cortical bone is reduced. These are the characteristic symptoms of osteopetrosis. Several mouse models for osteopetrosis have been described, in which the defects occur in very different genes, all of which appear to affect osteoclast differentiation. Among them are the osteopetrotic mouse (op), in which a defect in the gene for the colony-stimulating factor1 (Csf1) results in nonautonomous early block of osteoclast differentiation (Wiktor-Jedrzejczak et al., 1990; Yoshida et al., 1990). In the microphthalmia mouse (mi), a mutation in a novel HLH zipper protein impairs osteoclast differentiation due to an intrinsic defect in these cells (Hodgkinson et al., 1993). Mutations in cfos and csrc are other examples of defects in osteoclast precursors that result in osteopetrosis (Grigoriadis et al., 1994; Lowe et al., 1993; Soriano et al., 1991).
Whereas in osteopetrosis excess bone is formed, in osteoporosis, the bone mass is reduced as a result of an imbalance between bone resorption and bone formation. The equilibrium between these two processes is to a large extent controlled via endocrine factors such as estrogen and corticosteroids. In addition, the association of common alleles of the vitamin D receptor gene with bone mass in humans has long been a subject of intense study (Morrison et al., 1994); for review see: (Eisman, 1999). The vitamin D receptor, which belongs to the nuclear hormone receptor family, and its ligand, vitamin D, are known to be key regulators of bone and calcium homeostasis, consistent with a potential involvement of vitamin D receptor alleles in the development of osteoporosis.
Recent data implicate Hox genes in the regulation of bone formation. Cao’s group has shown that in osteoblasts, Hoxc8 interacts with the BMP signaling pathway (Shi et al., 1999). Upon BMP stimulation of osteoblasts, osteopontin gene expression is upregulated. Hoxc8 represses this activity in a concentration-dependent manner, by forming complexes with smad1 (Yang et al., 2000), a well-known component of the TGFβ and BMP-signaling pathways (Attisano et al., 1994; Massague, 1998). Smad6 can be recruited as a co-repressor with the Hoxc8 protein (Bai et al., 2000). Homeobox binding sites have been reported in the promoters of a number of osteoblast-expressed genes, including the NCAM (Boersma et al., 1999), bone sialoprotein (Kim et al., 1994; Li and Sodek, 1993), and osteocalcin genes (Towler et al., 1994). Activity of the rat osteocalcin gene promoter has been shown to be dependent on the homeobox binding motif (Hoffmann et al., 1996; Towler et al., 1994). Thus, homeobox transcription factors may act positively or negatively on gene expression in the developing skeleton (Franceschi, 1999). Taken together, these experiments identify a role for Hox transcription factors in developmental processes that reach well into adulthood.
Those areas of the skeleton where cartilage persists, such as in the joints and in intervertebral disks, are also subject to a coordinated equilibrium of opposing processes, in this case, of cartilage production and metabolism. Degradation of cartilage is found in arthritic diseases, the most prevalent form of which is osteoarthritis. Osteoarthritis is characterized by degeneration of cartilage and subsequent damage of joints (Oddis, 1996). Hereditary forms of the disease have been associated with collagen gene mutations (Olsen, 1995; Vandenberg, 1993), but most cases of arthritis are idiopathic and are more likely caused by exogenous factors other than abnormalities in the structural molecules themselves (Williams and Jimenez, 1995; Wordsworth, 1995). The increased incidence of osteoarthritis in women (Hughes and Dunlop, 1995), for example, may implicate hormonal regulation. Generally, osteoarthritis is characterized by an imbalance in production and metabolism of cartilage (Brinckerhoff, 1992; Testa et al., 1994). Recently, a signaling defective TGFβ type II receptor was shown to cause in osteoarthritis in mice (Serra et al., 1997a) implicating molecules of developmental processes (Serra and Chang, 2003) in arthritic disorders. Thus, a better understanding of the molecular mechanisms that control chondrogenic cell differentiation (Cancedda et al., 2000) will ultimately also help to address open questions surrounding deficiencies in cartilage homeostasis.
7. Towards a molecular framework of axial skeletogenesis
This review, albeit far from comprehensive, explicates an emerging general concept of axial skeleton development. It is becoming increasingly clear that regulatory mechanisms and molecules that are involved in other developmental processes during embryogenesis are also involved in skeletal development. In fact, they often operate in a dual role, early during specification of the mesodermal derivatives and later during chondrogenesis. However, while the general molecular pathways are shared, the specific roles for tissue-differentiation and morphological outcome within the developing skeleton are unique. This suggests that the actions of each molecular pathway are modulated according to their developmental context.
This consideration allows us to extend the model originally developed for Hox genes to other developmental genes in the axial skeleton. This model postulates that the same genes play multiple roles in skeletogenesis, in specification of morphogenetic events early, and in tissue-specific regulation of chondrocyte proliferation/differentiation at later stages of development. This would imply that regulatory pathways may control different biological processes depending on the cellular context in which they are expressed. In chondrocytes, for example, the two processes of proliferation and differentiation involve similar signaling pathways, but they may have opposite effects. BMP5 is a positive regulator of cell proliferation, but acts negatively on hypertrophic cells. Similarly, Fgfr3 acts both positively and negatively (see above). On the other hand, Cbfa1 acts positively throughout the entire pathway, while TGFβ has inhibitory effects on proliferating and hypertrophic cells. Thus, the action of signaling pathways is interpreted in the context of cellular potential and differentiation stage.
There are several ways in which such context-dependent roles could be accomplished. First, the molecular pathway may be involved in the regulation of different target molecules in early and late development. This could be determined by differential presence, subcellular localization, the proliferative status of the cell, or accessibility of chromatin to various transcription factors in different cell types (Duboule, 1995). In this scenario, a given signaling pathway would still interact with the same set of proteins, but the actual cellular environment in which these proteins function would differ with developmental stage. Second, each pathway may interact with different proteins in different cells. For example, the complement of other proteins may vary with cell type or time and thus lead to modulation of the outcome of signal transduction. The specific cell type, its proliferative or differentiative status, or signaling responses, may then determine which function prevails. These hypotheses are experimentally testable and generally imply that different cooperating factors and specific cellular responsiveness modulate the functional roles of regulatory molecules in early and late stages of skeletogenesis, and in various regions/elements of the skeleton. This also implies that cells have a framework according to which they sense and integrate opposing signals, as well as sense and integrate the action of transcriptional regulators. Elucidating this framework and the integration mechanisms will be the next important steps in understanding skeletal development and its perturbations in human skeletal variations and disorders.
D. Influence of Retinoic Acid as a Model Teratogen on Axial Skeleton Development
As discussed in the preceding sections, numerous genetic defects can lead to skeletal abnormalities. It is evident from several targeted alleles that the penetrance and expressivity of skeletal malformations is also dependent on the mouse strain backgrounds on which they are super-imposed, suggesting that modifier loci contribute to the manifestation of these complex phenotypes (Chen and Capecchi, 1997; Chen et al., 1998; Dunn et al., 1997; Favier et al., 1996; Fromental-Ramain et al., 1996a; Horan et al., 1994; Kostic and Capecchi, 1994; Lohnes et al., 1993; Mansour et al., 1993; Yan et al., 1997). The identification of such modifiers will help not only in understanding the molecular basis of skeletal variations, but also in discovering the targets for chemical teratogens that induce similar skeletal defects.
The best-studied teratogen in relation to skeletal defects is retinoic acid (RA), which severely affects vertebrate skeletal morphogenesis (Juchau, 1993). Among other defects, retinoic acid induces both anterior and posterior homeotic transformations of vertebrae, depending on the timepoint of administration (Kessel, 1992). Further analysis revealed, that RA induces changes in Hox gene expression (Kessel, 1992; Kessel and Gruss, 1991b; Krumlauf et al., 1993). Thus, retinoic acid acts upon a known patterning and regulatory system during skeletogenesis.
It is thought that the effects of RA are mediated through two families of receptors, the RA receptors (RAR) and the retinoid X receptors (RXR), which act as inducible transcriptional regulatory proteins and belong to the superfamily of nuclear receptors (reviewed in: (Chambon, 1994). These receptors activate transcription by binding to response elements of target genes predominantly in the form of RAR/RXR heterodimers. The RAR and RXR molecules are each encoded by three genes designated RARα, β, and γ and RXRα, β, and γ, respectively. Each gene generates multiple receptor isoforms with different N-terminal regions of the protein (α1, and α2, β1β4, and γ1 and γ2) through differential usage of alternative promoters and differential splicing. Recently, gene targeting has been used to generate mice deficient of all isoforms of RARα, RARβ and RARγ as well as mice deficient for the individual RAR and RXR isoforms (Li et al., 1993; Lohnes et al., 1993; Lufkin et al., 1993; Luo et al., 1995; Mendelsohn et al., 1994b). Interestingly, mutants deficient in individual isoforms appeared to be completely healthy (Li et al., 1993; Mendelsohn et al., 1994b). Mutants deficient for all isoforms of RARβ were also viable and fertile; however, they showed fusion of the ninth and tenth cranial ganglia with low penetrance (Luo et al., 1995). Mutants deficient for all RARα and RARγ isoforms displayed early postnatal lethality and some abnormalities such as testis degeneration in RARα mutant mice (Lufkin et al., 1993) and homeotic transformations of cervical vertebrae in RARγ mutants (Lohnes et al., 1993). When compound mutants for different RAR isoforms were generated by breeding of different RAR mutant mice, they were not viable and displayed multiple defects, among them the typical malformations associated with the vitamin A deficiency syndrome (Lohnes et al., 1995; Lohnes et al., 1994; Mendelsohn et al., 1994a). These experiments clearly establish that RARs participate in the transduction of the RA signal in vivo, but also suggest extensive functional redundancy between members of the RAR family, and possible interactions with the RXR receptors.
RXR mutants exhibit developmental defects too early to be informative for skeletogenesis (Wendling et al., 1999). However, compound mutants heterozygous for RAR/RXR combinations exhibit graded skeletal abnormalities in which different regions of the skeleton show differential susceptibility. For example, cervical vertebra C2 was more often affected than other cervical vertebrae (Kastner et al., 1997). These data establish RXRα, together with RARα or RARγ, as the major components involved in skeletal abnormalities in cervical vertebrae. While other regions of the axial skeleton were not investigated, it is conceivable that RAR/RXR heterodimers are involved in the interpretation of the retinoic acid signal in other regions of the skeleton as well (Rijli and Chambon, 1997). Thus, genetic evidence implicates the RAR/RXR heterodimers as the functional transducers of the retinoic acid signal during development (Kastner et al., 1997).
RARγ homozygous mutants are resistant to spina bifida induced in wildtype mice by retinoic acid treatment at 8.5 to 9.0 days of development (Iulianella and Lohnes, 1997). Resistance to the induction of skeletal defects was moderate with treatment on gestational day 7.3, but markedly enhanced with treatment at days 10.5 or 11.5 of pregnancy. These data support two important conclusions. First, different embryonic tissues may exhibit differential sensitivity to RA, and secondly, this sensitivity is temporally regulated. At the molecular level, this implicates various RAR/RXR receptor combinations in different tissues and at different timepoints. Thus, qualitative and quantitative aspects define the responsivity of cells in the skeleton to RA. The specific timing of interference may then determine the specific outcome for each affected tissue and cell type (Weston et al., 2003). Interestingly, the vertebral defects present in the RARγ-deficient mice could be rescued by retinoic acid treatment, suggesting that other receptor heterodimers are also involved in transducing the external signal, at least under conditions where RARγ is absent. Taken together, these studies provide insight into the molecular basis for temporally different RA-induced defects.
Definitive proof that endogenous RA is involved in normal skeletogenesis comes from the study of mice with a targeted deletion of the gene that encodes the enzyme retinaldehyde dehydrogenase2. Raldh2 is the major producer of retinoic acid in vivo, and consequently, Raldh2-deficient embryos do not turn, do not form limb buds, exhibit defective heart and craniofacial development, and their anterior-posterior axis is shortened (Niederreither et al., 1999). These data show that the RA synthesized by the embryo itself is required for vital developmental processes.
It has long been documented that the effects of RA on axial skeleton development are mediated by the Hox genes (Kessel, 1992). In particular, retinoic acid response elements (RARE) were found in the vicinity of a number of Hox genes, the best studied of which are Hoxd4 (Popperl and Featherstone, 1993; Zhang et al., 1997a), Hoxb4 (Gould et al., 1998), Hoxb1 (Langston et al., 1997; Marshall et al., 1994), and Hoxa1 (Dupe et al., 1997; Langston and Gudas, 1992; Langston et al., 1997). These RARE elements have been shown to be critical to the establishment of proper boundaries of Hox gene expression domains. Furthermore, it is suggested that the maintenance of Hox gene expression is also dependent on the RARE (Gould et al., 1998; Zhang et al., 1997a). Dupe et al. (Dupe et al., 1997) have shown that mutation of the RARE in the Hoxa1 gene leads to altered Hoxa1 expression in vivo, resulting in craniofacial defects similar to those found in Hoxa1 null mutants. These data provide evidence that RA is critical for proper Hox gene expression. Interestingly, treatment of the mutant animals with exogenous RA further impacts Hoxa1 expression, suggesting the involvement of additional RAREs or of other factors in the regulation of Hoxa1 expression that are also sensitive to RA (Dupe et al., 1997).
Obvious candidates for such factors are the upstream regulators for the Hox cluster genes, molecules of the trithorax and polycomb families (Hanson et al., 1999). Just as in Drosophila, the mammalian homologs are reciprocally involved in the regulation of Hox gene expression. For example, in Mll heterozygous mutants, Hoxa7 and Hoxc9 expression was shifted more posteriorly, resulting in homeotic transformations of vertebrae, while expression was completely abolished in homozygous mutants (Yu et al., 1995), which exhibited embryonic lethality. These results establish Mll as absolutely required for the expression of Hox genes. Thus, Mll acts as a positive upstream regulator. Conversely, mice with targeted disruptions of the polycomb homologs implicate these molecules as negative upstream regulators, or repressors of Hox gene expression. Bmi1 mutants develop posterior transformations along the entire anterior-posterior axis (van der Lugt et al., 1994), as do mice deficient for mel18 (Akasaka et al., 1996). M33-deficiency also results in posterior transformations, which are further enhanced by concomitant deficiency for Bmi1 (Bel et al., 1998). The results demonstrate that the polycomb-type molecules Bmi1, mel18 and M33 are involved in controlling the expression domains of Hox genes in mice (van Lohuizen, 1998). These upstream regulators antagonize each other as demonstrated by normalization of axial vertebral transformations in mice deficient for both Mll and Bmi1 (Hanson et al., 1999). The influence of RA on this system is documented for M33 mutant mice in which RA sensitivity and temporal response of a RARE-LacZ transgene are changed (Bel-Vialar et al., 2000). This may be achieved through M33 in controlling the accessibility of RAREs in Hox clusters, although how this results in altered temporal regulation of gene expression is not yet clear.
Taken together, these studies show that exogenous RA disrupts developmental processes that are normally controlled by endogenous RA. As endogenous retinoid signaling is mediated by Hox transcription factors, the interference from exogenous RA is also mediated through this regulatory system. However, while Hox genes mediate some of the effects of RA, there are presumably other transcriptional targets for nuclear receptors of the RAR and RXR families. For the axial skeleton, the genetic experiments provide strong evidence that Hox genes are major mediators for the vertebral variations induced by retinoic acid. Recent experimental evidence from chicken implicating retinoic acid in left-right determination (reviewed by (Brent, 2005) suggests the possibility that this signaling system might be involved in unilateral skeletal abnorlaities. Further experiments are needed to establish whether Hox genes or their upstream regulatory factors are sensitive also to other teratogens that affect the development of the axial skeleton.
E. Conclusions
From the above discussion, the following major conclusions arise:
The vertebrate skeleton develops through a series of steps that successively restrict tissue differentiation potential. Consequently, interference with early steps, through genetic or experimental manipulation, will affect the skeleton and the entire developing embryo more dramatically, resulting in lethality in extreme cases. In turn, the sequence of events suggests that the phases most relevant to human non-lethal skeletal variation are the stages after gastrulation, such as somite patterning and subsequent growth of skeletal elements, in which alterations may be restricted to selected regions of the skeleton.
Different regions of the skeleton appear to be differentially sensitive to perturbations, even within the axial skeleton. This may, in part, reflect the differential expression of particular genes, and their restricted function, in certain compartments of the skeleton. These findings indicate that skeletal variation is intricately linked to the region- and cell-type specific roles of individual gene and of entire regulatory pathways.
