Abstract
BACKGROUND
Hox transcription factors are well known for their role in skeletal patterning in vertebrates. They regulate gene expression during the development of cartilage, the precursor to mature bone. We previously reported that overexpression of the homeobox genes Hoxc8 and Hoxd4 results in severe cartilage defects, reduced proteoglycan content, accumulation of immature chondrocytes, and decreased maturation to hypertrophy. We have also shown that Hoxd4 transgenic mice whose diets were supplemented with folate had their skeletal development restored. Since folate is required for growth and differentiation of chondrocytes, we hypothesized that the beneficial effect of folate in Hoxd4 transgenic mice might indicate a local deficiency in folate utilization, possibly caused by deregulation of genes encoding folate transport proteins or folate metabolic enzymes.
METHODS
We assayed the prevalence of transcripts for 22 folate transport proteins and metabolizing enzymes, here collectively referred to as folate pathway genes. Quantitative real-time PCR was performed on cDNA samples derived from RNA isolated from primary chondrocytes of individual rib cartilages from Hoxd4 and Hoxc8 transgenic mice, respectively.
RESULTS
This study shows that the Hox transgenes produce overexpression of Hoxd4 and Hoxc8 in primary chondrocytes from perinatal transgenic mice. However, no differences were found in expression levels of the folate pathway genes in transgenic cells compared to littermate controls.
CONCLUSIONS
Our results provide evidence that folate pathway genes are only indirect targets of Hox transgene overexpression in our transgenic animals. These expression studies provide a baseline for future studies into the role of folate metabolism in chondrocyte differentiation.
Keywords: chondrocytes, homeobox genes, hypertrophy, nutritional supplementation, gene-environment interaction, cartilage maturation, transcription factor, folic acid, expression profiling, embryonic development, cell differentiation, extracellular matrix, proteoglycan, rib cage
INTRODUCTION
Hox genes encode transcription factors that are involved in patterning the individual elements of the developing skeleton (Capecchi, 1996). They also play a role in the regulation of cartilage differentiation prior to overt bone formation (Yokouchi et al., 1995; Knezevic et al., 1997; Yueh et al., 1998), and in gene regulation in osteoblastic cells (Shi et al., 1999; Yang et al., 2000).
We analyzed cartilage from Hoxc8 and Hoxd4 transgenic mice generated in the VP16-based binary system for trans-gene activation (Kappen, 1999). Overexpression of the homeobox genes Hoxd4 and Hoxc8 results in severe cartilage defects and perinatal lethality (Yueh et al., 1998; Kappen et al., 2004). The cartilage of the ribs in transgenic animals is weak and structurally insufficient, resulting in pulmonary failure and death shortly after birth. Vertebral and rib cartilage contain an accumulation of proliferating chondrocytes, indicating that cartilage maturation is affected by overexpression of Hoxc8 and Hoxd4, respectively, with reduced progression of chondrocytes to hypertrophy. These results implicate Hox genes as important regulators of cartilage development and differentiation.
Folic acid is a water-soluble vitamin in the B-complex group and an essential nutrient. Supplementation of folate in the diet of pregnant mothers is known to reduce the risk for neural tube defects (Czeizel, 1996) and craniofacial anomalies (Czeizel et al., 1999). The effects of folate on skeletal development are poorly understood. Cell culture experiments show that primary chondrocytes isolated from neonatal ribs need folate to proliferate and differentiate to hypertrophy (Kappen et al., 2004). We previously showed that the cartilage defects induced by overexpression of Hoxd4 can be rescued by folate supplementation (Kappen et al., 2004). Alcian Blue staining of cartilage in ribs and vertebral column was restored, and rigidity of the skeleton was improved (Kappen et al., 2004). Taken together with an earlier report of beneficial action of folate on cranial bone development in the Cart-1-mutant mouse (Zhao et al., 1996), these are the first reports implicating folate in skeletal development in mice genetically predisposed to defects in the skeletal system.
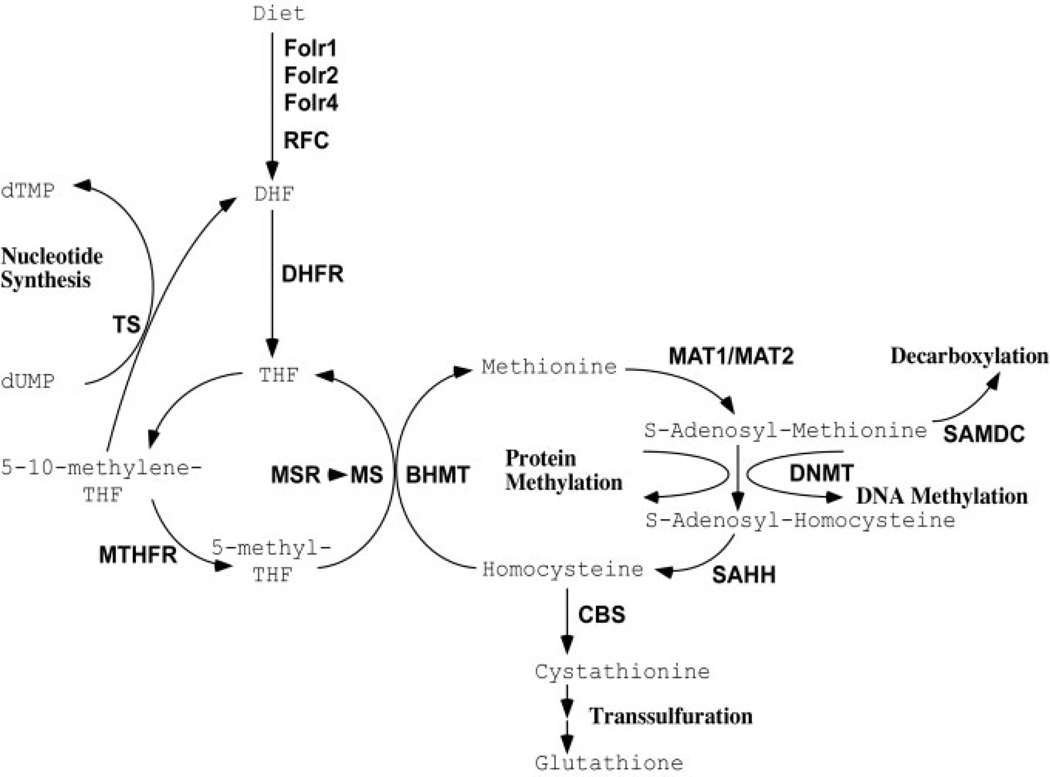
Given that the Hoxd4-induced cartilage defects respond to folate supplementation and that chondrocytes require folate for proliferation and differentiation, we hypothesized that the observed defects in chondrocyte proliferation and differentiation in Hoxd4 and Hoxc8 transgenic mice might be due to local folate deficiency. Local folate deficiency could arise in transgenic chondrocytes if the expression of enzymes that metabolize folate is altered by the overexpression of the Hox transgenes. The metabolism of folate affects multiple cellular processes, including methylation of DNA and proteins, and their synthesis. Any one of the cellular processes involving folate or its metabolites (for a schematic, see Fig. 1) might be affected in delayed maturation of chondrocytes. For example, defective DNA synthesis could be responsible for the apparently longer cell cycle in Hoxc8 transgenic chondrocytes (Cormier et al., 2003); similarly, reduced protein synthesis would be consistent with reduced proteoglycan content of Hoxc8 transgenic cartilage (Yueh et al., 1998), and other transcriptional changes could be associated with altered DNA or protein methylation. Finally, an elevated rate of elimination of folate from the metabolic cycle could also account for reduced availability of folate in chondrocytes. To investigate the proposition that folate pathway genes might be directly regulated by Hox transcription factors, we assayed the expression of folate pathway genes in chondrocytes from Hoxd4 and Hoxc8 transgenic mice.
Figure 1.
Folate pathway. Diagram of folate metabolic pathway compiled from the literature.
MATERIALS AND METHODS
Transgenic Mice
We used mice created by the VP16-dependent binary system (Kappen, 1999) for expression of Hox transgenes. The characterization of the phenotypes and similarities of defects in Hoxd4 and Hoxc8 transgenic mice have been published (Yueh et al., 1998; Kappen et al., 2004). In brief, the transactivator transgenic line (TA) harbors the transgene encoding VP16 under the control of the developmentally regulated promoter from the Hoxc8 gene. The other line, the transresponder (TR), harbors a Hox transgene under the control of an immediate early (IE) promoter from the ICP4 gene of herpes simplex virus 1. Activation of the IE promoter of the TR transgene requires the presence of VP16 protein. All transgenes were on a homogenous FVB inbred genetic background.
Hoxc8 transgenic mice are obtained from crosses of the T239 transactivator line (TA/TA +/+) to mice carrying both the Hoxc8-VP16 TA and the IE-Hoxc8 TR transgenes in hemizygous configuration (TA/+ TR/+). From these matings, we obtained 4 different genotypes: TA/+ +/+, TA/TA +/+, TA/+ TR/+, and TA/TA TR/+, the first 2 of which do not contain the Hoxc8 TR transgene and therefore served as littermate controls. The Hoxc8 transgenic mice are born with open eyes, a condition already evident at the time of collection, providing an easy visual ascertainment criterion for the expression of the Hoxc8 TR transgene (Yueh et al., 1998). Hoxd4 transgenic mice were generated by crossing the same transactivator strain (T239) to an IE-Hoxd4 hemizygous transresponder strain (+/+ TR/+). Lack of eyelid closure is also fully concordant with Hoxd4 transgene status (Kappen et al., 2004). The results presented here are classified by the control genotype containing at least one TA but no TR locus (TA/+ +/+, or TA/TA +/+, also referred to as TA only), and the experimental genotype containing at least one TA and one TR locus (TA/+ TR/+ or TA/TA TR/+, also referred to as TA + TR).
Genotyping was performed by semiquantitative PCR (Rundle et al., 1998; Yueh et al., 1998) on DNA isolated from tails of individual animal specimen. All samples were processed with tracking of information on individual and family of origin.
Preparation of Primary Chondrocytes from Transgenic Mice
At 18.5 days of gestation (the morning with presence of a vaginal plug being counted as 0.5 days), pregnant dams were sacrificed following standard laboratory procedures, and the embryos (day 18.5 of gestation) were collected. Individual rib cages were dissected as described previously (Cormier et al., 2003). Cells were transferred into Trizol reagent (Invitrogen, Carlsbad, CA) and RNA was extracted as follows: 20% vol chloroform was added, and samples were shaken vigorously by hand for 15 sec, followed by centrifugation at 12,000 rpm for 15 min at 4°C. The upper aqueous layer was removed to fresh tubes, and 5 µg glycogen was added to assist precipitation, which was performed with 0.5 ml isopropyl alcohol/ml of Trizol. Tubes were placed at −20°C for 20 min followed by centrifugation at 12,000 rpm for 15 min at 4°C. The supernatant was removed and the pellet was washed with 500 µl of 70% isopropyl alcohol. After final centrifugation at 7500 rpm for 5 min at 4°C, the supernatant was removed, and the pellet was air dried for 5 to 10 min and dissolved in 100µl of ultra pure DNase/RNase-free water.
Complementary DNA was obtained by reverse transcription (1st Strand Synthesis System for RT-PCR; Invitrogen, Carlsbad, CA) of at least 5 µg of RNA of each sample, following the supplier’s instructions. Purification of cDNA was accomplished using QIAquick PCR purification columns (Qiagen, Valencia, CA). RNA as well as cDNA concentrations were measured with a NanoDrop ND-1000 Spectrophotometer (NanoDrop Technologies, Rockland, DE).
Primers
Primers for amplification were designed using Primer Express software (Applied Biosystems, Foster City, CA). For all primer pairs, the same parameters were used: Tm (melting temperature) requirements: minimum Tm 58°C; maximum Tm 60°C; optimal Tm 59°C; GC content requirements: minimum GC content 30%; maximum GC content 80%; length requirements: minimum length 9; maximum length 40; optimal length 20 base pairs; amplicon requirements: minimum Tm 0°C; maximum Tm 85°C; minimum length 50; maximum length 150 base pairs. Primers for the gene Gapdh were used as provided by Applied Biosystems. The locations and sequences of primers are listed in Table 1. Where possible, the expected product amplicon was designed to span an exon/exon junction so that amplification of potentially contaminating genomic DNA would be excluded.