Similar molecules and signal transduction pathways are involved in skeletal development at early and at late stages. This may make it difficult to trace particular skeletal variations to unique insults. At the same time, the re-employment of molecules points to the importance of gene regulatory mechanisms in the developing skeleton that need to be coordinated in space, time, and with reference to specific cell types. In addition, as similar molecules are used at multiple times, specificity in regulating the unique development of individual skeletal elements is likely achieved through combinations of principal components and pathways.
Genetic manipulation in mice produces numerous phenotypes that resemble inherited or congenital human skeletal syndromes. In this fashion, molecular mouse genetics approaches provide fundamental insight into the etiology and pathogenesis of human skeletal malformations. Over 50 genes have been identified to date that play a critical role for proper skeleton development, attesting to the power of the comparative genetic approach.
The genetic approaches define the earliest timepoint at which a particular gene product is required developmentally. Thus, the current phenotype descriptions can refer to only one critical temporal phase of expression for each gene. Studies have yet to be conducted that allow to track, at the genetic and molecular level, the response of a given molecular pathway to insults at different times. Experimentally, this could be achieved through systems that enable temporal regulation of gene deletion or activation, as well as through gene expression profiling on a genomic scale.
As similar skeletal variations can be induced by chemical treatment and genetic manipulation, it is tempting to deduce that certain genes or regulatory pathways are the targets of teratogen interference. This has been well demonstrated for retinoic acid, but remains to be confirmed for other drugs and agents. The availability of mouse models now suggests molecular starting points for the characterization of specific teratogenic events in skeletal development.
Mouse genetics also provides a means to identify genetic loci that responsible for teratogen susceptibility. The availability of various genetically manipulated loci now allows assessment of teratogens on sensitized genetic backgrounds. Such investigations will ultimately lead to a better understanding of the contribution of genetic variation to the manifestations of skeletal variations in experimental animals and humans.
Acknowledgments
We thank our laboratory colleagues for their contributions and Dr. J. Michael Salbaum for continued discussion. The help of Dr. G. Basu and Ms. T. Davis with assembling the references, and secretarial assistance by Ms. R. McCaw and Ms. Schrack are gratefully acknowledged.
References
- Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development. 1993;117:1183–1198. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- Akasaka T, Kanno M, Balling R, Mieza MA, Taniguchi M, Koseki H. A role for mel-18, a Polycomb group-related vertebrate gene, during the anteroposterior specification of the axial skeleton. Development. 1996;122:1513–1522. doi: 10.1242/dev.122.5.1513. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Chaboissier MC, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amizuka N, Henderson JE, Hoshi K, Warshawsky H, Ozawa H, Goltzman D, Karaplis AC. Programmed cell death of chondrocytes and aberrant chondrogenesis in mice homozygous for parathyroid hormone-related peptide gene deletion. Endocrinology. 1996;137:5055–5067. doi: 10.1210/endo.137.11.8895380. [DOI] [PubMed] [Google Scholar]
- Amling M, Neff L, Tanaka S, Inoue D, Kuida K, Weir E, Philbrick WM, Broadus AE, Baron R. Bcl-2 lies downstream of parathyroid hormone-related peptide in a signaling pathway that regulates chondrocyte maturation during skeletal development. J Cell Biology. 1997;136:205–213. doi: 10.1083/jcb.136.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ang SL, Rossant J. HNF-3 beta is essential for node and notochord formation in mouse development. Cell. 1994;78:561–574. doi: 10.1016/0092-8674(94)90522-3. [DOI] [PubMed] [Google Scholar]
- Aszodi A, Chan D, Hunziker E, Bateman JF, Fassler R. Collagen II is essential for the removal of the notochord and the formation of intervertebral discs. J Cell Biol. 1998;143:1399–1412. doi: 10.1083/jcb.143.5.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attisano L, Wrana JL, Lopez-Casillas F, Massague J. TGF-beta receptors and actions. Biochim Biophys Acta. 1994;1222:71–80. doi: 10.1016/0167-4889(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Augustine K, Liu ET, Sadler TW. Antisense attenuation of Wnt-1 and Wnt-3a expression in whole embryo culture reveals roles for these genes in craniofacial, spinal cord, and cardiac morphogenesis. Dev Genet. 1993;14:500–520. doi: 10.1002/dvg.1020140611. [DOI] [PubMed] [Google Scholar]
- Aulehla A, Johnson RL. Dynamic expression of lunatic fringe suggests a link between notch signaling and an autonomous cellular oscillator driving somite segmentation. Dev Biol. 1999;207:49–61. doi: 10.1006/dbio.1998.9164. [DOI] [PubMed] [Google Scholar]
- Bagnall KM, Higgins SJ, Sanders EJ. The contribution made by a single somite to the vertebral column: experimental evidence in support of resegmentation using the chick-quail chimaera model. Development. 1988;103:69–85. doi: 10.1242/dev.103.1.69. [DOI] [PubMed] [Google Scholar]
- Bagnall KM, Higgins SJ, Sanders EJ. The contribution made by cells from a single somite to tissues within a body segment and assessment of their integration with similar cells from adjacent segments. Development. 1989;107:931–943. doi: 10.1242/dev.107.4.931. [DOI] [PubMed] [Google Scholar]
- Bai S, Shi X, Yang X, Cao X. Smad6 as a transcriptional corepressor. J Biol Chem. 2000;275:8267–8270. doi: 10.1074/jbc.275.12.8267. [DOI] [PubMed] [Google Scholar]
- Baker J, Liu JP, Robertson EJ, Efstratiadis A. Role of insulin-like growth factors in embryonic and postnatal growth. Cell. 1993;75:73–82. [PubMed] [Google Scholar]
- Balling R, Deutsch U, Gruss P. undulated, a mutation affecting the development of the mouse skeleton, has a point mutation in the paired box of Pax1. Cell. 1988;55:531–535. doi: 10.1016/0092-8674(88)90039-6. [DOI] [PubMed] [Google Scholar]
- Barbieri O, Astigiano S, Morini M, Tavella S, Schito A, Corsi A, Di Martino D, Bianco P, Cancedda R, Garofalo S. Depletion of cartilage collagen fibrils in mice carrying a dominant negative Col2a1 transgene affects chondrocyte differentiation. Am J Physiol Cell Physiol. 2003;285:C1504–1512. doi: 10.1152/ajpcell.00579.2002. [DOI] [PubMed] [Google Scholar]
- Barrantes IB, Elia AJ, Wunsch K, De Angelis MH, Mak TW, Rossant J, Conlon RA, Gossler A, de la Pompa JL. Interaction between Notch signalling and Lunatic fringe during somite boundary formation in the mouse. Curr Biol. 1999;9:470–480. doi: 10.1016/s0960-9822(99)80212-7. [DOI] [PubMed] [Google Scholar]
- Beckers J, Schlautmann N, Gossler A. The mouse rib-vertebrae mutation disrupts anterior-posterior somite patterning and genetically interacts with a delta1 null allele. Mech Dev. 2000;95:35–46. doi: 10.1016/s0925-4773(00)00323-3. [DOI] [PubMed] [Google Scholar]
- Beier F, Leask TA, SH, Chow C, Taylor AC, Lee RJ, Pestell RG, Ballock RT, LuValle P. Cell cycle control genes in chondrocyte proliferation and differentiation. Matrix Biology. 1999;18:109–120. doi: 10.1016/s0945-053x(99)00009-8. [DOI] [PubMed] [Google Scholar]
- Bel S, Core N, Djabali M, Kieboom K, Van der Lugt N, Alkema MJ, Van Lohuizen M. Genetic interactions and dosage effects of Polycomb group genes in mice. Development. 1998;125:3543–3551. doi: 10.1242/dev.125.18.3543. [DOI] [PubMed] [Google Scholar]
- Bel-Vialar S, Core N, Terranova R, Goudot V, Boned A, Djabali M. Altered retinoic acid sensitivity and temporal expression of Hox genes in polycomb-M33 deficient mice. Dev Biol. 2000;224:238–249. doi: 10.1006/dbio.2000.9791. [DOI] [PubMed] [Google Scholar]
- Bellairs R, Curtis AS, Sanders EJ. Cell adhesiveness and embryonic differentiation. J Embryol Exp Morphol. 1978;46:207–213. [PubMed] [Google Scholar]
- Bellairs R. The mechanism of somite segmentation in the chick embryo. J Embryol Exp Morphol. 1979;51:227–243. [PubMed] [Google Scholar]
- Beppu H, Kawabata M, Hamamoto T, Chytil A, Minowa O, Noda T, Miyazono K. BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev Biol. 2000;221:249–258. doi: 10.1006/dbio.2000.9670. [DOI] [PubMed] [Google Scholar]
- Bessho Y, Kageyama R. Oscillations, clocks and segmentation. Curr Opin Genet Dev. 2003;13:379–384. doi: 10.1016/s0959-437x(03)00083-2. [DOI] [PubMed] [Google Scholar]
- Bettenhausen B, Hrabe de Angelis M, Simon D, Guenet JL, Gossler A. Transient and restricted expression during mouse embryogenesis of Dll1, a murine gene closely related to Drosophila Delta. Development. 1995;121:2407–2418. doi: 10.1242/dev.121.8.2407. [DOI] [PubMed] [Google Scholar]
- Bi W, Deng JM, Zhang Z, Behringer RR, DeCrombrugghe B. Sox9 is required for cartilage formation. Nat Genet. 1999;22:85–89. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- Bi W, Huang W, Whitworth DJ, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Haploinsufficiency of Sox9 results in defective cartilage primordia and premature skeletal mineralization. Proc Natl Acad Sci USA. 2001;98:6698–6703. doi: 10.1073/pnas.111092198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitgood MJ, McMahon AP. Hedgehog and Bmp genes are coexpressed at many diverse sites of cell-cell interaction in the mouse embryo. Developmental Biology. 1995;172:126–138. doi: 10.1006/dbio.1995.0010. [DOI] [PubMed] [Google Scholar]
- Blechschmidt E. Die vorgeburtlichen Entwicklungsstadien des Menschen. Basel: Karger; 1961. [Google Scholar]
- Bloom W, Fawcett DW. A textbook of histology. Philadelphia: Saunders; 1968. pp. 223–262. [Google Scholar]
- Blum M, Gaunt SJ, Cho KW, Steinbeisser H, Blumberg B, Bittner D, De Robertis EM. Gastrulation in the mouse: the role of the homeobox gene goosecoid. Cell. 1992;69:1097–1106. doi: 10.1016/0092-8674(92)90632-m. [DOI] [PubMed] [Google Scholar]
- Boersma CJ, Bloemen M, Hendriks JM, van Berkel EA, Olijve W, van Zoelen EJ. Homeobox proteins as signal transduction intermediates in regulation of NCAM expression by recombinant human bone morphogenetic protein-2 in osteoblast-like cells. Mol Cell Biol Res Commun. 1999;1:117–124. doi: 10.1006/mcbr.1999.0115. [DOI] [PubMed] [Google Scholar]
- Boulet AM, Capecchi MR. Targeted Disruption of Hoxc-4 causes Esophageal Defects and Vertebral Transformations. Developmental Biology. 1996;177:232–249. doi: 10.1006/dbio.1996.0159. [DOI] [PubMed] [Google Scholar]
- Boulet AM, Capecchi MR. Duplication of the Hoxd11 gene causes alterations in the axial and appendicular skeleton of the mouse. Dev Biol. 2002;249:96–107. doi: 10.1006/dbio.2002.0755. [DOI] [PubMed] [Google Scholar]
- Brakebusch C, Hirsch E, Potoncnik A, Fassler R. Genetic analysis of beta1 integrin function: confirmed, new and revised roles for a crucial family of cell adhesion molecules. J Cell Sci. 1997;110:2895–2904. doi: 10.1242/jcs.110.23.2895. [DOI] [PubMed] [Google Scholar]
- Brand-Saberi B, Ebensperger C, Wilting J, Balling R, Christ B. The ventralizing effect of the notochord on somite differentiation in chick embryos. Anat Embryol. 1993;188:239–245. doi: 10.1007/BF00188215. [DOI] [PubMed] [Google Scholar]
- Brand-Saberi B, Christ B. Evolution and development of distinct cell lineages derived from somites. Curr Top Dev Biol. 2000;48:1–42. doi: 10.1016/s0070-2153(08)60753-x. [DOI] [PubMed] [Google Scholar]
- Branford WW, Benson GV, Ma L, Maas RL, Potter SS. Characterization of Hoxa10/Hoxa-11 transheterozygotes reveals functional redundancy and regulatory interactions. Dev Biol. 2000;224:373–387. doi: 10.1006/dbio.2000.9809. [DOI] [PubMed] [Google Scholar]
- Brent AE. Somite formation: where left meets right. Curr Biol. 2005;15:R468–470. doi: 10.1016/j.cub.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Brinckerhoff CE. Regulation of metalloproteinase gene expression: implications for osteoarthritis. Crit Rev Eukaryot Gene Expr. 1992;2:145–164. [PubMed] [Google Scholar]
- Brown D, Wagner D, Li X, Richardson JA, Olson EN. Dual role of the basic helix-loop-helix transcription factor scleraxis in mesoderm formation and chondrogenesis during mouse embryogenesis. Development. 1999;126:4317–4329. doi: 10.1242/dev.126.19.4317. [DOI] [PubMed] [Google Scholar]
- Brunet LJ, McMahon JA, McMahon AP, Harland RM. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280:1455–1457. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- Bulman MP, Kusumi K, Frayling TM, McKeown C, Garrett C, Lander ES, Krumlauf R, Hattersley AT, Ellard S, Turnpenny PD. Mutations in the human delta homologue, DLL3, cause axial skeletal defects in spondylocostal dysostosis. Nat Genet. 2000;24:438–441. doi: 10.1038/74307. [DOI] [PubMed] [Google Scholar]
- Burgess R, Cserjesi P, Ligon KL, Olson EN. Paraxis: a basic helix-loop-helix protein expressed in paraxial mesoderm and developing somites. Dev Biol. 1995;168:296–306. doi: 10.1006/dbio.1995.1081. [DOI] [PubMed] [Google Scholar]
- Burke AC, Nowicki JL. A new view of patterning domains in the vertebrate mesoderm. Dev Cell. 2003;4:159–165. doi: 10.1016/s1534-5807(03)00033-9. [DOI] [PubMed] [Google Scholar]
- Byers PH, Steiner RD. Osteogenesis imperfecta. Annu Rev Med. 1992;43:269–282. doi: 10.1146/annurev.me.43.020192.001413. [DOI] [PubMed] [Google Scholar]
- Cancedda R, Descalzi Cancedda F, Castagnola P. Chondrocyte differentiation. Int Rev Cytol. 1995;159:265–358. doi: 10.1016/s0074-7696(08)62109-9. [DOI] [PubMed] [Google Scholar]
- Cancedda R, Castagnola P, Cancedda FD, Dozin B, Quarto R. Developmental control of chondrogenesis and osteogenesis. Int J Dev Biol. 2000;44:707–714. [PubMed] [Google Scholar]
- Candia AF, Hu J, Crosby J, Lalley PA, Noden D, Nadeau JH, Wright CV. Mox-1 and Mox-2 define a novel homeobox gene subfamily and are differentially expressed during early mesodermal patterning in mouse embryos. Development. 1992;116:1123–1136. doi: 10.1242/dev.116.4.1123. [DOI] [PubMed] [Google Scholar]