Table 1.
Genes Investigated in This Study: Primer Location, Sequences, and Efficiency*
| Abbreviation | Full name (alternative abbreviation) | Accession # | Forward primer position |
Reverse primer position |
Exon-exon boundary? |
|---|---|---|---|---|---|
| Bhmt | Betaine-homocysteine methyltransferase | NM_016668 | 458–478 | 545–523 | NO |
| Cbs | Cystathionine beta-synthase | NM_144855 | 1086–1106 | 1161–1184 | YES |
| Dhfr | Dihydrofolate reductase | NM_010049 | 438–460 | 558–538 | YES |
| Dnmt1 | DNA methyltransferase (cytosine-5) 1 | NM_010066 | 384–413 | 493–470 | NO |
| Dnmt2 | DNA methyltransferase 2 | NM_010067 | 208–236 | 287–268 | YES |
| Dnmt3a | DNA methyltransferase 3A | NM_007872 | 751–769 | 874–849 | YES |
| Dnmt3b | DNA methyltransferase 3B | NM_010068 | 1542–1563 | 1646–1622 | YES |
| Folr1 | Folate receptor 1 | NM_008034 | 519–536 | 585–606 | YES |
| Folr2 | Folate receptor 2 | NM_008035 | 402–424 | 473–492 | YES |
| Folr4 | Folate receptor 4 | NM_022888 | 946–968 | 1022–1003 | NO |
| Gapd | Glyceraldehyde-3-phosphate dehydrogenase | NM_008084 | 649–669 | 751–733 | NO |
| Hoxc8 | Homeobox c8 | NM_010466 | 610–631 | 725–702 | YES |
| Hoxd4 | Homeobox d4 | NM_010469 | 1658–1678 | 1748–1722 | YES |
| Mat1a | Methionine adenosyl-transferase 1, alpha | NM_133653 | 1162–1182 | 1246–1226 | YES |
| Mat2a | Methionine adenosyl-transferase 2, alpha | NM_145569 | 500–525 | 580–560 | NO |
| Ms | Methionine synthase (Mtr) | XM_138431 | 2309–2329 | 2459–2439 | YES |
| Msr | Methionine synthase reductase (Mtrr) | NM_172480 | 444–462 | 522–502 | YES |
| Mthfr | 5, 10-methyleneletrahydrofolate reductase | NM_010840 | 1608–1627 | 1690–1674 | YES |
| Nat1 | N-acetyl transferase 1 | NM_008673 | 921–943 | 1024–997 | NO |
| Nat2 | N-acetyl transferase 2 | NM_010874 | 194–218 | 276–257 | NO |
| Nat3 | N-acetyl transferase 3 | NM_008674 | 598–622 | 717–693 | NO |
| Rfc1 | Reduced folate carrier (Slc19a1) | NM_031196 | 294–315 | 344–322 | YES |
| Amd1 | S-adenosylmethionine decarboxylase 1 | NM_009665 | 650–674 | 745–719 | NO |
| Sahh | S-adenosyl homocysteine hydrolase (Ahcy) | L32836 | 555–578 | 622–605 | YES |
| Tysy | Thymidylate synthase (Tyms) | NM_021288 | 234–255 | 313–291 | YES |
| Abbreviation | Forward primer; sequence | Reverse primer; sequence | Amplification rate |
Efficiency (±SD) |
Number of samples |
| Bhmt | GAACGTGGACTTCCTCATTGC | GGGCTTACCAGATGCTTTTAAGG | 1.95 | 0.95 (±0.24) | 21 |
| Cbs | GACGGAGCAAACAGCCTATGA | CATTGCTCTTGAACCACCTATCC | 1.59 | 0.59 (±0.22) | 24 |
| Dhfr | AACCAGGCCACCTTAGACTCTTT | GAGAGGACGCCTGGGTATTCT | 2.20 | 1.20 (±0.07) | 22 |
| Dnmt1 | GGAAACCAAATTACATAAAGAGGAATTATC | GAGTGAGAGTGTGTGTTCCGTTCT | 1.99 | 0.99 (±0.19) | 21 |
| Dnmt2 | AGACTTTGACAAGCTATCTTTCAATATGA | CCCCCTGTAGGCCAATTCTT | 1.66 | 0.66 (±0.12) | 22 |
| Dnmt3a | CGACCCATGCCAAGACTCA | TTACTGCAATTACCTTGGCTTTCTTC | 1.32 | 0.32 (±0.10) | 18 |
| Dnmt3b | AGAAGAACCCTGTGTCCTTCCA | GATAGCCGTCCTCATCATACATGTA | 1.78 | 0.78 (±0.10) | 21 |
| Folr1 | CGGGCCCTGAGGACAATT | TTATGTGCTTCCTGGCTTGTGT | 1.38 | 0.38 (±0.27) | 32 |
| Folr2 | GACAAGCTGCATGACCAGTGTAG | GACGGGAGTCAGCCTTGTGT | 1.63 | 0.63 (±0.31) | 38 |
| Folr4 | CCTTCTCTCTGTGCCTGTTGTTC | GGCCCAGAAGGATCAGGAAA | 1.82 | 0.82 (±0.15) | 41 |
| Gapd | CCAGAACATCATCCCTGCATC | GGTAGGAACACGGAAGGCC | 1.83 | 0.83 (±0.11) | 78 |
| Hoxc8 | CGAAGGACAAGGCCACTTAAAT | AGGTCTGATACCGGCTGTAAGTTT | 1.84 | 0.84 (±0.18) | 62 |
| Hoxd4 | TTCGGTGAACCCCAACTACAC | AAATTCCTTTTCCAGTTCTAGGACTTG | 1.33 | 0.33 (±0.13) | 55 |
| Mat1a | CAGGTGTCCTATGCCATTGGT | GCTCCCGCTCAGTCTTATTGG | 1.80 | 0.80 (±0.23) | 21 |
| Mat2a | TGAAGAGTGTATGCCTTTAACCATTG | AATGTACCATTGCGGCGTAGT | 1.66 | 0.66 (±0.17) | 40 |
| Ms | AAGCTGCTCTGGACCACAAAG | TGGGTTCTTGAAGTGGTTGCT | 1.78 | 0.78 (±0.12) | 22 |