- Capecchi MR. Function of Homeobox Genes in Skeletal Development. Ann NY Acad Sci. 1996;785:34–37. doi: 10.1111/j.1749-6632.1996.tb56241.x. [DOI] [PubMed] [Google Scholar]
- Carpenter EM, Goddard JM, Chisaka O, Manley NR, Capecchi MR. Loss of hoxa1 (hox-1.6) function results in the reorganization of the murine hindbrain. Development. 1993;118:1063–1075. doi: 10.1242/dev.118.4.1063. [DOI] [PubMed] [Google Scholar]
- Cash DE, Bock CB, Schughart K, Linney E, Underhill TM. Retinoic acid receptor alpha function in vertebrate limb skeletogenesis: a modulator of chondrogenesis. J Cell Biol. 1997;136:445–457. doi: 10.1083/jcb.136.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambon P. The retinoid signaling pathway: molecular and genetic analyses. Semin Cell Biol. 1994;5:115–125. doi: 10.1006/scel.1994.1015. [DOI] [PubMed] [Google Scholar]
- Charite J, de Graaff W, Shen SB, Deschamps J. Ectopic expression of hoxb-8 causes duplication of the zpa in the forelimb and homeotic transformation of axial structures. Cell. 1994;78:589–601. doi: 10.1016/0092-8674(94)90524-x. [DOI] [PubMed] [Google Scholar]
- Chawengsaksophak K, James R, Hammond VE, Kontgen F, Beck F. Homeosis and intestinal tumours in Cdx2 mutant mice. Nature. 1997;386:84–87. doi: 10.1038/386084a0. [DOI] [PubMed] [Google Scholar]
- Chawengsaksophak K, de Graaff W, Rossant J, Deschamps J, Beck F. Cdx2 is essential for axial elongation in mouse development. Proc Natl Acad Sci USA. 2004;101:7641–7645. doi: 10.1073/pnas.0401654101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Capecchi MR. Targeted mutations in hoxa-9 and hoxb-9 reveal synergistic interactions. Dev Biol. 1997;181:186–196. doi: 10.1006/dbio.1996.8440. [DOI] [PubMed] [Google Scholar]
- Chen F, Greer J, Capecchi MR. Analysis of Hoxa7/Hoxb7 mutants suggests periodicity in the generation of the different sets of vertebrae. Mech Dev. 1998;77:49–57. doi: 10.1016/s0925-4773(98)00126-9. [DOI] [PubMed] [Google Scholar]
- Cheney CM, Lash JW. An increase in cell-cell adhesion in the chick segmental plate results in a meristic pattern. J Embryol Exp Morphol. 1984;79:1–10. [PubMed] [Google Scholar]
- Chevallier A. Role of the somitic mesoderm in the development of the rib cage of bird embryos. I. Origin of the sternal component and conditions for the development of the ribs. J Embryol Exp Morphol. 1975;33:291–311. [PubMed] [Google Scholar]
- Chin YE, Kitagawa M, Su WC, You ZH, Iwamoto Y, Fu XY. Cell growth arrest and induction of cyclin-dependent kinase inhibitor p21 WAF1/CIP1 mediated by STAT1. Science. 1996;272:719–722. doi: 10.1126/science.272.5262.719. [DOI] [PubMed] [Google Scholar]
- Chong SW, Jiang YJ. Off limits--integrins holding boundaries in somitogenesis. Trends Cell Biol. 2005;15:453–457. doi: 10.1016/j.tcb.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Christ B, Jacob HJ, Jacob M. Experimental analysis of somitogenesis in the chick embryo. Z Anat Entwicklungsgesch. 1972;138:82–97. [PubMed] [Google Scholar]
- Christ B, Ordahl CP. Early stages of chick somite development. Anat Embryol. 1995;191:381–396. doi: 10.1007/BF00304424. [DOI] [PubMed] [Google Scholar]
- Christ B, Wilting J. From somites to vertebral column. Anat Anz. 1992;174:23–32. doi: 10.1016/s0940-9602(11)80337-7. [DOI] [PubMed] [Google Scholar]
- Chung UI, Lanske B, Lee K, Li E, Kronenberg H. The parathyroid hormone/parathyroid hormone-related peptide receptor coordinates endochondral bone development by directly controlling chondrocyte differentiation. Proc Natl Acad Sci USA. 1998;95:13030–13035. doi: 10.1073/pnas.95.22.13030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobrinik D, Lee MH, Hannon G, Mulligan G, Bronson RT, Dyson N, Harlow E, Beach D, Weinberg RA, Jacks T. Shared role of the pRB-related p130 and p107 proteins in limb development. Genes Dev. 1996;10:1633–1644. doi: 10.1101/gad.10.13.1633. [DOI] [PubMed] [Google Scholar]
- Coffin JD, Florkiewicz RZ, Neumann J, Mort-Hopkins T, Dorn GW, Lightfoot P, German R, Howles PN, Kier A, O’Toole BA, et al. Abnormal bone growth and selective translational regulation in basic fibroblast growth factor (FGF-2) transgenic mice. Mol Biol Cell. 1995;6:1861–1873. doi: 10.1091/mbc.6.12.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SE, Levorse JM, Tilghman SM, Vogt TF. Clock regulatory elements control cyclic expression of Lunatic fringe during somitogenesis. Dev Cell. 2002;3:75–84. doi: 10.1016/s1534-5807(02)00212-5. [DOI] [PubMed] [Google Scholar]
- Condie BG, Capecchi MR. Mice homozygous for a targeted disruption of hoxd-3 (hox-4.1) exhibit anterior transformations of the first and second cervical vertebrae, the atlas and the axis. Development. 1993;119:579–595. doi: 10.1242/dev.119.3.579. [DOI] [PubMed] [Google Scholar]
- Condie BG, Capecchi MR. Mice with targeted disruptions in the paralogous genes hoxa-3 and hoxd-3 reveal synergistic interactions. Nature. 1994;370:304–307. doi: 10.1038/370304a0. [DOI] [PubMed] [Google Scholar]
- Conlon RA, Reaume AG, Rossant J. Notch1 is required for the coordinate segmentation of somites. Development. 1995;121:1533–1545. doi: 10.1242/dev.121.5.1533. [DOI] [PubMed] [Google Scholar]
- Couly GF, Le Douarin NM. Mapping of the early neural primordium in quail-chick chimeras. I. Developmental relationships between placodes, facial ectoderm, and prosencephalon. Dev Biol. 1985;110:422–439. doi: 10.1016/0012-1606(85)90101-0. [DOI] [PubMed] [Google Scholar]
- Couly GF, Le Douarin NM. Mapping of the early neural primordium in quail-chick chimeras. II. The prosencephalic neural plate and neural folds: implications for the genesis of cephalic human congenital abnormalities. Dev Biol. 1987;120:198–214. doi: 10.1016/0012-1606(87)90118-7. [DOI] [PubMed] [Google Scholar]
- Couly GF, Coltey PM, Le Douarin NM. The developmental fate of the cephalic mesoderm in quail-chick chimeras. Development. 1992;114:1–15. doi: 10.1242/dev.114.1.1. [DOI] [PubMed] [Google Scholar]
- Cremer H, Lange R, Christoph A, Plomann M, Vopper G, Roes J, Brown R, Baldwin S, Kraemer P, Scheff S, et al. Inactivation of the N-CAM gene in mice results in size reduction of the olfactory bulb and deficits in spatial learning. Nature. 1994;367:455–459. doi: 10.1038/367455a0. [DOI] [PubMed] [Google Scholar]
- Crowe R, Zikherman J, Niswander L. Delta-1 negatively regulates the transition from prehypertrophic to hypertrophic chondrocytes during cartilage formation. Development. 1999;126:987–998. doi: 10.1242/dev.126.5.987. [DOI] [PubMed] [Google Scholar]
- Cserjesi P, Brown D, Ligon KL, Lyons GE, Copeland NG, Gilbert DJ, Jenkins NA, Olson EN. Scleraxis: a basic helix-loop-helix protein that prefigures skeletal formation during mouse embryogenesis. Development. 1995;121:1099–1110. doi: 10.1242/dev.121.4.1099. [DOI] [PubMed] [Google Scholar]
- Dale KJ, Pourquie O. A clock-work somite. Bioessays. 2000;22:72–83. doi: 10.1002/(SICI)1521-1878(200001)22:1<72::AID-BIES12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Davis AP, Capecchi MR. Axial homeosis and appendicular skeleton defects in mice with a targeted disruption of hoxd-11. Development. 1994;120:2187–2198. doi: 10.1242/dev.120.8.2187. [DOI] [PubMed] [Google Scholar]
- Davis AP, Witte DP, Hsieh-Li HM, Potter SS, Capecchi MR. Absence of radius and ulna in mice lacking hoxa-11 and hoxd-11. Nature. 1995;375:791–795. doi: 10.1038/375791a0. [DOI] [PubMed] [Google Scholar]
- Davis AP, Capecchi MR. A mutational analysis of the 5′ HoxD genes: dissection of genetic interactions during limb development in the mouse. Development. 1996;122:1175–1185. doi: 10.1242/dev.122.4.1175. [DOI] [PubMed] [Google Scholar]
- de Arcangelis A, Georges-Labouesse E. Integrin and ECM functions: roles in vertebrate development. Trends Genet. 2000;16:389–395. doi: 10.1016/s0168-9525(00)02074-6. [DOI] [PubMed] [Google Scholar]
- de Crombrugghe B, Katzenstein P, Mukhopadhyay K, Lefebvre V, Zhou G, Garofalo S, Metsaranta M, Rosati R, Vuorio E. Transgenic mice with deficiencies in cartilage collagens: possible models for gene therapy. J Rheumatol -Suppl. 1995;43:140–142. [PubMed] [Google Scholar]
- de Crombrugghe B, Lefebvre V, Behringer RR, Bi W, Murakami S, Huang W. Transcriptional mechanisms of chondrocyte differentiation. Matrix Biol. 2000;19:389–394. doi: 10.1016/s0945-053x(00)00094-9. [DOI] [PubMed] [Google Scholar]
- Debeer P, Bacchelli C, Scambler PJ, De Smet L, Fryns JP, Goodman FR. Severe digital abnormalities in a patient heterozygous for both a novel missense mutation in HOXD13 and a polyalanine tract expansion in HOXA13. J Med Genet. 2002;39:852–856. doi: 10.1136/jmg.39.11.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca F, Baron J. Control of Bone Growth by Fibroblast Growth Factors. Trends in Endocrinology and Metabolism. 1999;10:61–65. doi: 10.1016/s1043-2760(98)00120-9. [DOI] [PubMed] [Google Scholar]
- Deng CX, Wynshaw-Boris A, Shen MM, Daugherty C, Ornitz DM, Leder P. Murine FGFR-1 is required for early postimplantation growth and axial organization. Genes Dev. 1994;8:3045–3057. doi: 10.1101/gad.8.24.3045. [DOI] [PubMed] [Google Scholar]
- Dequeant ML, Glynn E, Gaudenz K, Wahl M, Chen J, Mushegian A, Pourquie O. A complex oscillating network of signaling genes underlies the mouse segmentation clock. Science. 2006;314:1595–1598. doi: 10.1126/science.1133141. [DOI] [PubMed] [Google Scholar]
- Deschamps J, Meijlink F. Mammalian homeobox genes in normal development and neoplasia. Crit Rev Oncogen. 1992;3:117–173. [PubMed] [Google Scholar]
- Deutsch U, Dressler GR, Gruss P. Pax 1, a member of a paired box homologous murine gene family, is expressed in segmented structures during development. Cell. 1988;53:617–625. doi: 10.1016/0092-8674(88)90577-6. [DOI] [PubMed] [Google Scholar]
- Dickman ED, Rogers R, Conway SJ. Abnormal skeletogenesis occurs coincident with increased apoptosis in the Splotch (Sp2H) mutant: putative roles for Pax3 and PDGFRalpha in rib patterning. Anat Rec. 1999;255:353–361. doi: 10.1002/(SICI)1097-0185(19990701)255:3<353::AID-AR11>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Dietrich S, Schubert FR, Gruss P. Altered Pax gene expression in murine notochord mutants: the notochord is required to initiate and maintain ventral identity in the somite. Mech Dev. 1993;44:189–207. doi: 10.1016/0925-4773(93)90067-8. [DOI] [PubMed] [Google Scholar]
- Dietrich S, Gruss P. undulated phenotypes suggest a role of Pax-1 for the development of vertebral and extravertebral structures. Dev Biol. 1995;167:529–548. doi: 10.1006/dbio.1995.1047. [DOI] [PubMed] [Google Scholar]
- Dolle P, Dierich A, LeMeur M, Schimmang T, Schuhbaur B, Chambon P, Duboule D. Disruption of the Hoxd-13 gene induces localized heterochrony leading to mice with neotenic limbs. Cell. 1993;75:431–441. doi: 10.1016/0092-8674(93)90378-4. [DOI] [PubMed] [Google Scholar]
- Donoviel DB, Hadjantonakis AK, Ikeda M, Zheng H, Hyslop PS, Bernstein A. Mice lacking both presenilin genes exhibit early embryonic patterning defects. Genes Dev. 1999;13:2801–2810. doi: 10.1101/gad.13.21.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CJ, Little CD. Integrins play an essential role in somite adhesion to the embryonic axis. Dev Biol. 1991;143:418–421. doi: 10.1016/0012-1606(91)90092-h. [DOI] [PubMed] [Google Scholar]
- Duband JL, Dufour S, Hatta K, Takeichi M, Edelman GM, Thiery JP. Adhesion molecules during somitogenesis in the avian embryo. J Cell Biol. 1987;104:1361–1374. doi: 10.1083/jcb.104.5.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duboule D. Vertebrate Hox-genes and proliferation: an alternative pathway to homeosis? Curr Op Genet Dev. 1995;5:525–528. doi: 10.1016/0959-437x(95)90058-o. [DOI] [PubMed] [Google Scholar]
- Dubrulle J, Pourquie O. Coupling segmentation to axis formation. Development. 2004;131:5783–5793. doi: 10.1242/dev.01519. [DOI] [PubMed] [Google Scholar]
- Ducy P, Karsenty G. Genetic control of cell differentiation in the skeleton. Curr Op Cell Biol. 1998;10:614–619. doi: 10.1016/s0955-0674(98)80037-9. [DOI] [PubMed] [Google Scholar]
- Dunn NR, Winnier GE, Hargett LK, Schrick JJ, Fogo AB, Hogan BL. Haploinsufficient phenotypes in Bmp4 heterozygous null mice and modification by mutations in Gli3 and Alx4. Dev Biol. 1997;188:235–247. doi: 10.1006/dbio.1997.8664. [DOI] [PubMed] [Google Scholar]
- Dupe V, Davenne M, Brocard J, Dolle P, Mark M, Dierich A, Chambon P, Rijli FM. In vivo functional analysis of the Hoxa-1 3′ retinoic acid response element (3′RARE) Development. 1997;124:399–410. doi: 10.1242/dev.124.2.399. [DOI] [PubMed] [Google Scholar]
- Ebensperger C, Wilting J, Brand-Saberi B, Mizutani Y, Christ B, Balling R, Koseki H. Pax-1, a regulator of sclerotome development is induced by notochord and floor plate signals in avian embryos. Anat Embryol. 1995;191:297–310. doi: 10.1007/BF00534682. [DOI] [PubMed] [Google Scholar]
- Eisman JA. Genetics of osteoporosis. Endocr Rev. 1999;20:788–804. doi: 10.1210/edrv.20.6.0384. [DOI] [PubMed] [Google Scholar]
- Enomoto-Iwamoto M, Iwamoto M, Mukudai Y, Kawakami Y, Nohno T, Higuchi Y, Takemoto S, Ohuchi H, Noji S, Kurisu K. Bone morphogenetic protein signaling is required for maintenance of differentiated phenotype, control of proliferation, and hypertrophy in chondrocytes. J Cell Biol. 1998;140:409–418. doi: 10.1083/jcb.140.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein M, Pillemer G, Yelin R, Yisraeli JK, Fainsod A. Patterning of the embryo along the anterior-posterior axis: the role of the caudal genes. Development. 1997;124:3805–3814. doi: 10.1242/dev.124.19.3805. [DOI] [PubMed] [Google Scholar]
- Erlebacher A, Filvaroff EH, Gitelman SE, Derynck R. Toward a molecular understanding of skeletal development. Cell. 1995;80:371–378. doi: 10.1016/0092-8674(95)90487-5. [DOI] [PubMed] [Google Scholar]
- Fan CM, Tessier-Lavigne M. Patterning of mammalian somites by surface ectoderm and notochord: evidence for sclerotome induction by a hedgehog homolog. Cell. 1994;79:1175–1186. doi: 10.1016/0092-8674(94)90009-4. [DOI] [PubMed] [Google Scholar]
- Fan CM, Porter JA, Chiang C, Chang DT, Beachy PA, Tessier-Lavigne M. Long-range sclerotome induction by sonic hedgehog: direct role of the amino-terminal cleavage product and modulation by the cyclic AMP signaling pathway. Cell. 1995;81:457–465. doi: 10.1016/0092-8674(95)90398-4. [DOI] [PubMed] [Google Scholar]