| Msr | CTTGGAGCCCAGCGTTTCT | CAATCCACGGCTCTACCACAA | 1.98 | 0.98 (±0.08) | 22 |
| Mthfr | TGCGGGTCAACTACCACATC | CCACGTCACGGCATTGG | 1.71 | 0.71 (±0.16) | 30 |
| Nat1 | GGGCTCCACCTTTACAAGTAGGA | CGGTTTTCAGTACATCTTCTATTTCTTC | 2.05 | 1.05 (±0.25) | 26 |
| Nat2 | TGAAAACATTAACCGAAATCCTTCA | CATGGATTCCCCACAATGGA | 1.74 | 0.74 (±0.13) | 34 |
| Nat3 | TGTAGATAGTGCATTCCCATTTTCC | TACCAGGTTCCATTCTCTTCTCTCA | 1.46 | 0.46 (±0.21) | 37 |
| Rfc1 | TGGAACGTAAATTCACCAAGGA | GCATCGGAATGATCTCGTTAGTC | 1.85 | 0.85 (±0.14) | 26 |
| Amd1 | GGAAGAAATCGAGTTTCTTAATGCA | TCCAAAGTATACAAGTACCAGCAGTCA | 1.76 | 0.76 (±0.11) | 22 |
| Sahh | GTCACCAAGAGCAAGTTTGACAAC | GCCCGTTTGATGCCATCT | 1.94 | 0.94 (±0.07) | 22 |
| Tysy | TTTTGGAGGAGTTGTTGTGGTTT | CATCCCAGATTCTCACTCCCTTT | 1.36 | 0.36 (±0.15) | 37 |
Messenger RNA sequences for the investigated genes were taken from GenBank (accession numbers), and the positions corresponding to primer sequences are listed. Where possible, the amplicon spanned across an exon-exon junction. Sequences of the gene-specific primers are given in the lower half of the table (in 5′ to 3′ direction), as is the amplification rate achieved with each primer set. The amplification rate was calculated from the formula r = e + 1 (see Methods), where e was determined from the slope of the detection curve in the linear range. Mean e values (± SDs) were determined from the number of samples given in the last column.
Quantitative Real-Time PCR
Gene expression was evaluated in independent families of Hoxc8 and Hoxd4 transgenic mice and their nontransgenic littermates using the ABI Prism 7000 Instrument (Applied Biosystems). Each PCR reaction (25 µl) was performed on 4 ng of template cDNA, 100 nM each primer, and SYBR Green Master Mix (Applied Biosystems). The PCR reactions consisted of a denaturation step of 15 sec at 95°C, annealing for 2 min at 50°C, and extension for 1 min at 60°C, for a total of 40 cycles. Each cDNA sample was assayed in triplicate, and the cycle number at first detection of signal above threshold (Ct) was determined. Analysis was performed with ABI Prism 7000 SDS Software Version 1.0 (Applied Biosystems). Triplicate measurements for a given sample were averaged, and the values for each gene were then normalized to measurements for Gapdh cDNA in the same sample applying the formulas: Ctgene − CtGapdh ΔΔCt. Comparison of transgenic samples to nontransgenic littermate controls was achieved in a second subtraction that yielded the ΔΔCt values: ΔΔCt ΔΔCttransgenic − ΔCtcontrol. Amplification efficiencies were determined for each gene-specific reaction from the slope of the linear portion of the amplification reaction averaged over at least 10 samples and yielded the efficiencies and amplification rates shown in Table 1. Amplification rates did not differ between controls and transgenic samples; we thus did not see a need for further correction of the normalized ΔCt or ΔΔCt values, as this would only affect estimations of absolute levels of gene expression but not the relative levels in a comparison of experimental and control samples. Consequently, all data presented here have to be considered relative quantifications, and the results are therefore presented as ΔCt or ΔΔCt units. In order to estimate the “relative fold change” in expression level for comparisons of select samples, we used the formula f = rΔΔCt, with r representing amplification rate (r = amplification efficiency e + 1).
Statistical Evaluation
For statistical analyses, we used the software modules implemented in Microsoft Excel (Microsoft, Redmond, WA) as well as S-PLUS 6.2 for Windows (S-PLUS; Insightful Corp., Seattle, WA). Student t tests were conducted to analyze difference in gene expression between the controls and TR-containing transgenic mice.