- Faust C, Magnuson T. Genetic control of gastrulation in the mouse. Curr Opin Genet Dev. 1993;3:491–498. doi: 10.1016/0959-437x(93)90125-9. [DOI] [PubMed] [Google Scholar]
- Fassler R, Meyer M. Consequences of lack of beta 1 integrin gene expression in mice. Genes Dev. 1995;9:1896–908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- Favier B, LeMeur M, Chambon P, Dolle P. Axial skeleton homeosis and forelimb malformations in Hoxd-11 mutant mice. Proc Natl Acad Sci USA. 1995;92:310–314. doi: 10.1073/pnas.92.1.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favier B, Rijli FM, Fromental-Ramain C, Fraulob V, Chambon P, Dolle P. Functional cooperation between the non-paralogous genes Hoxa-10 and Hoxd11 in the developing forelimb and axial skeleton. Development. 1996;122:449–460. doi: 10.1242/dev.122.2.449. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Lichtler AC, Pan ZZ, Dealy CN, Upholt WB, Kosher RA. Ectopic expression of Msx-2 in posterior limb bud mesoderm impairs limb morphogenesis while inducing BMP-4 expression, inhibiting cell proliferation, and promoting apoptosis. Developmental Biology. 1998;197:12–24. doi: 10.1006/dbio.1998.8880. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Sumoy L, Gannon J, Sun H, Brown AM, Upholt WB, Kosher RA. The expression pattern of the Distal-less homeobox-containing gene Dlx-5 in the developing chick limb bud suggests its involvement in apical ectodermal ridge activity, pattern formation, and cartilage differentiation. Mechanisms of Development. 1995;52:257–264. doi: 10.1016/0925-4773(95)98113-o. [DOI] [PubMed] [Google Scholar]
- Franceschi RT. The developmental control of osteoblast-specific gene expression: role of specific transcription factors and the extracellular matrix environment. Crit Rev Oral Biol Med. 1999;10:40–57. doi: 10.1177/10454411990100010201. [DOI] [PubMed] [Google Scholar]
- Francomano CA, McIntosh I, Wilkin DJ. Bone dysplasias in man: molecular insights. Curr Op Genet Dev. 1996;6:301–308. doi: 10.1016/s0959-437x(96)80006-2. [DOI] [PubMed] [Google Scholar]
- Frisen L, Lagerstedt K, Tapper-Persson M, Kockum I, Nordenskjold A. A novel duplication in the HOXA13 gene in a family with atypical hand-foot-genital syndrome. J Med Genet. 2003;40:e49. doi: 10.1136/jmg.40.4.e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromental-Ramain C, Warot X, Lakkaraju S, Favier B, Haack H, Birling C, Dierich A, Dolle P, Chambon P. Specific and redundant functions of the paralogous Hoxa-9 and Hoxd-9 genes in forelimb and axial skeleton patterning. Development. 1996a;122:461–472. doi: 10.1242/dev.122.2.461. [DOI] [PubMed] [Google Scholar]
- Fromental-Ramain C, Warot X, Messadecq N, LeMeur M, Dolle P, Chambon P. Hoxa-13 and Hoxd-13 play a crucial role in the patterning of the limb autopod. Development. 1996b;122:2997–3011. doi: 10.1242/dev.122.10.2997. [DOI] [PubMed] [Google Scholar]
- Garofalo S, Metsaranta M, Ellard J, Smith C, Horton W, Vuorio E, de Crombrugghe B. Assembly of cartilage collagen fibrils is disrupted by overexpression of normal type II collagen in transgenic mice. Proc Natl Acad Sci USA. 1993;90:38253829. doi: 10.1073/pnas.90.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Qian YQ, Billeter M, Furukubo-Tokunaga K, Schier AF, Resendez-Perez D, Affolter M, Otting G, Wüthrich K. Homeodomain-DNA Recognition. Cell. 1994;78:211–223. doi: 10.1016/0092-8674(94)90292-5. [DOI] [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Gilbert SF. Developmental Biology. 4. Sunderland, MA: Sinauer Associates; 1994. [Google Scholar]
- Gimsrud CD, Romano PR, D’Souza M, Puzas JE, Reynolds PR, Rosier RN, O’Keefe RJ. BMP-6 is an autocrine stimulator of chondrocyte differentiation. J Bone Miner Res. 1999;14:475–482. doi: 10.1359/jbmr.1999.14.4.475. [DOI] [PubMed] [Google Scholar]
- Givol D, Yayon A. Complexity of FGF receptors: genetic basis for structural diversity and functional specificity. Faseb J. 1992;6:3362–3369. [PubMed] [Google Scholar]
- Gluecksohn-Schoenheimer S. The development of normal and homozygous Bachyury (T/T) mouse embryos in the extraembryonic coelom of the chick. Proc Natl Acad Sci USA. 1944;30:134–140. doi: 10.1073/pnas.30.6.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff DJ, Tabin CJ. Analysis of Hoxd-13 and Hoxd-11 misexprssion in chick limb buds reveals that Hox genes affect both bone condensation and growth. Development. 1997;124:627–636. doi: 10.1242/dev.124.3.627. [DOI] [PubMed] [Google Scholar]
- Goh KL, Yang JT, Hynes RO. Mesodermal defects and cranial neural crest apoptosis in alpha5 integrin-null embryos. Development. 1997;124:4309–4319. doi: 10.1242/dev.124.21.4309. [DOI] [PubMed] [Google Scholar]
- Goodman FR, Mundlos S, Muragaki Y, Donnai D, Giovannucci-Uzielli ML, Lapi E, Majewski F, McGaughran J, McKeown C, Reardon W, Upton J, Winter RM, Olsen BR, Scambler PJ. Synpolydactyly phenotypes correlate with size of expansions in HOXD13 polyalanine tract. Proc Natl Acad Sci USA. 1997;94:7458–7463. doi: 10.1073/pnas.94.14.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould A, Itasaki N, Krumlauf R. Initiation of rhombomeric Hoxb4 expression requires induction by somites and a retinoid pathway. Neuron. 1998;21:39–51. doi: 10.1016/s0896-6273(00)80513-9. [DOI] [PubMed] [Google Scholar]
- Goulding M, Sterrer S, Fleming J, Balling R, Nadeau J, Moore KJ, Brown SD, Steel KP, Gruss P. Analysis of the Pax-3 gene in the mouse mutant splotch. Genomics. 1993;17:355–363. doi: 10.1006/geno.1993.1332. [DOI] [PubMed] [Google Scholar]
- Goulding M, Lumsden A, Paquette AJ. Regulation of Pax-3 expression in the dermomyotome and its role in muscle development. Development. 1994;120:957–971. doi: 10.1242/dev.120.4.957. [DOI] [PubMed] [Google Scholar]
- Goumans MJ, Mummery C. Functional analysis of the TGFbeta receptor/Smad pathway through gene ablation in mice. Int J Dev Biol. 2000;44:253–265. [PubMed] [Google Scholar]
- Greenwald I, Rubin GM. Making a difference: the role of cell-cell interactions in establishing separate identities for equivalent cells. Cell. 1992;68:271–281. doi: 10.1016/0092-8674(92)90470-w. [DOI] [PubMed] [Google Scholar]
- Grigoriadis AE, Wang ZQ, Cecchini MG, Hofstetter W, Felix R, Fleisch HA, Wagner EF. c-Fos: a key regulator of osteoclast-macrophage lineage determination and bone remodeling. Science. 1994;266:443–448. doi: 10.1126/science.7939685. [DOI] [PubMed] [Google Scholar]
- Gruss P, Walther C. Pax in development. Cell. 1992;69:719–722. doi: 10.1016/0092-8674(92)90281-g. [DOI] [PubMed] [Google Scholar]
- Grüneberg H. The pathology of development. Oxford: Blackwell; 1963. [Google Scholar]
- Hald HJ, Danz B, Schwab R, Burmeister K, Bähren W. Röntgenologisch nachweisbare Wirbelsäulenveränderungen asymptomatischer junger Männer. Fortschr Röntgenstr. 1995;163:4–8. doi: 10.1055/s-2007-1015936. [DOI] [PubMed] [Google Scholar]
- Hall BK. Earliest evidence of cartilage and bone development in embryonic life. Clin Orthop. 1987;225:255–272. [PubMed] [Google Scholar]
- Hall BK, Miyake T. The membranous skeleton: the role of cell condensations in vertebrate skeletogenesis. Anat Embryol. 1992;186:107–124. doi: 10.1007/BF00174948. [DOI] [PubMed] [Google Scholar]
- Hall BK, Miyake T. Divide, accumulate, differentiate: cell condensation in skeletal development revisited. Int J Dev Biol. 1995;39:881–893. [PubMed] [Google Scholar]
- Hall BK. A role for epithelial-mesenchymal interactions in tail growth/morphogenesis and chondrogenesis in embryonic mice. Cells Tissues Organs. 2000;166:6–14. doi: 10.1159/000016703. [DOI] [PubMed] [Google Scholar]
- Hall BK, Miyake T. All for one and one for all: condensations and the initiation of skeletal development. Bioessays. 2000;22:138–147. doi: 10.1002/(SICI)1521-1878(200002)22:2<138::AID-BIES5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Hamada Y, Kadokawa Y, Okabe M, Ikawa M, Coleman JR, Tsujimoto Y. Mutation in ankyrin repeats of the mouse Notch2 gene induces early embryonic lethality. Development. 1999;126:3415–3424. doi: 10.1242/dev.126.15.3415. [DOI] [PubMed] [Google Scholar]
- Hanson RD, Hess JL, Yu BD, Ernst P, van Lohuizen M, Berns A, van der Lugt NM, Shashikant CS, Ruddle FH, Seto M, Korsmeyer SJ. Mammalian Trithorax and polycomb-group homologues are antagonistic regulators of homeotic development. Proc Natl Acad Sci USA. 1999;96:14372–14377. doi: 10.1073/pnas.96.25.14372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastbacka J, de la Chapelle A, Mahtani MM, Clines G, Reeve-Daly MP, Daly M, Hamilton BA, Kusumi K, Trivedi B, Weaver A, et al. The diastrophic dysplasia gene encodes a novel sulfate transporter: positional cloning by fine-structure linkage disequilibrium mapping. Cell. 1994;78:1073–1087. doi: 10.1016/0092-8674(94)90281-x. [DOI] [PubMed] [Google Scholar]
- Heino J. The collagen receptor integrins have distinct ligand recognition and signaling functions. Matrix Biol. 2000;19:319–323. doi: 10.1016/s0945-053x(00)00076-7. [DOI] [PubMed] [Google Scholar]
- Herrmann BG, Labeit S, Poustka A, King TR, Lehrach H. Cloning of the T gene required in mesoderm formation in the mouse. Nature. 1990;343:617–622. doi: 10.1038/343617a0. [DOI] [PubMed] [Google Scholar]
- Herrmann BG. Expression pattern of the Brachyury gene in whole-mount TWis/TWis mutant embryos. Development. 1991;113:913–917. doi: 10.1242/dev.113.3.913. [DOI] [PubMed] [Google Scholar]
- Herrmann BG, Kispert A. The T genes in embryogenesis. Trends Genet. 1994;10:280–286. doi: 10.1016/0168-9525(90)90011-t. [DOI] [PubMed] [Google Scholar]
- Hickok NJ, Haas AR, Tuan RS. Regulation of chondrocyte differentiation and maturation. Microsc Res Tech. 1998;43:174–190. doi: 10.1002/(SICI)1097-0029(19981015)43:2<174::AID-JEMT9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Hiltunen A, Metsaranta M, Virolainen P, Aro HT, Vuorio E. Retarded chondrogenesis in transgenic mice with a type II collagen defect results in fracture healing abnormalities. Dev Dyn. 1994;200:340–349. doi: 10.1002/aja.1002000409. [DOI] [PubMed] [Google Scholar]
- Hodgkinson CA, Moore KJ, Nakayama A, Steingrimsson E, Copeland NG, Jenkins NA, Arnheiter H. Mutations at the mouse microphthalmia locus are associated with defects in a gene encoding a novel basic-helix-loop-helix-zipper protein. Cell. 1993;74:395–404. doi: 10.1016/0092-8674(93)90429-t. [DOI] [PubMed] [Google Scholar]
- Hoffmann HM, Beumer TL, Rahman S, McCabe LR, Banerjee C, Aslam F, Tiro JA, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Bone tissue-specific transcription of the osteocalcin gene: role of an activator osteoblast-specific complex and suppressor hox proteins that bind the OC box. J Cell Biochem. 1996;61:310–324. doi: 10.1002/(sici)1097-4644(19960501)61:2<310::aid-jcb14>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Constantini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. 2. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1994. [Google Scholar]
- Hogan BL. Bone morphogenetic proteins in development. Current Opinion in Genetics & Development. 1996;6:432–438. doi: 10.1016/s0959-437x(96)80064-5. [DOI] [PubMed] [Google Scholar]
- Holley SA, Geisler R, Nüsslein-Volhard C. Control of her1 expression during zebrafish somitogenesis by a delta-dependent oscillator and an independent wave-front activity. Genes Dev. 2000;14:1678–1690. [PMC free article] [PubMed] [Google Scholar]
- Holley SA, Julich D, Rauch GJ, Geisler R, Nüsslein-Volhard C. her1 and the notch pathway function within the oscillator mechanism that regulates zebrafish somitogenesis. Development. 2002;129:1175–1183. doi: 10.1242/dev.129.5.1175. [DOI] [PubMed] [Google Scholar]
- Horan GS, Wu K, Wolgemuth DJ, Behringer RR. Homeotic transformation of cervical vertebrae in Hoxa-4 mutant mice. Proc Natl Acad Sci USA. 1994;91:1264412648. doi: 10.1073/pnas.91.26.12644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan GS, Kovacs EN, Behringer RR, Featherstone MS. Mutations in paralogous Hox genes result in overlapping homeotic transformations of the axial skeleton: evidence for unique and redundant function. Dev Biol. 1995a;169:359–372. doi: 10.1006/dbio.1995.1150. [DOI] [PubMed] [Google Scholar]
- Horan GS, Ramirez-Solis R, Featherstone MS, Wolgemuth DJ, Bradley A, Behringer RR. Compound mutants for the paralogous hoxa-4, hoxb-4, and hoxd-4 genes show more complete homeotic transformations and a dose-dependent increase in the number of vertebrae transformed. Genes Dev. 1995b;9:1667–1677. doi: 10.1101/gad.9.13.1667. [DOI] [PubMed] [Google Scholar]
- Huang R, Zhi Q, Wilting J, Christ B. The fate of somitocoele cells in avian embryos. Anat Embryol. 1994;190:243–250. doi: 10.1007/BF00234302. [DOI] [PubMed] [Google Scholar]
- Huang R, Zhi Q, Brand-Saberi B, Christ B. New experimental evidence for somite resegmentation. Anat Embryol. 2000;202:195–200. doi: 10.1007/s004290000110. [DOI] [PubMed] [Google Scholar]
- Hughes SL, Dunlop D. The prevalence and impact of arthritis in older persons. Arth Care Res. 1995;8:257–264. doi: 10.1002/art.1790080409. [DOI] [PubMed] [Google Scholar]
- Iimura T, Oida S, Takeda K, Maruoka Y, Sasaki S. Changes In Homeobox-Containing Gene Expression During Ectopic Bone Formation Induced By Bone Morphogenetic Protein. Biochem Biophy Res Comm. 1994;201:980–987. doi: 10.1006/bbrc.1994.1798. [DOI] [PubMed] [Google Scholar]
- Inada M, Yasui T, Nomura S, Miyake S, Deguchi K, Himeno M, Sato M, Yamagiwa H, Kimura T, Yasui N, Ochi T, Endo N, Kitamura Y, Kishimoto T, Komori T. Maturational disturbance of chondrocytes in Cbfa1-deficient mice. Dev Dynamics. 1999;214:279–290. doi: 10.1002/(SICI)1097-0177(199904)214:4<279::AID-AJA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Ingram JL, Stodgell CJ, Hyman SL, Figlewicz DA, Weitkamp LR, Rodier PM. Discovery of allelic variants of HOXA1 and HOXB1: genetic susceptibility to autism spectrum disorders. Teratology. 2000;62:393–405. doi: 10.1002/1096-9926(200012)62:6<393::AID-TERA6>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Innis JW, Goodman FR, Bacchelli C, Williams TM, Mortlock DP, Sateesh P, Scambler PJ, McKinnon W, Guttmacher AE. A HOXA13 allele with a missense mutation in the homeobox and a dinucleotide deletion in the promoter underlies Guttmacher syndrome. Hum Mutat. 2002;19:573–574. doi: 10.1002/humu.9036. [DOI] [PubMed] [Google Scholar]