RESULTS
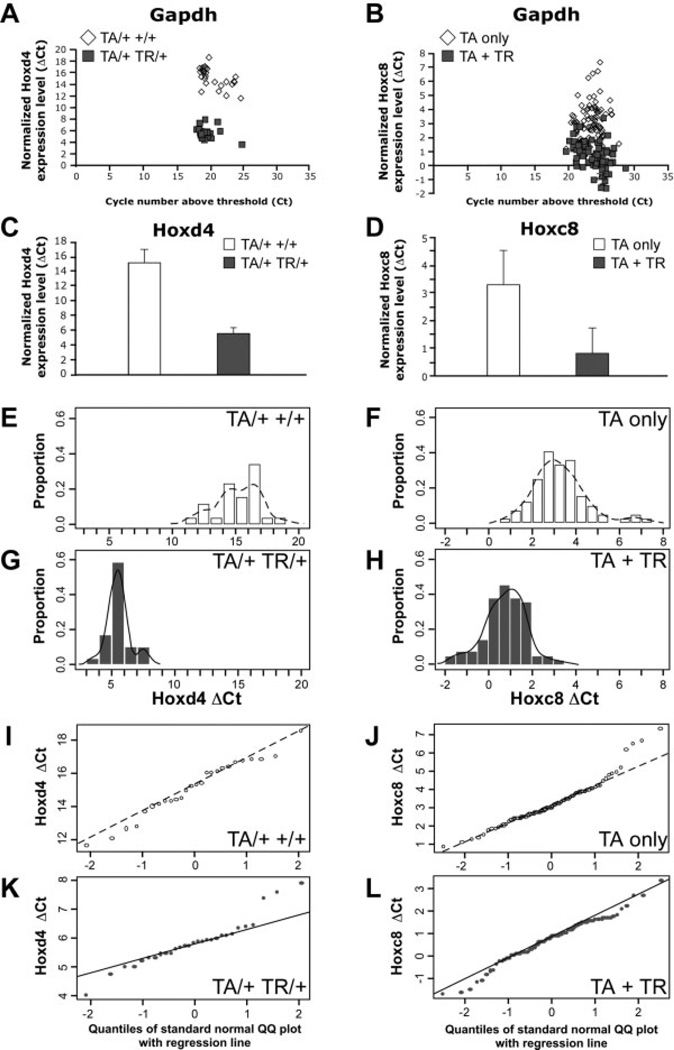
In the first assays, we measured the levels of expression of Hoxc8 and Hoxd4 in respective control and transgenic animals. Hoxd4-transgenic and control animals were generated in crosses of parents with the genotypes TA/TA +/+ and +/+ TR/+. Six different families with a total of 29 TR transgenic progeny (TA/+ TR/+) and 26 control progeny (TA/+ +/+) were investigated. The mean cycle number above the detection threshold level (Ct) for Hoxd4 expression in control samples was 35.8 ± 1.7 (mean ± SD); this relatively high number indicates relatively low to absent expression of Hoxd4 in non-transgenic control chondrocytes. The mean Ct number in TR-containing samples was 25.3 ± 1.8. This was lower than in controls, indicating that the expression level of Hoxd4 is higher in these transgenic samples. Normalized to Gapdh expression in each sample (Fig. 2A), the mean of the ΔCt was 15.2 ± 1.7 for transgenic Hoxd4 samples, compared to 5.6 ± 0.9 in the controls (Fig. 2C), demonstrating a statistically significantly elevated expression of the Hoxd4 gene in transgenic animals (P < .0001). On average, Hoxd4 transgene activation leads to 15.4-fold higher levels of Hoxd4 expression in transgenic chondrocytes with very low expression in control samples. To visualize the distribution of values in each experimental group, we ordered ΔCt values into bins of equal intervals and plotted the fraction of observations that fell into each bin interval in histograms (Fig. 2E and G). As shown in Figure 2E, 73% of the ΔCt values in control samples were between 14 and 17. In transgenic samples (Fig. 2G), the distribution is centered in the 4 to 6 interval (72% of ΔCt values), illustrating the elevated Hoxd4 expression levels in Hoxd4 transgenic samples. Overlaid on the histograms are nonparametric estimates of the probability density functions. Each density curve describes the overall pattern of a distribution that has an area of exactly 1 underneath it. From the density graphs for Hoxd4 transgenic and control samples (Fig. 2E and G), it is evident that there is no overlap of Hoxd4 gene expression levels between controls and transgenic mice, demonstrating that Hoxd4 is overexpressed only in TR-containing mice.
Figure 2.
Increased expression of the homeobox genes Hoxd4 and Hoxc8 in chondrocytes from transgenic mice. The expression of Hoxd4 was measured by quantitative RT-PCR (qRT-PCR) in 56 samples from 6 different families, and expression of Hoxc8 was assayed in 163 samples from 23 families. The Ct value is the cycle number for detection above threshold, and the ΔCt value is the Ct value normalized to concurrent determination of Gapdh expression in each sample. The Ct value for Gapdh does not differ between controls (open symbols, open bars) and transgenic (filled symbols, filled bars) samples in Hoxd4 (A) and Hoxc8 (B) families. A low ΔCt value indicates a high level of expression and a high ΔCt value indicates low level of expression, respectively, of Hoxd4 (A,C) or Hoxc8 (B,D) in these samples. Histograms show the distributions for the Hoxd4 (E,G) and Hoxc8 (F,H) expression levels, separately for the controls (TA only; E,F) and the transgenic mice (TR-containing; G,H). The histograms are scaled as proportions of total data points, depicting probability density; the sum of the bar heights multiplied by bar widths will equal 1 (note the different scale of x-axis between E/F and G/H, respectively). A nonparametric estimate of the probability density function is overlaid in solid line. Normal quantile plots (I–L) indicate that the data are distributed normally, as the points lie close to a straight line.
Results for 23 families with 85 Hoxc8 transgenic (TA + TR) and 78 control individuals (TA only) also showed a significant overexpression of the Hoxc8 transgene (Fig. 2B, D, and H). The Ct number for the Hoxc8 gene in controls was 26.8 ±2.2, representing the normal level of Hoxc8 expression in chondrocytes, compared to 24.5 ±1.7 in TR-carrying samples. Normalized to the expression levels of Gapdh in each sample, the mean ΔCt value was 3.3 ±1.2 for the Hoxc8 transgenic animals compared to 0.78 ±0.94 for the control samples (P < .0001), demonstrating significantly increased expression of Hoxc8 in these transgenic mice (Fig. 2D). On average, Hoxc8 expression in Hoxc8 transgenic primary chondrocytes is elevated by 2.52 cycles, or 4.6-fold over the mean of the controls. The significant differences of the ΔCt values between transgenics and controls demonstrate that our RT-PCR system is able to identify increased expression of genes of interest (Table 2) in our animal models.
Table 2.