- Iulianella A, Lohnes D. Contribution of retinoic acid receptor gamma to retinoid-induced craniofacial and axial defects. Dev Dyn. 1997;209:92–104. doi: 10.1002/(SICI)1097-0177(199705)209:1<92::AID-AJA9>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Iwata T, Chen L, Li C, Ovchinnikov DA, Behringer RR, Francomano CA, Deng CX. A neonatal lethal mutation in FGFR3 uncouples proliferation and differentiation of growth plate chondrocytes in embryos. Hum Mol Genet. 2000;9:16031613. doi: 10.1093/hmg/9.11.1603. [DOI] [PubMed] [Google Scholar]
- Jabs EW, Li X, Scott AF, Meyers G, Chen W, Eccles M, Mao JI, Charnas LR, Jackson CE, Jaye M. Jackson-Weiss and Crouzon syndromes are allelic with mutations in fibroblast growth factor receptor 2. Nat Genet. 1994;8:275–279. doi: 10.1038/ng1194-275. [DOI] [PubMed] [Google Scholar]
- Jacenko O, Olsen BR, Warman ML. Of mice and men: heritable skeletal disorders [editorial] Am J Hum Genet. 1994;54:163–168. [PMC free article] [PubMed] [Google Scholar]
- Jacob M, Jacob JH, Christ B. The early differentiation of the perinotochordal connective tissue. A scanning and transmission electron microscopic study on chick embryos. Experientia. 1975;31:1083–1086. doi: 10.1007/BF02326973. [DOI] [PubMed] [Google Scholar]
- Jeanotte L, Lemieux M, Charron J, Poirier F, Robertson EJ. Specification of axial identity in the mouse: role of the Hoxa-5 (Hox1.3) gene. Genes Dev. 1993;7:20852096. doi: 10.1101/gad.7.11.2085. [DOI] [PubMed] [Google Scholar]
- Jegalian BG, DeRobertis EM. Homeotic transformations in the mouse induced by overexpression of a human Hox3.3 transgene. Cell. 1992;71:901–910. doi: 10.1016/0092-8674(92)90387-r. [DOI] [PubMed] [Google Scholar]
- Jena N, Martin-Seisdedos C, McCue P, Croce CM. BMP7 null mutation inmice: developmental defects in skeleton, kidney and eye. Exp Cell Res. 1997;230:28–37. doi: 10.1006/excr.1996.3411. [DOI] [PubMed] [Google Scholar]
- Johnson D, Kan SH, Oldridge M, Trembath RC, Roche P, Esnouf RM, Giele H, Wilkie AO. Missense mutations in the homeodomain of HOXD13 are associated with brachydactyly types D and E. Am J Hum Genet. 2003;72:984–997. doi: 10.1086/374721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson RL, Laufer E, Riddle RD, Tabin C. Ectopic expression of Sonic hedgehog alters dorsal-ventral patterning of somites. Cell. 1994;79:1165–1173. doi: 10.1016/0092-8674(94)90008-6. [DOI] [PubMed] [Google Scholar]
- Jostes B, Walther C, Gruss P. The murine paired box gene, Pax7, is expressed specifically during the development of the nervous and muscular system. Mech Dev. 1990;33:27–37. doi: 10.1016/0925-4773(90)90132-6. [DOI] [PubMed] [Google Scholar]
- Joyner AL. Gene targeting: A Practical Appraoch. Oxford: Oxford University Press; 1993. [Google Scholar]
- Juchau MR. Chemical teratogenesis. Prog Drug Res. 1993;41:9–50. doi: 10.1007/978-3-0348-7150-1_3. [DOI] [PubMed] [Google Scholar]
- Juliano RL, Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993;120:577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jüppner H. Role of parathyroid hormone-related peptide and Indian hedgehog in skeletal development. Pediatr Nephrol. 2000;14:606–611. doi: 10.1007/s004670000343. [DOI] [PubMed] [Google Scholar]
- Kameda T, Watanabe H, Iba H. C-Jun and JunD suppress maturation of chondrocytes. Cell Growth Differ. 1997;8:495–503. [PubMed] [Google Scholar]
- Kameda T, Koike C, Saitoh K, Kuroiwa A, Iba H. Developmental patterning in chondrocyte cultures by morphogenetic gradients: BMP indeces expression of Indian hedgehog and noggin. Genes Cells. 1999;4:175–184. doi: 10.1046/j.1365-2443.1999.00250.x. [DOI] [PubMed] [Google Scholar]
- Kan SH, Johnson D, Giele H, Wilkie AO. An acceptor splice site mutation in HOXD13 results in variable hand, but consistent foot malformations. Am J Med Genet A. 2003;121:69–74. doi: 10.1002/ajmg.a.20103. [DOI] [PubMed] [Google Scholar]
- Kappen C, Ruddle FH. Evolution of a regulatory gene family: HOM/HOX genes. Curr Op Genet Dev. 1993;3:931–938. doi: 10.1016/0959-437x(93)90016-i. [DOI] [PubMed] [Google Scholar]
- Kappen C. Early and late functions of homeobox genes in the development of the axial skeleton. In: Buckwalter JAMGE, Sandell LJ, Trippel SB, editors. Skeletal Growth and Development: Clinical Issues and Basic Science Advances. Rosemont, IL: American Academy of Orthopedic Surgeons; 1998. pp. 147–162. [Google Scholar]
- Kappen C. Disruption of the homeobox gene Hoxb-6 results in increased numbers of early erythrocyte progenitors. Am J Hematol. 2000;65:111–118. doi: 10.1002/1096-8652(200010)65:2<111::aid-ajh4>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Kappen C, Mello MA, Finnell RH, Salbaum JM. Folate modulates cartilage defects in Hoxd-4 transgenic mice. Genesis. 2004;39:115–166. doi: 10.1002/gene.20036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaplis AC, Luz A, Glowacki J, Bronson RT, Tybulewicz VL, Kronenberg HM, Mulligan RC. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 1994;8:277–289. doi: 10.1101/gad.8.3.277. [DOI] [PubMed] [Google Scholar]
- Karaplis AC, Kronenberg HM. Physiological roles for parathyroid hormone-related protein: lessons from gene knockout mice. Vit Horm. 1996;52:177–193. doi: 10.1016/s0083-6729(08)60411-2. [DOI] [PubMed] [Google Scholar]
- Karaplis AC, He B, Nguyen MT, Young ID, Semeraro D, Ozawa H, Amizuka N. Inactivating mutation in the human parathyroid hormone receptor type 1 gene in Blomstrand chondrodysplasia. Endocrinology. 1998;139:5255–5258. doi: 10.1210/endo.139.12.6522. [DOI] [PubMed] [Google Scholar]
- Karp SJ, Schipani E, St-Jacques B, Hunzelman J, Kronenberg H, McMahon AP. Indian hedgehog coordinates endochondral bone growth and morphogenesis via parathyroid hormone related-protein-dependent and -independent pathways. Development. 2000;127:543–548. doi: 10.1242/dev.127.3.543. [DOI] [PubMed] [Google Scholar]
- Karperien M, van Dijk TB, Hoeijmakers T, Cremers F, Abou-Samra AB, Boonstra J, de Laat SW, Defize LH. Expression pattern of parathyroid hormone/parathyroid hormone related peptide receptor mRNA in mouse postimplantation embryos indicates involvement in multiple developmental processes. Mech Dev. 1994;47:29–42. doi: 10.1016/0925-4773(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Karperien M, van der Harten HJ, van Schooten R, Farih-Sips H, den Hollander NS, Kneppers SL, Nijweide P, Papapoulos SE, Lowik CW. A frame-shift mutation in the type I parathyroid hormone (PTH)/PTH- related peptide receptor causing Blomstrand lethal osteochondrodysplasia. J Clin Endocrinol Metab. 1999;84:3713–3720. doi: 10.1210/jcem.84.10.6033. [DOI] [PubMed] [Google Scholar]
- Kastner P, Mark M, Ghyselinck N, Krezel W, Dupe V, Grondona JM, Chambon P. Genetic evidence that the retinoid signal is transduced by heterodimeric RXR/RAR functional units during mouse development. Development. 1997;124:313–326. doi: 10.1242/dev.124.2.313. [DOI] [PubMed] [Google Scholar]
- Katagiri T, Boorla S, Frendo JL, Hogan BL, Karsenty G. Skeletal abnormalities in doubly heterozygous Bmp4 and Bmp7 mice. Dev Genet. 1998;22:340–348. doi: 10.1002/(SICI)1520-6408(1998)22:4<340::AID-DVG4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Kaufman MH, Bard JBL. The anatomical basis of mouse development. San Diego, London: Academic Press; 1999. Somites and their derivatives; pp. 51–59. [Google Scholar]
- Kaur S, Singh G, Stock JL, Schreiner CM, Kier AB, Yager KL, Mucenski ML, Scott WJJ, Potter SS. Dominant mutation of the murine Hox-2.2 gene results in developmental abnormalities. J Exp Zool. 1992;264:323–336. doi: 10.1002/jez.1402640311. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Ishikawa T, Shimabara M, Tanda N, Enomoto-Iwamoto M, Iwamoto M, Kuwana T, Ueki A, Noji S, Nohno T. BMP signaling during bone pattern determination in the developing limb. Development. 1996;122:3557–3566. doi: 10.1242/dev.122.11.3557. [DOI] [PubMed] [Google Scholar]
- Kerszberg M, Wolpert L. A clock and trail model for somite formation, specialization and polarization. J Theor Biol. 2000;205:505–510. doi: 10.1006/jtbi.2000.2085. [DOI] [PubMed] [Google Scholar]
- Kessel M, Balling R, Gruss P. Variations of cervical vertebrae after expression of a Hox -1.1 transgene in mice. Cell. 1990;61:301–308. doi: 10.1016/0092-8674(90)90810-2. [DOI] [PubMed] [Google Scholar]
- Kessel M, Gruss P. Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell. 1991;67:89–104. doi: 10.1016/0092-8674(91)90574-i. [DOI] [PubMed] [Google Scholar]
- Kessel M. Respecification of vertebral identities by retinoic acid. Development. 1992;115:487–501. doi: 10.1242/dev.115.2.487. [DOI] [PubMed] [Google Scholar]
- Keynes RJ, Stern CD. Mechanisms of vertebrate segmentation. Development. 1988;103:413–429. doi: 10.1242/dev.103.3.413. [DOI] [PubMed] [Google Scholar]
- Kieny M, Mauger A, Sengel P. Early regionalization of somitic mesoderm as studied by the development of axial skeleton of the chick embryo. Dev Biol. 1972;28:142–161. doi: 10.1016/0012-1606(72)90133-9. [DOI] [PubMed] [Google Scholar]
- Kim IS, Otto F, Zabel B, Mundlos S. Regulation of chondrocyte differentiation by Cbfa1. Mech Dev. 1999;80:159–170. doi: 10.1016/s0925-4773(98)00210-x. [DOI] [PubMed] [Google Scholar]
- Kim RH, Shapiro HS, Li JJ, Wrana JL, Sodek J. Characterization of the human bone sialoprotein (bsp) gene and its promoter sequence. Matrix Biology. 1994;14:31–40. doi: 10.1016/0945-053x(94)90027-2. [DOI] [PubMed] [Google Scholar]
- King JA, Marker PC, Seung KJ, Kingsley DM. BMP5 and the molecular, skeletal, and soft-tissue alterations in short ear mice. Dev Biol. 1994;166:112–122. doi: 10.1006/dbio.1994.1300. [DOI] [PubMed] [Google Scholar]
- Kingsley DM, Bland AE, Grubber JM, Marker PC, Russell LB, Copeland NG, Jenkins NA. The mouse short ear skeletal morphogenesis locus is associated with defects in a bone morphogenetic member of the TGF beta superfamily. Cell. 1992;71:399–410. doi: 10.1016/0092-8674(92)90510-j. [DOI] [PubMed] [Google Scholar]
- Kingsley DM. The TGF-beta superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev. 1994a;8:133–146. doi: 10.1101/gad.8.2.133. [DOI] [PubMed] [Google Scholar]
- Kingsley DM. What do BMPs do in mammals? Clues from the mouse short-ear mutation. Trends Genet. 1994b;10:16–21. doi: 10.1016/0168-9525(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Knezevic V, DeSanto R, Schughart K, Huffstadt U, Chiang C, Mahon KA, Mackem S. Hoxd-12 differentially affects preaxial and postaxial chondrogenic branches in the limb and regulates Sonic hedgehog in a positive feedback loop. Development. 1997;124:4523–4536. doi: 10.1242/dev.124.22.4523. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Chung UI, Schipani E, Starbuck M, Karsenty G, Katagiri T, Goad DL, Lanske B, Kronenberg HM. PTHrP and Indian hedgehog control differentiation of growth plate chondrocytes at multiple steps. Development. 2002;129:2977–2986. doi: 10.1242/dev.129.12.2977. [DOI] [PubMed] [Google Scholar]
- Kokubu C, Wilm B, Kokubu T, Wahl M, Rodrigo I, Sakai N, Santagati F, Hayashizaki Y, Suzuki M, Yamamura K, Abe K, Imai K. Undulated short-tail deletion mutation in the mouse ablates Pax1 and leads to ectopic activation of neighboring Nkx2–2 in domains that normally express Pax1. Genetics. 2003;165:299–307. doi: 10.1093/genetics/165.1.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Koseki H, Wallin J, Wilting J, Mizutani Y, Kispert A, Ebensperger C, Herrmann BG, Christ B, Balling R. A role for Pax-1 as a mediator of notochordal signals during the dorsoventral specification of vertebrae. Development. 1993;119:649–660. doi: 10.1242/dev.119.3.649. [DOI] [PubMed] [Google Scholar]
- Koshida S, Kishimoto Y, Ustumi H, Shimizu T, Furutani-Seiki M, Kondoh H, Takada S. Integrinalpha5-dependent fibronectin accumulation for maintenance of somite boundaries in zebrafish embryos. Dev Cell. 2005;8:587–598. doi: 10.1016/j.devcel.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Kostic D, Capecchi MR. Targeted disruptions of the murine hoxa-4 and hoxa-6 genes result in homeotic transformations of components of the vertebral column. Mech Dev. 1994;46:231–247. doi: 10.1016/0925-4773(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Koziel L, Wuelling M, Schneider S, Vortkamp A. Gli3 acts as a repressor downstream of Ihh in regulating two distinct steps of chondrocyte differentiation. Development. 2005;132:5249–5260. doi: 10.1242/dev.02097. [DOI] [PubMed] [Google Scholar]
- Kronenberg HM, Lee K, Lanske B, Segre GV. Parathyroid hormone-related protein and Indian hedgehog control the pace of cartilage differentiation. J Endocrinol. 1997;154:S39–45. [PubMed] [Google Scholar]
- Kronenberg HM, Lanske B, Kovacs CS, Chung UI, Lee K, Segre GV, Schipani E, Jüppner H. Functional analysis of the PTH/PTHrP network of ligands and receptors. Rec Progr Horm Res. 1998;53:283–301. disc 301–283. [PubMed] [Google Scholar]
- Krumlauf R. Mouse Hox genetic functions. Curr Opin Genet Dev. 1993;3:621–625. doi: 10.1016/0959-437x(93)90098-a. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Hox Genes in Vertebrate Development. Cell. 1994;78:191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Krumlauf R, Marshall H, Studer M, Nonchev S, Sham MH, Lumsden A. Hox homeobox genes and regionalisation of the nervous system. J Neurobiol. 1993;24:1328–1340. doi: 10.1002/neu.480241006. [DOI] [PubMed] [Google Scholar]
- Kume T, Jiang H, Topczewska JM, Hogan BL. The murine winged helix transcription factors, Foxc1 and Foxc2, are both required for cardiovascular development and somitogenesis. Genes Dev. 2001;15:2470–2482. doi: 10.1101/gad.907301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KM, Pang MK, Zhou S, Cowan SK, Kong RY, Pfordte T, Olsen BR, Sillence DO, Tam PP, Cheah KS. Abnormal compartmentalization of cartilage matrix components in mice lacking collagen X: implications for function. J Cell Biol. 1997;136:459–471. doi: 10.1083/jcb.136.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langston AW, Gudas LJ. Identification of a retinoic acid responsive enhancer 3′ of the murine homeobox gene Hox-1.6. Mech Dev. 1992;38:217–227. doi: 10.1016/0925-4773(92)90055-o. [DOI] [PubMed] [Google Scholar]
- Langston AW, Thompson JR, Gudas LJ. Retinoic acid-responsive enhancers located 3′ of the Hox A and Hox B homeobox gene clusters. Functional analysis. J Biol Chem. 1997;272:2167–2175. doi: 10.1074/jbc.272.4.2167. [DOI] [PubMed] [Google Scholar]
- Lanske B, Karaplis AC, Lee K, Luz A, Vortkamp A, Pirro A, Karperien M, Defize LHK, Ho C, Mulligan RC, Abou-Samra AB, Juppner H, Segre GV, Kronenberg HM. PTH/PTHrP receptor in early development and Indian hedgehog-regulated bone growth. Science. 1996;273:663–666. doi: 10.1126/science.273.5275.663. [DOI] [PubMed] [Google Scholar]
- Lanske B, Kronenberg HM. Parathyroid hormone-related peptide (PTHrP) and parathyroid hormone (PTH)/PTHrP receptor. Crit Rev Eukaryot Gene Expr. 1998;8:297–320. doi: 10.1615/critreveukargeneexpr.v8.i3-4.40. [DOI] [PubMed] [Google Scholar]
- Lardelli M, Lendahl U. Motch A and motch B--two mouse Notch homologues coexpressed in a wide variety of tissues. Exp Cell Res. 1993;204:364–372. doi: 10.1006/excr.1993.1044. [DOI] [PubMed] [Google Scholar]
- Lardelli M, Dahlstrand J, Lendahl U. The novel Notch homologue mouse Notch 3 lacks specific epidermal growth factor-repeats and is expressed in proliferating neuroepithelium. Mech Dev. 1994;46:123–136. doi: 10.1016/0925-4773(94)90081-7. [DOI] [PubMed] [Google Scholar]
- Lash JW, Seitz AW, Cheney CM, Ostrovsky D. On the role of fibronectin during the compaction stage of somitogenesis in the chick embryo. J Exp Zool. 1984;232:197–206. doi: 10.1002/jez.1402320207. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, de Crombrugghe B. Toward understanding SOX9 function in chondrocyte differentiation. Matrix Biol. 1998;16:529–540. doi: 10.1016/s0945-053x(98)90065-8. [DOI] [PubMed] [Google Scholar]
- Lefebvre V, Li P, de Crombrugghe B. A new long form of Sox5 (L-Sox5), Sox6 and Sox9 are coexpressed in chondrogenesis and cooperatively activate the type II collagen gene. EMBO J. 1998;17:5718–5733. doi: 10.1093/emboj/17.19.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire P, Garrett N, Gurdon JB. Expression cloning of Siamois, a Xenopus homeobox gene expressed in dorsal-vegetal cells of blastulae and able to induce a complete secondary axis. Cell. 1995;81:85–94. doi: 10.1016/0092-8674(95)90373-9. [DOI] [PubMed] [Google Scholar]
- LeMouellic H, Lallemand Y, Blanchet P, Boullier D, Brulet P. Genetic Analysis of Hox-3.1, a Mouse Homeo-Gene. Gene Transf Gene Ther. 1989;87:243–253. [Google Scholar]
- LeMouellic H, Lallemand Y, Brulet P. Homeosis in the mouse induced by a null mutation in the Hox-3.1 gene. Cell. 1992;69:251–264. doi: 10.1016/0092-8674(92)90406-3. [DOI] [PubMed] [Google Scholar]
- Li E, Sucov HM, Lee KF, Evans RM, Jaenisch R. Normal development and growth of mice carrying a targeted disruption of the alpha 1 retinoic acid receptor gene. Proc Natl Acad Sci USA. 1993;90:1590–1594. doi: 10.1073/pnas.90.4.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Sodek J. Cloning and characterization of the rat bone sialoprotein gene promoter. Biochem J. 1993;289:625–629. doi: 10.1042/bj2890625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SW, Prockop DJ, Helminen H, Fassler R, Lapvetelainen T, Kiraly K, Peltarri A, Arokoski J, Lui H, Arita M, et al. Transgenic mice with targeted inactivation of the Col2 alpha 1 gene for collagen II develop a skeleton with membranous and periosteal bone but no endochondral bone. Genes Dev. 1995a;9:2821–2830. doi: 10.1101/gad.9.22.2821. [DOI] [PubMed] [Google Scholar]
- Li Y, Lacerda DA, Warman ML, Beier DR, Yoshioka H, Ninomiya Y, Oxford JT, Morris NP, Andrikopoulos K, Ramirez F, et al. A fibrillar collagen gene, Col11a1, is essential for skeletal morphogenesis. Cell. 1995b;80:423–430. doi: 10.1016/0092-8674(95)90492-1. [DOI] [PubMed] [Google Scholar]
- Liu P, Wakamiya M, Shea MJ, Albrecht U, Behringer RR, Bradley A. Requirement for Wnt3 in vertebrate axis formation. Nat Genet. 1999;22:361–365. doi: 10.1038/11932. [DOI] [PubMed] [Google Scholar]
- Lohnes D, Kastner P, Dierich A, Mark M, LeMeur M, Chambon P. Function of retinoic acid receptor gamma in the mouse. Cell. 1993;73:643–658. doi: 10.1016/0092-8674(93)90246-m. [DOI] [PubMed] [Google Scholar]
- Lohnes D, Mark M, Mendelsohn C, Dolle P, Dierich A, Gorry P, Gansmuller A, Chambon P. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994;120:2723–2748. doi: 10.1242/dev.120.10.2723. [DOI] [PubMed] [Google Scholar]
- Lohnes D, Mark M, Mendelsohn C, Dolle P, Decimo D, Lemeur M, Dierich A, Gorry P, Chambon P. Developmental Roles Of the Retinoic Acid Receptors. J Steroid Biochem Mol Biol. 1995;53:475–486. doi: 10.1016/0960-0760(95)00094-g. [DOI] [PubMed] [Google Scholar]
- Lowe C, Yoneda T, Boyce BF, Chen H, Mundy GR, Soriano P. Osteopetrosis in Src-deficient mice is due to an autonomous defect of osteoclasts. Proc Natl Acad Sci USA. 1993;90:4485–4489. doi: 10.1073/pnas.90.10.4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu MF, Cheng HT, Lacy AR, Kern MJ, Argao EA, Potter SS, Olson EN, Martin JF. Paired-related homeobox genes cooperate in handplate and hindlimb zeugopod morphogenesis. Dev Biol. 1999;205:145–157. doi: 10.1006/dbio.1998.9116. [DOI] [PubMed] [Google Scholar]
- Lufkin T, Mark M, Hart CP, Dolle P, LeMeur M, Chambon P. Homeotic transformation of the occipital bones of the skull by ectopic expression of a homeobox gene. Nature. 1992;359:835–841. doi: 10.1038/359835a0. [DOI] [PubMed] [Google Scholar]
- Lufkin T, Lohnes D, Mark M, Dierich A, Gorry P, Gaub MP, LeMeur M, Chambon P. High postnatal lethality and testis degeneration in retinoic acid receptor alpha mutant mice. Proc Natl Acad Sci USA. 1993;90:7225–7229. doi: 10.1073/pnas.90.15.7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Pasceri P, Conlon RA, Rossant J, Giguere V. Mice lacking all isoforms of retinoic acid receptor beta develop normally and are susceptible to the teratogenic effects of retinoic acid. Mech Dev. 1995;53:61–71. doi: 10.1016/0925-4773(95)00424-6. [DOI] [PubMed] [Google Scholar]
- Lyon MF, Rastan S, Brown SDM. Genetic variants and strains of the laboratory mouse. Oxford, New York, Tokyo: Oxford University Press; 1996. [Google Scholar]
- Mahmood R, Kiefer P, Guthrie S, Dickson C, Mason I. Multiple roles for FGF-3 during cranial neural development in the chicken. Development. 1995;121:1399–1410. doi: 10.1242/dev.121.5.1399. [DOI] [PubMed] [Google Scholar]
- Manley NR, Capecchi MR. Hox group 3 paralogous genes act synergistically in the formation of somitic and neural crest-derived structures. Dev Biol. 1997;192:274–288. doi: 10.1006/dbio.1997.8765. [DOI] [PubMed] [Google Scholar]
- Mansour SL, Goddard JM, Capecchi MR. Mice homozygous for a targeted disruption of the proto-oncogene int-2 have developmental defects in the tail and inner ear. Development. 1993;117:13–28. doi: 10.1242/dev.117.1.13. [DOI] [PubMed] [Google Scholar]
- Marazzi G, Wang Y, Sassoon D. Msx2 is a transcriptional regulator in the BMP4mediated programmed cell death pathway. Dev Biol. 1997;186:127–138. doi: 10.1006/dbio.1997.8576. [DOI] [PubMed] [Google Scholar]
- Maroto M, Dale JK, Dequeant ML, Petit AC, Pourquie O. Synchronised cycling gene oscillations in presomitic mesoderm cells require cell-cell contact. Int J Dev Biol. 2005;49:309–315. doi: 10.1387/ijdb.041958mm. [DOI] [PubMed] [Google Scholar]
- Marshall H, Studer M, Popperl H, Aparicio S, Kuroiwa A, Brenner S, Krumlauf R. A conserved retinoic acid response element required for early expression of the homeobox gene hoxb-1. Nature. 1994;370:567–571. doi: 10.1038/370567a0. [DOI] [PubMed] [Google Scholar]
- Mason IJ. The ins and outs of fibroblast growth factors. Cell. 1994;78:547–552. doi: 10.1016/0092-8674(94)90520-7. [DOI] [PubMed] [Google Scholar]
- Massague J. TGF-beta signal transduction. Ann Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- McGinnis W, Krumlauf R. Homeobox genes and axial patterning. Cell. 1992;68:283–302. doi: 10.1016/0092-8674(92)90471-n. [DOI] [PubMed] [Google Scholar]
- McGinnis W, Kuziora M. The molecular architects of body design. Scientific American. 1994;270:58–61. 64–56. doi: 10.1038/scientificamerican0294-58. [DOI] [PubMed] [Google Scholar]
- McLain K, Schreiner C, Yager KL, Stock JL, Potter SS. Ectopic expression of Hox-2.3 induces craniofacial and skeletal malformations in transgenic mice. Mech Dev. 1992;39:3–16. doi: 10.1016/0925-4773(92)90021-b. [DOI] [PubMed] [Google Scholar]
- McMahon AP, Gavin BJ, Parr B, Bradley A, McMahon JA. The Wnt family of cell signalling molecules in postimplantation development of the mouse. Ciba Found Symp. 1992;165:199–212. doi: 10.1002/9780470514221.ch12. [DOI] [PubMed] [Google Scholar]
- Medina-Martinez O, Bradley A, Ramirez-Solis R. A large targeted deletion of Hoxb1-Hoxb9 produces a series of single-segment anterior homeotic transformations. Dev Biol. 2000;222:71–83. doi: 10.1006/dbio.2000.9683. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C, Lohnes D, Decimo D, Lufkin T, LeMeur M, Chambon P, Mark M. Function of the retinoic acid receptors (RARs) during development (II). Multiple abnormalities at various stages of organogenesis in RAR double mutants. Development. 1994a;120:2749–2771. doi: 10.1242/dev.120.10.2749. [DOI] [PubMed] [Google Scholar]
- Mendelsohn C, Mark M, Dolle P, Dierich A, Gaub MP, Krust A, Lampron C, Chambon P. Retinoic acid receptor beta 2 (RAR beta 2) null mutant mice appear normal. Dev Biol. 1994b;166:246–258. doi: 10.1006/dbio.1994.1311. [DOI] [PubMed] [Google Scholar]
- Menkes B, Sandor S. Somitogenesis: regulation potentcies, sequence determination and primordial interactions. In: Ede DA, Hinchcliffe JR, Bails M, editors. Vertebrate limb and somite morphogenesis. Cambridge: Cambridge University Press; 1977. pp. 405–420. [Google Scholar]
- Mo R, Freer AM, Zinyk DL, Crackower MA, Michaud J, Heng HH, Chik KW, Shi XM, Tsui LC, Cheng SH, Joyner AL, Hui C. Specific and redundant functions of Gli2 and Gli3 zinc finger genes in skeletal patterning and development. Development. 1997;124:113–123. doi: 10.1242/dev.124.1.113. [DOI] [PubMed] [Google Scholar]
- Moloney DJ, Panin VM, Johnston SH, Chen J, Shao L, Wilson R, Wang Y, Stanley P, Irvine KD, Haltiwanger RS, Vogt TF. Fringe is a glycosyltransferase that modifies Notch. Nature. 2000;406:369–375. doi: 10.1038/35019000. [DOI] [PubMed] [Google Scholar]
- Morgan BA, Tabin CJ. The role of homeobox genes in limb development. Curr Op Genet Dev. 1993;3:668–674. doi: 10.1016/0959-437x(93)90105-x. [DOI] [PubMed] [Google Scholar]
- Morrison NA, Qi JC, Tokita A, Kelly PJ, Crofts L, Nguyen TV, Sambrook PN, Eisman JA. Prediction of bone density from vitamin D receptor alleles. Nature. 1994;367:284–287. doi: 10.1038/367284a0. [DOI] [PubMed] [Google Scholar]
- Mortlock DP, Post LC, Innes JW. The molecular basis of hypodactyly (Hd): a deletion in Hoxa-13 leads to arrest of digital arch formation. Nature Genet. 1996;13:284–289. doi: 10.1038/ng0796-284. [DOI] [PubMed] [Google Scholar]
- Mortlock DP, Innis JW. Mutation of HOXA13 in hand-foot-genital syndrome. Nature Genet. 1997;15:179–180. doi: 10.1038/ng0297-179. [DOI] [PubMed] [Google Scholar]
- Muenke M, Schell U, Hehr A, Robin NH, Losken HW, Schinzel A, Pulleyn LJ, Rutland P, Reardon W, Malcolm S, et al. A common mutation in the fibroblast growth factor receptor 1 gene in Pfeiffer syndrome. Nat Genet. 1994;8:269–274. doi: 10.1038/ng1194-269. [DOI] [PubMed] [Google Scholar]
- Mundlos S, Huang LF, Selby P, Olsen BR. Cleidocranial dysplasia in mice. Ann NY Acad Sci. 1996;785:301–302. doi: 10.1111/j.1749-6632.1996.tb56290.x. [DOI] [PubMed] [Google Scholar]
- Mundlos S, Olsen BR. Heritable diseases of the skeleton. Part I: Molecular insights into skeletal development-transcription factors and signaling pathways. FASEB J. 1997a;11:125–132. doi: 10.1096/fasebj.11.2.9039954. [DOI] [PubMed] [Google Scholar]
- Mundlos S, Olsen BR. Heritable diseases of the skeleton. Part II: Molecular insights into skeletal development-matrix components and their homeostasis. FASEB J. 1997b;11:227–233. [PubMed] [Google Scholar]
- Muragaki Y, Mundlos S, Upton J, Olsen BR. Altered growth and branching pattern in synpolydactyly caused by mutations in HOXD13. Science. 1996;272:548–515. doi: 10.1126/science.272.5261.548. [DOI] [PubMed] [Google Scholar]
- Naski MC, Wang Q, Xu J, Ornitz DM. Graded activation of fibroblast growth factor receptor 3 by mutations causing achondroplasia and thanatophoric dysplasia. Nature Genet. 1996;13:233–237. doi: 10.1038/ng0696-233. [DOI] [PubMed] [Google Scholar]
- Naski MC, Colvin JS, Coffin JD, Ornitz DM. Repression of hedgehog signaling and BMP4 expression in growth plate cartilage by fibroblast growth factor receptor 3. Development. 1998;125:4977–4988. doi: 10.1242/dev.125.24.4977. [DOI] [PubMed] [Google Scholar]
- Nerlich AG, Freisinger P, Bonaventure J. Radiological and histological variants of thanatophoric dysplasia are associated with common mutations in FGFR-3. Ameri J Med Genet. 1996;63:155–160. doi: 10.1002/(SICI)1096-8628(19960503)63:1<155::AID-AJMG27>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Neubüser A, Koseki H, Balling R. Characterization and developmental expression of Pax9, a paired-box- containing gene related to Pax1. Dev Biol. 1995;170:701–716. doi: 10.1006/dbio.1995.1248. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Subbarayan V, Dolle P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- Norris WE, Stern CD, Keynes RJ. Molecular differences between the rostral and caudal halves of the sclerotome in the chick embryo. Development. 1989;105:541–548. doi: 10.1242/dev.105.3.541. [DOI] [PubMed] [Google Scholar]
- Nüsslein-Volhard C, Wieschaus E. Mutations affecting segment number and polarity in Drosophila. Nature. 1980;287:795–801. doi: 10.1038/287795a0. [DOI] [PubMed] [Google Scholar]
- Oddis CV. New perspectives on osteoarthritis. American Journal of Medicine. 1996;100:10S–15S. doi: 10.1016/s0002-9343(97)89541-1. [DOI] [PubMed] [Google Scholar]
- Olsen BR. Mutations in collagen genes resulting in metaphyseal and epiphyseal dysplasias. Bone. 1995;17:45S–49S. doi: 10.1016/8756-3282(95)00208-u. [DOI] [PubMed] [Google Scholar]
- Ordahl CP, Le Douarin NM. Two myogenic lineages within the developing somite. Development. 1992;114:339–353. doi: 10.1242/dev.114.2.339. [DOI] [PubMed] [Google Scholar]
- Ornitz DM. FGF signaling in the developing endochondral skeleton. Cytokine Growth Fact Rev. 2005;16:205–213. doi: 10.1016/j.cytogfr.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard DS., Jr Chick somite determination: the role of factors in young somites and the segmental plate. J Exp Zool. 1978;203:295–306. doi: 10.1002/jez.1402030212. [DOI] [PubMed] [Google Scholar]