Functional Role of Folate Pathway Genes*
| Abbreviation | Enzyme | EC | Reaction catalyzed | Pathway |
|---|---|---|---|---|
| Bhmt | Betaine-homocysteine S-methyltransferase | 2.1.1.5 | Trimethylammonioacetate + L-homocysteine = dimethylglycine + L-methionine | Methyl transfer |
| Cbs | Cystathionine beta-synthase | 4.2.1.22 | L-serine + L-homocysteine = cystathionine + H2O | Transsulfuration |
| Dhfr | Dihydrofolate reductase | 1.5.1.3 | 5,6,7,8-tetrahydrofolate + NADP(+) = 7,7-dihydrofolate + NADPH | Folate metabolism |
| Dnmt | DNA methyltransferase | 2.1.1.37 | S-adenosyl-L-methionine + DNA = S-adenosyl-L-homocysteine + DNA containing 5-methylcytosine | DNA Methylation |
| Mat | Methionine adenosyltransferase | 2.5.1.6 | ATP + L-methionine + H2O = phosphate + diphosphate + S-adenosyl-L-methionine | Methyl transfer |
| Ms | Methionine synthase | 2.1.1.13 | 5-methyltetrahydrofolate + L-homocysteine = tetrahydrofolate + L-methionine | Methylation |
| Msr | Methionine synthase reductase | 1.16.1.8 | 2 [methionine synthase]-methylcob(l)alamin + 2 S-adenosylhomocysteine + NADP (+) = 2 [methionine synthase]-cob (l)alamin + NADPH + 2 S-adenosyl-L-methionine | Methylation |
| Mthfr | Methylenetetrahydrofolate reductase | 1.5.1.20 | 5-methyltetrahydrofolate + NADP(+) = 5, 10-methylenetetrahydrofolate + NADPH | Folate metabolism |
| Nat | Arylamine N-acetyltransferase | 2.3.1.5 | Acetyl-CoA + an arylamine = CoA + an N-acetylarylamine | Acetyl transfer |
| Amd | S-adenosylmethionine decarboxylase | 4.1.1.50 | S-adenosyl-L-methionine = (5-deoxy-5-adenosyl) (3-aminopropyl) methylsulfonium salt + CO2 | Decarboxylation |
| Sahh | S-adenosyl-L-homocysteine hydrolase | 3.3.1.1 | S-adenosyl-L-homocysteine + H2O = adenosine + L-homocysteine | Methionine cycle |
| Tysy | Thymidylate synthase | 2.1.1.45 | 5, 10-methylenetetrahydrofolate + dUMP = dihydrofolate + dTMP | Nucleotide synthesis |
| Folr | Folate receptor | Binds to folate and reduced folic acid derivates and mediates delivery of 5-methyltetrahydrofolate to interior of cells | Folate metabolism | |
| Rfc | Reduced folate carrier | Transporter for the intake of folate | Folate transport |
Information on folate pathway genes assayed in this study was assembled from the NCBI (www.ncbi.nlm.nih.gov) and BRENDA (www.brenda.uni-koeln.de) databases.
The histograms in Figure 2E, G, F, and H reveal, for each control or transgenic group, that individual measurements of ΔCt were distributed over a range of values. Quantile-quantile plots (Q-Q plots) were used to assess normality of the distributions. For Hoxd4 transgenic as well as control samples, the points on a Q-Q plot were close to a straight line (Fig. 2I and K), indicating that the data are distributed normally. The Q-Q plots for Hoxc8 transgenic and respective control samples (Fig. 2J and L) again showed that the results followed a normal distribution.
The histograms for Hoxc8 transgenic and control progeny (Fig. 2F and H) show that there was some overlap of Hoxc8 gene expression levels between the controls and transgenic samples. This was expected, since the Hoxc8 gene is normally expressed in rib chondrocytes, and Hoxc8 transgene activation elevates the overall Hoxc8 expression levels (Rundle et al., 1998). If animals were grouped strictly based upon the levels of Hoxc8 expression, the overlap between the groups of nontransgenic and transgenic animals would cause concern about potential misclassification. If a cutoff value of 2 is used to divide the mice into control and transgenic group, 9 control