- Parr BA, McMahon AP. Wnt genes and vertebrate development. Curr Opin Genet Dev. 1994;4:523–528. doi: 10.1016/0959-437x(94)90067-d. [DOI] [PubMed] [Google Scholar]
- Pathi S, Rutenberg JB, Johnson RL, Vortkamp A. Interaction of Ihh and BMP/noggin signaling during cartilage differentiation. Dev Biol. 1999;209:239–253. doi: 10.1006/dbio.1998.9181. [DOI] [PubMed] [Google Scholar]
- Patten BM. Human Embryology. 2. New York: Blackiston; 1953. [Google Scholar]
- Perrimon N, McMahon AP. Negative feedback mechanisms and their role during pattern formation. Cell. 1999;97:13–16. doi: 10.1016/s0092-8674(00)80710-2. [DOI] [PubMed] [Google Scholar]
- Peters H, Wilm B, Sakai N, Imai K, Maas R, Balling R. Pax1 and Pax9 synergistically regulate vertebral column development. Development. 1999;126:53995408. doi: 10.1242/dev.126.23.5399. [DOI] [PubMed] [Google Scholar]
- Placzek M, Jessell TM, Dodd J. Induction of floor plate differentiation by contact-dependent, homeogenetic signals. Development. 1993;117:205–218. doi: 10.1242/dev.117.1.205. [DOI] [PubMed] [Google Scholar]
- Placzek M, Tessier-Lavigne M, Yamada T, Jessell T, Dodd J. Mesodermal control of neural cell identity: floor plate induction by the notochord. Science. 1990;250:985–988. doi: 10.1126/science.2237443. [DOI] [PubMed] [Google Scholar]
- Placzek M, Yamada T, Tessier-Lavigne M, Jessell T, Dodd J. Control of dorsoventral pattern in vertebrate neural development: induction and polarizing properties of the floor plate. Development Suppl. 1991:105–122. [PubMed] [Google Scholar]
- Pollock RA, Jay G, Bieberich CJ. Altering the boundaries of Hox3.1 expression: evidence for antipodal gene regulation. Cell. 1992;71:911–923. doi: 10.1016/0092-8674(92)90388-s. [DOI] [PubMed] [Google Scholar]
- Pöpperl H, Featherstone MS. Identification of a retinoic acid response element upstream of the murine Hox-4.2 gene. Mol Cell Biol. 1993;13:257–265. doi: 10.1128/mcb.13.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JA, von Kessler DP, Ekker SC, Young KE, Lee JJ, Moses K, Beachy PA. The product of hedgehog autoproteolytic cleavage active in local and long-range signalling. Nature. 1995;374:363–366. doi: 10.1038/374363a0. [DOI] [PubMed] [Google Scholar]
- Pourquie O, Coltey M, Teillet MA, Ordahl C, Le Douarin NM. Control of dorsoventral patterning of somitic derivatives by notochord and floor plate. Proc Natl Acad Sci USA. 1993;90:5242–5246. doi: 10.1073/pnas.90.11.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probstmeier R, Bilz A, Schneider SJ. Expression of the neural cell adhesion molecule and polysialic acid during early mouse embryogenesis. J Neurosci Res. 1994;37:324–335. doi: 10.1002/jnr.490370305. [DOI] [PubMed] [Google Scholar]
- Radice GL, Rayburn H, Matsunami H, Knudsen KA, Takeichi M, Hynes RO. Developmental defects in mouse embryos lacking N-cadherin. Dev Biol. 1997;181:64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- Ramirez-Solis R, Zheng H, Whiting J, Krumlauf R, Bradley A. Hoxb-4 (hox-2.6) mutant mice show homeotic transformation of a cervical vertebra and defects in the closure of the sternal rudiments. Cell. 1993;73:279–294. doi: 10.1016/0092-8674(93)90229-j. [DOI] [PubMed] [Google Scholar]
- Rancourt DE, Tsuzuki T, Capecchi MR. Genetic interaction between hoxb-5 and hoxb-6 is revealed by nonallelic noncomplementation. Genes Dev. 1995;9:108–122. doi: 10.1101/gad.9.1.108. [DOI] [PubMed] [Google Scholar]
- Rauber AA, Kopsch F. Lehrbuch und Atlas. Stuttgart: Thieme; 1987. Anatomie des Menschen. [Google Scholar]
- Rawls A, Wilson-Rawls J, Olson EN. Genetic regulation of somite formation. Curr Top Dev Biol. 2000;47:131–154. doi: 10.1016/s0070-2153(08)60724-3. [DOI] [PubMed] [Google Scholar]
- Reaume AG, Conlon RA, Zirngibl R, Yamaguchi TP, Rossant J. Expression analysis of a Notch homologue in the mouse embryo. Dev Biol. 1992;154:377–387. doi: 10.1016/0012-1606(92)90076-s. [DOI] [PubMed] [Google Scholar]
- Reddi AH. Regulation of cartilage and bone differentiation by bone morphogenetic proteins. Curr Opin Cell Biol. 1992;4:850–855. doi: 10.1016/0955-0674(92)90110-x. [DOI] [PubMed] [Google Scholar]
- Reddi AH. Bone and cartilage differentiation. Curr Opin Genet Dev. 1994;4:737–744. doi: 10.1016/0959-437x(94)90141-o. [DOI] [PubMed] [Google Scholar]
- Reichenberger E, Aigner T, von der Mark K, Stoss H, Bertling W. In situ hybridization studies on the expression of type X collagen in fetal human cartilage. Dev Biol. 1991;148:562–572. doi: 10.1016/0012-1606(91)90274-7. [DOI] [PubMed] [Google Scholar]
- Reimold AM, Grusby MJ, Kosaras B, Fries JW, Mori R, Maniwa S, Clauss IM, Collins T, Sidman RL, Glimcher MJ, Glimcher LH. Chondrodysplasia and neurological abnormalities in ATF-2-deficient mice. Nature. 1996;379:262–265. doi: 10.1038/379262a0. [DOI] [PubMed] [Google Scholar]
- Remak R. Untersuchungen über die Entwicklung der Wirbeltiere. Berlin: Reimer; 1855. [Google Scholar]
- Rijli FM, Mark M, Lakkaraju S, Dierich A, Dolle P, Chambon P. A homeotic transformation is generated in the rostral branchial region of the head by disruption of Hoxa-2, which acts as a selector gene. Cell. 1993;75:1333–1349. doi: 10.1016/0092-8674(93)90620-6. [DOI] [PubMed] [Google Scholar]
- Rijli FM, Dolle P, Fraulob V, LeMeur M, Chambon P. Insertion of a targeting construct in a Hoxd-10 allele can influence the control of Hoxd-9 expression. Dev Dyn. 1994;201:366–377. doi: 10.1002/aja.1002010408. [DOI] [PubMed] [Google Scholar]
- Rijli FM, Matyas R, Pellegrini M, Dierich A, Gruss P, Dolle P, Chambon P. Cryptochordism and homeotic transformations of spinal nerves and vertebrae in Hoxa-10 mutant mice. Proc Natl Acad Sci USA. 1995;92:8185–8189. doi: 10.1073/pnas.92.18.8185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rijli FM, Chambon P. Genetic interactions of Hox genes in limb development: learning from compound mutants. Cur Op Genet Dev. 1997;7:481–487. doi: 10.1016/s0959-437x(97)80074-3. [DOI] [PubMed] [Google Scholar]
- Rivera-Perez JA, Mallo M, Gendron-Maguire M, Gridley T, Behringer RR. Goosecoid is not an essential component of the mouse gastrula organizer but is required for craniofacial and rib development. Development. 1995;121:3005–3012. doi: 10.1242/dev.121.9.3005. [DOI] [PubMed] [Google Scholar]
- Rivera-Perez JA, Wakamiya M, Behringer RR. Goosecoid acts cell autonomously in mesenchyme-derived tissues during craniofacial development. Development. 1999;126:3811–3821. doi: 10.1242/dev.126.17.3811. [DOI] [PubMed] [Google Scholar]
- Roelink H, Porter JA, Chiang C, Tanabe Y, Chang DT, Beachy PA, Jessell TM. Floor plate and motor neuron induction by different concentrations of the amino-terminal cleavage product of sonic hedgehog autoproteolysis. Cell. 1995;81:445–455. doi: 10.1016/0092-8674(95)90397-6. [DOI] [PubMed] [Google Scholar]
- Rosen V, Thies RS, Lyons K. Signaling pathways in skeletal formation: a role for BMP receptors. Ann N Y Acad Sci. 1996;785:59–69. doi: 10.1111/j.1749-6632.1996.tb56244.x. [DOI] [PubMed] [Google Scholar]
- Rugh R. The mouse: its reproduction and development. Burgess; Minneapolis: 1968. [Google Scholar]
- Ruoslahti E, Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991;64:867–869. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- Rutland P, Pulleyn LJ, Reardon W, Baraitser M, Hayward R, Jones B, Malcolm S, Winter RM, Oldridge M, Slaney SF, et al. Identical mutations in the FGFR2 gene cause both Pfeiffer and Crouzon syndrome phenotypes. Nat Genet. 1995;9(2):173–176. doi: 10.1038/ng0295-173. [DOI] [PubMed] [Google Scholar]
- Saegusa H, Takahashi N, Noguchi S, Suemori H. Targeted Disruption in the Mouse Hoxc-4 Locus Results in Axial Skleton Homeosis and Malformations of the Xiphoid Process. Dev Biol. 1996;174:55–64. doi: 10.1006/dbio.1996.0051. [DOI] [PubMed] [Google Scholar]
- Saga Y, Yagi T, Ikawa Y, Sakakura T, Aizawa S. Mice develop normally without tenascin. Genes Dev. 1992;6:1821–1831. doi: 10.1101/gad.6.10.1821. [DOI] [PubMed] [Google Scholar]
- Satokata I, Benson G, Maas R. Sexually dimorphic sterility phenotypes in Hoxa10-deficient mice. Nature. 1995;374:460–463. doi: 10.1038/374460a0. [DOI] [PubMed] [Google Scholar]
- Schipani E, Kruse K, Juppner H. A constitutively active mutant PTH-PTHrP receptor in Jansen-type metaphyseal chondrodysplasia. Science. 1995;268:98–100. doi: 10.1126/science.7701349. [DOI] [PubMed] [Google Scholar]
- Schipani E, Lanske B, Hunzelman J, Luz A, Kovacs CS, Lee K, Pirro A, Kronenberg HM, Jüppner H. Targeted expression of constitutively active receptors for parathyroid hormone and parathyroid hormone-related peptide delays endochondral bone formation and rescues mice that lack parathyroid hormone-related peptide. Proc Natl Acad Sci US A. 1997;94:13689–13694. doi: 10.1073/pnas.94.25.13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schipani E, Langman C, Hunzelman J, Le Merrer M, Loke KY, Dillon MJ, Silve C, Jüppner H. A novel parathyroid hormone (PTH)/PTH-related peptide receptor mutation in Jansen’s metaphyseal chondrodysplasia. J Clin Endocrinol Metab. 1999;84:3052–3057. doi: 10.1210/jcem.84.9.6000. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Notch and presenilins in vertebrates and invertebrates: implications for neuronal development and degeneration. Curr Opin Neurobiol. 2000;10:50–57. doi: 10.1016/s0959-4388(99)00054-9. [DOI] [PubMed] [Google Scholar]
- Serra R, Johnson M, Filvaroff EH, LaBorde J, Sheehan DM, Derynck R, Moses HL. Expression of a truncated, kinase-defective TGF-beta type II receptor in mouse skeletal tissue promotes terminal chondrocyte differentiation and osteoarthritis. J Cell Biol. 1997;139:541–552. doi: 10.1083/jcb.139.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra R, Chang C. TGF-beta signaling in human skeletal and patterning disorders. Birth Defects Res C Embryo Today. 2003;69:333–351. doi: 10.1002/bdrc.10023. [DOI] [PubMed] [Google Scholar]
- Shashikant CS, Utset MF, Violette SM, Wise TL, Einat P, Einat M, Pendleton JW, Schughart K, Ruddle FH. Homeobox genes in mouse development. Crit Rev Eukaryot Gene Expr. 1991;1:207–245. [PubMed] [Google Scholar]
- Sheppard D. In vivo functions of integrins: lessons from null mutations in mice. Matrix Biol. 2000;19:203–209. doi: 10.1016/s0945-053x(00)00065-2. [DOI] [PubMed] [Google Scholar]
- Shi X, Yang X, Chen D, Chang Z, Cao X. Smad1 interacts with homeobox DNA-binding proteins in bone morphogenetic protein signaling. J Biol Chem. 1999;274:13711–13717. doi: 10.1074/jbc.274.19.13711. [DOI] [PubMed] [Google Scholar]
- Shiang R, Thompson LM, Zhu YZ, Church DM, Fielder TJ, Bocian M, Winokur ST, Wasmuth JJ. Mutations in the transmembrane domain of FGFR3 cause the most common genetic form of dwarfism, achondroplasia. Cell. 1994;78:335–342. doi: 10.1016/0092-8674(94)90302-6. [DOI] [PubMed] [Google Scholar]
- Small KM, Potter SS. Homeotic transformations and limb defects in Hox A11 mutant mice. Genes Dev. 1995;7:2318–2328. doi: 10.1101/gad.7.12a.2318. [DOI] [PubMed] [Google Scholar]
- Smits P, Lefebvre V. Sox5 and Sox6 are required for notochord extracellular matrix sheath formation, notochord cell survival and development of the nucleus pulposus of intervertebral discs. Development. 2003;130:1135–1148. doi: 10.1242/dev.00331. [DOI] [PubMed] [Google Scholar]
- Solloway MJ, Dudley AT, Bikoff EK, Lyons KM, Hogan BL, Robertson EJ. Mice lacking Bmp6 function. Dev Genet. 1998;22:321–339. doi: 10.1002/(SICI)1520-6408(1998)22:4<321::AID-DVG3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Solursh M, Fisher M, Meier S, Singley CT. The role of extracellular matrix in the formation of the sclerotome. J Embryol Exp Morphol. 1979;54:75–98. [PubMed] [Google Scholar]
- Soriano P, Montgomery C, Geske R, Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Sreenath TL, Pollock RA, Bieberich CJ. Functional specificity of Hoxa-4 in vertebral patterning lies outside of the homeodomain. Proc Natl Acad Sci USA. 1996;93:9636–9640. doi: 10.1073/pnas.93.18.9636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Jacques B, Hammerschmidt M, McMahon AP. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev. 1999;13:2072–2086. doi: 10.1101/gad.13.16.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern CD, Keynes RJ. Interactions between somite cells: the formation and maintenance of segment boundaries in the chick embryo. Development. 1987;99:261–272. doi: 10.1242/dev.99.2.261. [DOI] [PubMed] [Google Scholar]
- Stern CD. Vertebrate gastrulation. Curr Opin Genet Dev. 1992;2:556–561. doi: 10.1016/s0959-437x(05)80171-6. [DOI] [PubMed] [Google Scholar]
- Storm EE, Huynh TV, Copeland NG, Jenkins NA, Kingsley DM, Lee SJ. Limb alterations in brachypodism mice due to mutations in a new member of the TGF beta-superfamily. Nature. 1994;368:639–643. doi: 10.1038/368639a0. [DOI] [PubMed] [Google Scholar]
- Stott D, Kispert A, Herrmann BG. Rescue of the tail defect of Brachyury mice. Genes Dev. 1993;7:197–203. doi: 10.1101/gad.7.2.197. [DOI] [PubMed] [Google Scholar]
- Su WC, Kitagawa M, Xue N, Xie B, Garofalo S, Cho J, Deng C, Horton WA, Fu XY. Activation of Stat1 by mutant fibroblast growth-factor receptor in thanatophoric dysplasia type II dwarfism. Nature. 1997;386:288–292. doi: 10.1038/386288a0. [DOI] [PubMed] [Google Scholar]
- Suemori H, Takahashi N, Noguchi S. Hoxc-9 mutant mice show anterior transformation of the vertebrae and malformation of the sternum and ribs. Mech Dev. 1995;51:265–273. doi: 10.1016/0925-4773(95)00371-1. [DOI] [PubMed] [Google Scholar]
- Suemori H, Noguchi S. Hox C cluster genes are dispensable for overall body plan of mouse embryonic development. Dev Biol. 2000;220:333–342. doi: 10.1006/dbio.2000.9651. [DOI] [PubMed] [Google Scholar]
- Swiatek PJ, Lindsell CE, del Amo FF, Weinmaster G, Gridley T. Notch1 is essential for postimplantation development in mice. Genes Dev. 1994;8:707–719. doi: 10.1101/gad.8.6.707. [DOI] [PubMed] [Google Scholar]
- Takada S, Stark KL, Shea MJ, Vassileva G, McMahon JA, McMahon AP. Wnt-3a regulates somite and tailbud formation in the mouse embryo. Genes Dev. 1994;8:174–189. doi: 10.1101/gad.8.2.174. [DOI] [PubMed] [Google Scholar]
- Tam PP, Goldman D, Camus A, Schoenwolf GC. Early events of somitogenesis in higher vertebrates: allocation of precursor cells during gastrulation and the organization of a meristic pattern in the paraxial mesoderm. Curr Top Dev Biol. 2000;47:1–32. doi: 10.1016/s0070-2153(08)60720-6. [DOI] [PubMed] [Google Scholar]
- Tanabe Y, Jessell TM. Diversity and pattern in the developing spinal cord. Science. 1996;274:1115–1123. doi: 10.1126/science.274.5290.1115. [DOI] [PubMed] [Google Scholar]
- Tang SJ, Hoodless PA, Lu Z, Breitman ML, McInnes RR, Wrana JL, Buchwald M. The Tlx-2 homeobox gene is a downstream target of BMP signalling and is required for mouse mesoderm development. Development. 1998;125:1877–1887. doi: 10.1242/dev.125.10.1877. [DOI] [PubMed] [Google Scholar]
- Testa V, Capasso G, Maffulli M, Sgambato A, Ames PR. Proteases and antiproteases in cartilage homeostasis. A brief review. Clin Orthop Rel Res. 1994;308:79–84. [PubMed] [Google Scholar]
- Thondury G. Entwicklungsgeschichte und Fehlbildungen der Wirbelsäule. Stuttgart: Hippokrates; 1985. [Google Scholar]
- Tischfield MA, Bosley TM, Salih MA, Alorainy IA, Sener EC, Nester MJ, Oystreck DT, Chan WM, Andrews C, Erickson RP, Engle EC. Homozygous HOXA1 mutations disrupt human brainstem, inner ear, cardiovascular and cognitive development. Nat Genet. 2005;37:1035–1037. doi: 10.1038/ng1636. [DOI] [PubMed] [Google Scholar]
- Towler DA, Bennett CD, Rodan GA. Activity of the rat osteocalcin basal promoter in osteoblastic cells is dependent upon homeodomain and CP1 binding motifs. Mol Endocrinol. 1994;8:614–624. doi: 10.1210/mend.8.5.7914673. [DOI] [PubMed] [Google Scholar]
- Trout JJ, Buckwalter JA, Moore KC, Landas SK. Ultrastructure of the human intervertebral disc. I. Changes in notochordal cells with age. Tissue Cell. 1982;14:359–369. doi: 10.1016/0040-8166(82)90033-7. [DOI] [PubMed] [Google Scholar]
- Tsumaki N, Tanaka K, Arikawa-Hirasawa E, Nakase T, Kimura T, Thomas JT, Ochi T, Luyten FP, Yamada Y. Role of CDMP-1 in skeletal morphogenesis: promotion of mesenchymal cell recruitment and chondrocyte differentiation. J Cell Biol. 1999;144:161–173. doi: 10.1083/jcb.144.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utsch B, Becker K, Brock D, Lentze MJ, Bidlingmaier F, Ludwig M. A novel stable polyalanine [poly(A)] expansion in the HOXA13 gene associated with hand-footgenital syndrome: proper function of poly(A)-harbouring transcription factors depends on a critical repeat length? Hum Genet. 2002;110:488–494. doi: 10.1007/s00439-002-0712-8. [DOI] [PubMed] [Google Scholar]
- van der Lugt NM, Domen J, Linders K, van Roon M, Robanus-Maandag E, te Riele H, van der Valk M, Deschamps J, Sofroniew M, van Lohuizen M, et al. Posterior transformation, neurological abnormalities, and severe hematopoietic defects in mice with a targeted deletion of the bmi-1 proto-oncogene. Genes Dev. 1994;8:757–769. doi: 10.1101/gad.8.7.757. [DOI] [PubMed] [Google Scholar]
- van der Rest M, Garrone R. Collagen family of proteins. Faseb J. 1991;5(13):28142823. [PubMed] [Google Scholar]
- van Lohuizen M. Functional analysis of mouse Polycomb group genes. Cell Mol Life Sci. 1998;54:71–79. doi: 10.1007/s000180050126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Straaten HW, Hekking JW, Wiertz-Hoessels EJ, Thors F, Drukker J. Effect of the notochord on the differentiation of a floor plate area in the neural tube of the chick embryo. Anat Embryol. 1988;177:317–324. doi: 10.1007/BF00315839. [DOI] [PubMed] [Google Scholar]
- Vandenberg P. Molecular basis of heritable connective tissue disease. Biochem Med Metab Biol. 1993;49:1–12. doi: 10.1006/bmmb.1993.1001. [DOI] [PubMed] [Google Scholar]
- Verbout AJ. The development of the vertebral column. Adv Anat Embryol Cell Biol. 1985;90:1–122. doi: 10.1007/978-3-642-69983-2. [DOI] [PubMed] [Google Scholar]
- Vikkula M, Metsaranta M, Ala-Kokko L. Type II collagen mutations in rare and common cartilage diseases. Ann Med. 1994;26:107–114. doi: 10.3109/07853899409147337. [DOI] [PubMed] [Google Scholar]
- Vogan KJ, Epstein DJ, Trasler DG, Gros P. The splotch-delayed (Spd) mouse mutant carries a point mutation within the paired box of the Pax-3 gene. Genomics. 1993;17:364–369. doi: 10.1006/geno.1993.1333. [DOI] [PubMed] [Google Scholar]
- Vortkamp A, Lee K, Lanske B, Segre GV, Kronenberg HM, Tabin C. Regulation of rate of cartilage differentiation by indian hedgehog and PTH-related peptide. Science. 1996;273:613–622. doi: 10.1126/science.273.5275.613. [DOI] [PubMed] [Google Scholar]
- Vortkamp A. Defining the skeletal elements. Curr Biol. 1997;7:R104–107. doi: 10.1016/s0960-9822(06)00049-2. [DOI] [PubMed] [Google Scholar]
- Wai AW, Ng LJ, Watanabe H, Yamada Y, Tam PP, Cheah KS. Disrupted expression of matrix genes in the growth plate of the mouse cartilage matrix deficiency (cmd) mutant. Dev Genet. 1998;22:349–358. doi: 10.1002/(SICI)1520-6408(1998)22:4<349::AID-DVG5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Wallin J, Mizutani Y, Imai K, Miyashita N, Moriwaki K, Taniguchi M, Koseki H, Balling R. A new Pax gene, Pax-9, maps to mouse chromosome 12. Mamm Genome. 1993;4:354–358. doi: 10.1007/BF00360584. [DOI] [PubMed] [Google Scholar]
- Wallin J, Wilting J, Koseki H, Fritsch R, Christ B, Balling R. The role of Pax-1 in axial skeleton development. Development. 1994;120:1109–1121. doi: 10.1242/dev.120.5.1109. [DOI] [PubMed] [Google Scholar]
- Wang Y, Spatz MK, KK, Hayk H, Avivi A, Gorivodsky M, Pines M, Yayon A, Lonai P, Givol D. A mouse model for achondroplasia produced by targeting fibroblast growth factor receptor 3. Proc Natl Acad Sci USA. 1999;96:4455–4460. doi: 10.1073/pnas.96.8.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZQ, Ovitt C, Grigoriadis AE, Mohle-Steinlein U, Ruther U, Wagner EF. Bone and haematopoietic defects in mice lacking c-fos. Nature. 1992;360:741–745. doi: 10.1038/360741a0. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Saitoh K, Kameda T, Murakami M, Niikura Y, Okazaki S, Morishita Y, Mori S, Yokouchi Y, Kuroiwa A, Iba H. Chondrocytes as a specific target of ectopic Fos expression in early development. Proc Natl Acad Sci USA. 1997;94:39943999. doi: 10.1073/pnas.94.8.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Pan ZQ, Schreiber-Agus N, DePinho RA, Hurwitz J, Xiong Y. Suppression of cell transformation by the cyclin-dependent kinase inhibitor p57KIP2 requires binding to proliferating cell nuclear antigen. Proc Natl Acad Sci USA. 1998;95:1392–1397. doi: 10.1073/pnas.95.4.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe H, Yamada Y. Mice lacking link protein develop dwarfism and craniofacial abnormalities. Nature Genet. 1999;21:225–229. doi: 10.1038/6016. [DOI] [PubMed] [Google Scholar]
- Weinstein DC, Ruiz i Altaba A, Chen WS, Hoodless P, Prezioso VR, Jessell TM, Darnell JE., Jr The winged-helix transcription factor HNF-3 beta is required for notochord development in the mouse embryo. Cell. 1994;78:575–588. doi: 10.1016/0092-8674(94)90523-1. [DOI] [PubMed] [Google Scholar]
- Weir EC, Philbrick WM, Amling M, Neff LA, Baron R, Broadus AE. Targeted overexpression of parathyroid hormone-related peptide in chondrocytes causes chondrodysplasia and delayed endochondral bone formation. Proc Natl Acad Sci USA. 1996;93:10240–10245. doi: 10.1073/pnas.93.19.10240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendling O, Chambon P, Mark M. Retinoid X receptors are essential for early mouse development and placentogenesis. Proc Natl Acad Sci USA. 1999;96:547–551. doi: 10.1073/pnas.96.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston AD, Hoffman LM, Underhill TM. Revisiting the role of retinoid signaling in skeletal development. Birth Defects Res C Embryo Today. 2003;69:156–173. doi: 10.1002/bdrc.10010. [DOI] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W, Bartocci A, Ferrante AW, Jr, Ahmed-Ansari A, Sell KW, Pollard JW, Stanley ER. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci USA. 1990;87:4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild A, Kalff-Suske M, Vortkamp A, Bornholdt D, Konig R, Grzeschik KH. Point mutations in human GLI3 cause Greig syndrome. Hum Mol Genet. 1997;6:1979–1984. doi: 10.1093/hmg/6.11.1979. [DOI] [PubMed] [Google Scholar]
- Wilkin DJ, Szabo JK, Cameron R, Henderson S, Bellus GA, Mack ML, Kaitila I, Loughlin J, Munnich A, Sykes B, Bonaventure J, Francomano CA. Mutations in fibroblast growth-factor receptor 3 in sporadic cases of achondroplasia occur exclusively on the paternally derived chromosome. Amer J Hum Genet. 1998;63:711–716. doi: 10.1086/302000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DG, Bhatt S, Herrmann BG. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature. 1990;343:657–659. doi: 10.1038/343657a0. [DOI] [PubMed] [Google Scholar]
- Williams BA, Ordahl CP. Pax-3 expression in segmental mesoderm marks early stages in myogenic cell specification. Development. 1994;120:785–796. doi: 10.1242/dev.120.4.785. [DOI] [PubMed] [Google Scholar]
- Williams CJ, Jimenez SA. Heritable diseases of cartilage caused by mutations in collagen genes. J Rheumatol - Suppl. 1995;43:28–33. [PubMed] [Google Scholar]
- Willing MC, Pruchno CJ, Byers PH. Molecular heterogeneity in osteogenesis imperfecta type I. Am J Med Genet. 1993;45:223–227. doi: 10.1002/ajmg.1320450214. [DOI] [PubMed] [Google Scholar]
- Wilm B, Dahl E, Peters H, Balling R, Imai K. Targeted disruption of Pax1 defines its null phenotype and proves haploinsufficiency. Proc Natl Acad Sci USA. 1998;95:8692–8697. doi: 10.1073/pnas.95.15.8692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Wordsworth P. Genes and arthritis. Brit Med Bull. 1995;51:249–266. doi: 10.1093/oxfordjournals.bmb.a072959. [DOI] [PubMed] [Google Scholar]
- Wozney JM, Rosen V. Bone morphogenetic protein and bone morphogenetic protein gene family in bone formation and repair. Clin Orthop Rel Res. 1998;346:26–37. [PubMed] [Google Scholar]
- Yamaguchi M, Nakamoto M, Honda H, Nakagawa T, Fujita H, Nakamura T, Hirai H, Narumiya S, Kakizuka A. Retardation of skeletal development and cervical abnormalities in transgenic mice expressing a dominant-negative retinoic acid receptor in chondrogenic cells. Proc Natl Acad Sci USA. 1998;95:7491–7496. doi: 10.1073/pnas.95.13.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi TP, Conlon RA, Rossant J. Expression of the fibroblast growth factor receptor FGFR-1/flg during gastrulation and segmentation in the mouse embryo. Dev Biol. 1992;152:75–88. doi: 10.1016/0012-1606(92)90157-c. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Harpal K, Henkemeyer M, Rossant J. Fgfr-1 is required for embryonic growth and mesodermal patterning during mouse gastrulation. Genes Dev. 1994;8:3032–3044. doi: 10.1101/gad.8.24.3032. [DOI] [PubMed] [Google Scholar]
- Yan Y, Frisen J, Lee MH, Massague J, Barbacid M. Ablation of the CDK inhibitor p57Kip2 results in increased apoptosis and delayed differentiation during mouse development. Genes Dev. 1997;11:973–983. doi: 10.1101/gad.11.8.973. [DOI] [PubMed] [Google Scholar]
- Yang C, Li SW, Helminen HJ, Khillan JS, Bao Y, Prockop DJ. Apoptosis of chondrocytes in transgenic mice lacking collagen II. Exp Cell Res. 1997;235:370–373. doi: 10.1006/excr.1997.3692. [DOI] [PubMed] [Google Scholar]
- Yang X, Ji X, Shi X, Cao X. Smad1 domains interacting with Hoxc-8 induce osteoblast differentiation. J Biol Chem. 2000;275:1065–1072. doi: 10.1074/jbc.275.2.1065. [DOI] [PubMed] [Google Scholar]
- Yi SE, Daluiski A, Pederson R, Rosen V, Lyons KM. The type I BMP receptor BMPRIB is required for chondrogenesis in the mouse limb. Development. 2000;127:621–630. doi: 10.1242/dev.127.3.621. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Hayashi S, Kunisada T, Ogawa M, Nishikawa S, Okamura H, Sudo T, Shultz LD. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990;345:442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- Yu BD, Hess JL, Horning SE, Brown GA, Korsmeyer SJ. Altered Hox expression and segmental identity in Mll-mutant mice. Nature. 1995;378:505–508. doi: 10.1038/378505a0. [DOI] [PubMed] [Google Scholar]
- Yueh YG, Gardner DP, Kappen C. Evidence for regulation of cartilage differentiation by the homeobox gene Hoxc-8. Proc Natl Acad Sci USA. 1998;95:99569961. doi: 10.1073/pnas.95.17.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakany J, Duboule D. Synpolydactyly In Mice With a Targeted Deficiency In the Hoxd Complex. Nature. 1996;384:69–71. doi: 10.1038/384069a0. [DOI] [PubMed] [Google Scholar]
- Zakany J, Gerard M, Favier B, Potter SS, Duboule D. Functional equivalence and rescue among group 11 Hox gene products in vertebral patterning. Developmental Biology. 1996;176:325–328. doi: 10.1006/dbio.1996.0137. [DOI] [PubMed] [Google Scholar]
- Zeng L, Kempf H, Murtaugh LC, Sato ME, Lassar AB. Shh establishes an Nkx3.2/Sox9 autoregulatory loop that is maintained by BMP signals to induce somitic chondrogenesis. Genes Dev. 2002;16:1990–2005. doi: 10.1101/gad.1008002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Pöpperl H, Morrison A, Kovacs EN, Prideaux V, Schwarz L, Krumlauf R, Rossant J, Featherstone MS. Elements both 5′ and 3′ to the murine Hoxd4 gene establish anterior borders of expression in mesoderm and neurectoderm. Mech Dev. 1997a;67:49–58. doi: 10.1016/s0925-4773(97)00104-4. [DOI] [PubMed] [Google Scholar]
- Zhang H, Hu G, Wang H, Sciavolino P, Iler N, Shen MM, Abate-Shen C. Heterodimerization of Msx and Dlx homeoproteins results in functional antagonism. Mol Cell Biol. 1997b;17:2920–2932. doi: 10.1128/mcb.17.5.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N, Gridley T. Defects in somite formation in lunatic fringe-deficient mice. Nature. 1998;394:374–377. doi: 10.1038/28625. [DOI] [PubMed] [Google Scholar]
- Zhang P, Liegeois NJ, Wong C, Finegold M, Hou H, Thompson JC, Silverman A, Harper JW, DePinho RA, Elledge SJ. Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith-Wiedemann syndrome. Nature. 1997c;387:151–158. doi: 10.1038/387151a0. [DOI] [PubMed] [Google Scholar]
- Zhou G, Lefebvre V, Zhang Z, Eberspaecher H, de Crombrugghe B. Three high mobility group-like sequences within a 48-base pair enhancer of the Col2a1 gene are required for cartilage-specific expression in vivo. J Biol Chem. 1998;273:1498914997. doi: 10.1074/jbc.273.24.14989. [DOI] [PubMed] [Google Scholar]
- Zhou X, Sasaki H, Lowe L, Hogan BL, Kuehn MR. Nodal is a novel TGF-beta-like gene expressed in the mouse node during gastrulation. Nature. 1993;361:543–547. doi: 10.1038/361543a0. [DOI] [PubMed] [Google Scholar]