mice, and 5 TR-containing mice would be misassigned, representing fractions of 12% and 6%, respectively. Conversely, 88% and 94% of animals, respectively, would be classified correctly, providing a 90% confidence level to classification solely on the basis of Hoxc8 gene expression levels. In contrast, the Hoxd4 transgene is activated in cells that do not normally express Hoxd4. Consequently, discrimination between controls and Hoxd4 transgenic individuals by Hoxd4 expression levels was absolute. Nevertheless, we used genotyping for transgene status to unequivocally classify control and transgenic samples. Since it is conceivable that any changes in folate pathway gene expression are dependent on the relative level of transgene expression, we correlated all measurements to transgene expression levels in each individual sample.
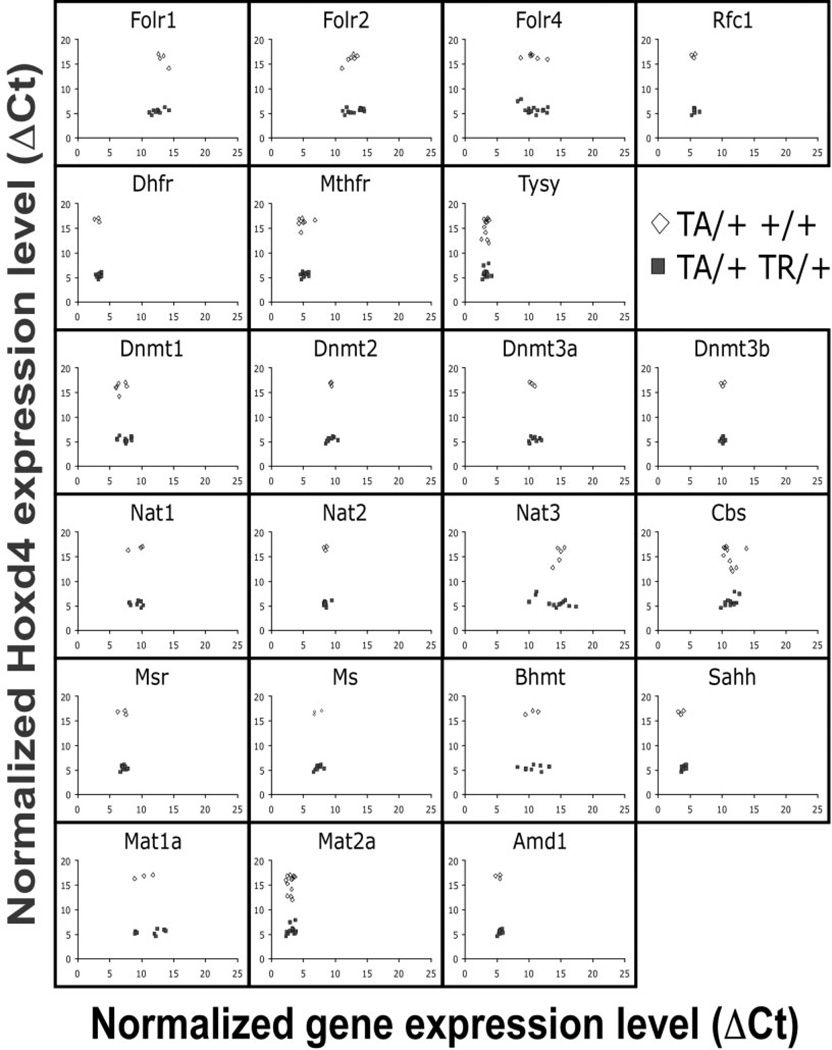
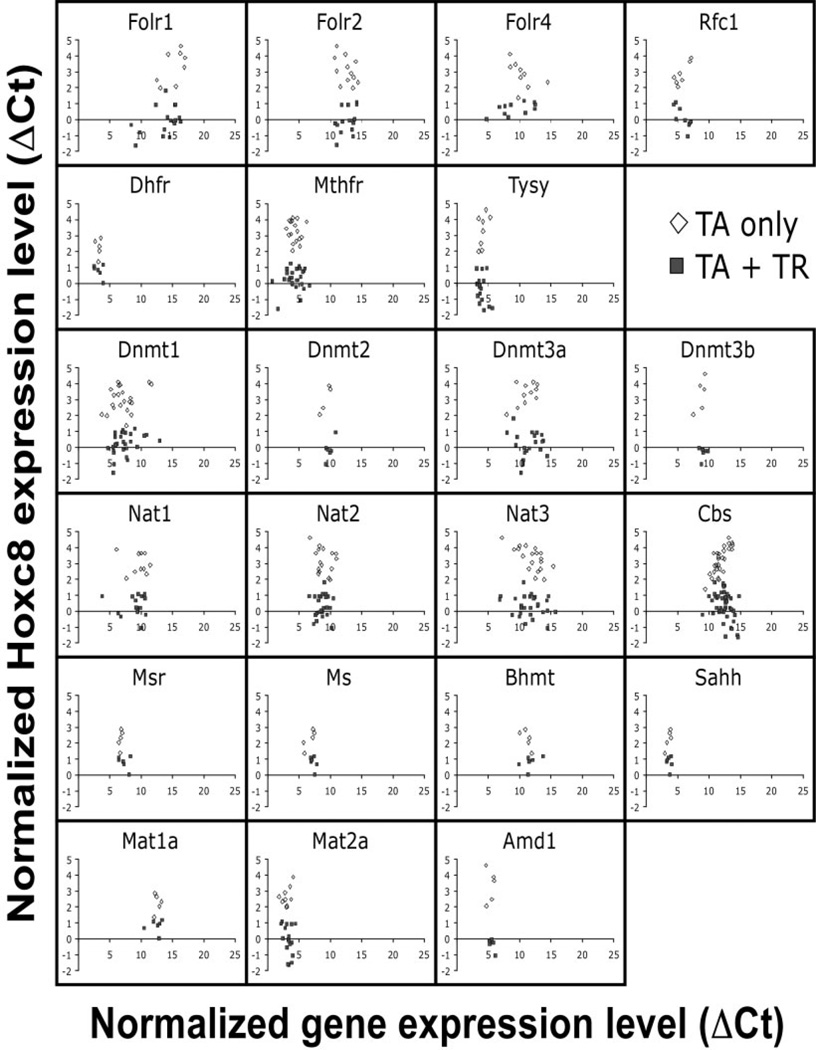
Genes of the folate pathway investigated in this study are listed in Table 2. Expression of each gene was assayed by quantitative real-time PCR in triplicates on chondrocytes from individual rib cages of our Hoxd4 and Hoxc8 transgenic animals. For the Hoxd4 transgenic line, we investigated at least 3 different control and 8 TR-containing samples (Fig. 3A). At least 5 controls and 6 TR-containing samples were investigated for the Hoxc8 transgenic line (Fig. 3B). The results are plotted as ΔCt (expression level for each gene normalized to Gapdh) relative to the ΔCt values for Hoxd4 or Hoxc8 gene expression, in each sample. Lower ΔCt values indicate higher gene expression levels; high ΔCt values correspond to lower expression levels. Each data point represents the average of triplicate measurements for an individual animal. Animals carrying a TR transgene (Fig. 3A and B; filled symbols) always had a lower ΔCt value for expression of the Hoxd4 and Hoxc8 transgene, indicating that Hoxd4 and Hoxc8 gene expression is higher than in non-transgenic littermate controls (Fig. 3A and B; open symbols). Individual genes in the folate pathway were found expressed at different relative levels; for example, the low ΔCt values for Dhfr indicate high overall expression, the high ΔCt values for Cbs and Nat3 indicate that expression of these genes is generally at very low levels or absent in chondrocytes. These results show that the quantitative PCR assay detects folate pathway gene expression in primary chondrocytes with high fidelity. The close clustering of data points for each gene in the X-axis dimension demonstrates high consistency of measurements between animals, with higher levels of expression for Dhfr, Tysy, Sahh, and Mat2a, intermediate expression for Dnmt, Nat1, and Nat2, and low expression or absence of Folr1, Nat3, Mat1a, and Cbs.
Figure 3.
Expression of folate pathway genes in Hoxd4 and Hoxc8 transgenic chondrocytes. Quantitative RT-PCR was performed in triplicate on mRNA of primary chondrocytes from rib cages of individual Hoxd4 (A) and Hoxc8 (B) transgenic mouse embryos isolated at 18.5 days of gestation. Gapdh cDNA levels in each sample were used to standardize measurements. The results are plotted as cycle number above the detection threshold (Ct) for each primer pair relative to Gapdh-normalized Hoxd4 and Hoxc8 gene expression (ΔCt) in each sample. Low ΔCt values reflect higher relative expression levels, and high ΔCt values reflect low relative levels of gene expression. Each symbol represents an individual animal (open symbols = controls; filled symbols = TR-containing samples).
The comparison of folate pathway gene expression between transgenic and control animals revealed no significant differences for the ΔCt values of folate pathway genes. All 22 genes investigated were expressed at the same relative levels in Hoxd4 transgenics compared to nontransgenic controls; the same observation pertains to Hoxc8 transgenic animals. Table 3 summarizes all results as means of ΔCt values. No significant differences in expression of folate pathway genes were detected between transgenics and respective controls; there were also no differences between just the 2 control groups or the transgenic groups from the 2 strains.
Table 3.
Normalized Expression of Folate Pathway Genes in Hoxd4 and Hoxc8 Transgenic Animals*
|
Hoxd4 |
Hoxc8 |
|||
|---|---|---|---|---|
| Gene | TA/+ +/+ | TA/+ TR/+ | TA only | TA + TR |
| Bhmt | 10.4 ± 0.99 (n = 3) | 10.6 ± 1.61 (n = 8) | 11.1 ± 0.74 (n = 5) | 11.6 ± 1.25 (n = 6) |
| Cbs | 11.41 ± 1.25 (n = 12) | 11.38 ± 0.88 (n = 11) | 11.77 ± 1.11 (n = 27) | 12.50 ± 1.28 (n = 47) |
| Dhfr | 3.01 ± 0.41 (n = 3) | 3.3 ± 0.33 (n = 8) | 3.2 ± 0.35 (n = 5) | 3.23 ± 0.65 (n = 6) |
| Dnmt | 8.19 ± 0.65 (n = 3) | 8.95 ± 0.56 (n = 8) | 8.11 ± 0.47 (n = 5) | 8.48 ± 0.57 (n = 6) |
| Dnmt1 | 6.1 ± 0.49 (n = 6) | 6.62 ± 0.55 (n = 12) | 6.74 ± 2.0 (n = 18) | 6.89 ± 1.88 (n = 28) |
| Dnmt2 | 9.31 ± 0.11 (n = 3) | 9.23 ± 0.48 (n = 8) | 9.55 ± 1.05 (n = 5) | 9.82 ± 0.59 (n = 6) |
| Dnmt3a | 10.46 ± 0.36 (n = 3) | 10.79 ± 0.74 (n = 8) | 10.85 ± 1.54 (n = 13) | 11.45 ± 1.78 (n = 20) |
| Dnmt3b | 10.07 ± 0.27 (n = 3) | 10.07 ± 0.27 (n = 8) | 8.67 ± 0.75 (n = 5) | 9.05 ± 0.48 (n = 6) |
| Folr1 | 13.33 ± 0.78 (n = 4) | 12.73 ± 1.10 (n = 10) | 14.68 ± 1.98 (n = 8) | 13.83 ± 2.54 (n = 17) |
| Folr2 | 12.57 ± 0.85 (n = 7) | 12.75 ± 1.37 (n = 12) | 12.45 ± 1.42 (n = 12) | 12.52 ± 1.74 (n = 17) |
| Folr4 | 9.91 ± 2.24 (n = 7) | 10.68 ± 1.46 (n = 14) | 10.34 ± 1.72 (n = 10) | 9.07 ± 2.59 (n = 12) |
| Mat1a | 10.3 ± 1.42 (n = 3) | 11.35 ± 2.01 (n = 8) | 12.55 ± 0.49 (n = 5) | 12.35 ± 1.03 (n = 6) |
| Mat2a | 3.01 ± 0.46 (n = 12) | 3.18 ± 0.52 (n = 14) | 3.08 ± 0.72 (n = 10) | 3.17 ± 0.64 (n = 18) |
| Ms | 7.04 ± 0.65 (n = 3) | 7.28 ± 0.54 (n = 8) | 6.67 ± 0.83 (n = 5) | 7.24 ± 0.37 (n = 6) |
| Msr | 7.02 ± 0.68 (n = 3) | 7.17 ± 0.39 (n = 8) | 6.72 ± 0.21 (n = 5) | 7.26 ± 0.8 (n = 6) |
| Mthfr | 4.95 ± 0.86 (n = 7) | 5.1 ± 0.39 (n = 12) | 4.44 ± 1.14 (n = 19) | 4.4 ± 1.58 (n = 28) |
| Nat1 | 9.22 ± 1.22 (n = 3) | 9.1 ± 0.89 (n = 8) | 9.08 ± 2.3 (n = 11) | 9.05 ± 1.84 (n = 16) |
| Nat2 | 8.41 ± 0.23 (n = 3) | 8.48 ± 0.4 (n = 8) | 8.95 ± 1.37 (n = 16) | 8.78 ± 1.03 (n = 25) |
| Nat3 | 14.13 ± 1.93 (n = 6) | 14.01 ± 2.13 (n = 12) | 11.97 ± 1.86 (n = 19) | 11.54 ± 2.06 (n = 27) |
| Rfc | 5.48 ± 0.3 (n = 3) | 5.68 ± 0.35 (n = 8) | 6.1 ± 1.74 (n = 8) | 6.07 ± 1.28 (n = 10) |
| Amd1 | 5.19 ± 0.4 (n = 3) | 5.42 ± 0.28 (n = 8) | 5.29 ± 0.63 (n = 5) | 5.48 ± 0.39 (n = 6) |
| Sahh | 3.51 ± 0.42 (n = 3) | 3.88 ± 0.32 (n = 8) | 3.51 ± 0.41 (n = 5) | 3.62 ± 0.31 (n = 6) |
| Tysy | 3.28 ± 0.37 (n = 11) | 3.23 ± 0.37 (n = 14) | 4.09 ± 0.62 (n = 8) | 3.9 ± 0.68 (n = 18) |
| Hoxd4 | 15.2 ± 1.7 (n = 26) | 5.6 ± 0.9 (n = 29) | 13.7 ± 0.83 (n = 14) | 14.5 ± 1.2 (n = 20) |
| Hoxc8 | 2.9 ± 0.61 (n = 11) | 3.0 ± 0.65 (n = 15) | 3.3 ± 1.2 (n = 78) | 0.78 ± 0.94 (n = 85) |
ΔCt values were determined separately for all controls (TA only) and TR-containing Hoxd4 and Hoxc8 transgenic animals, and means were calculated for all animals tested in each group (n = from 3 to 85) as indicated. Generally, larger standard deviations were found in cases of lower expression level (high ΔCt value) with correlation coefficients noted where significant at P = .05 (Fig. 5).
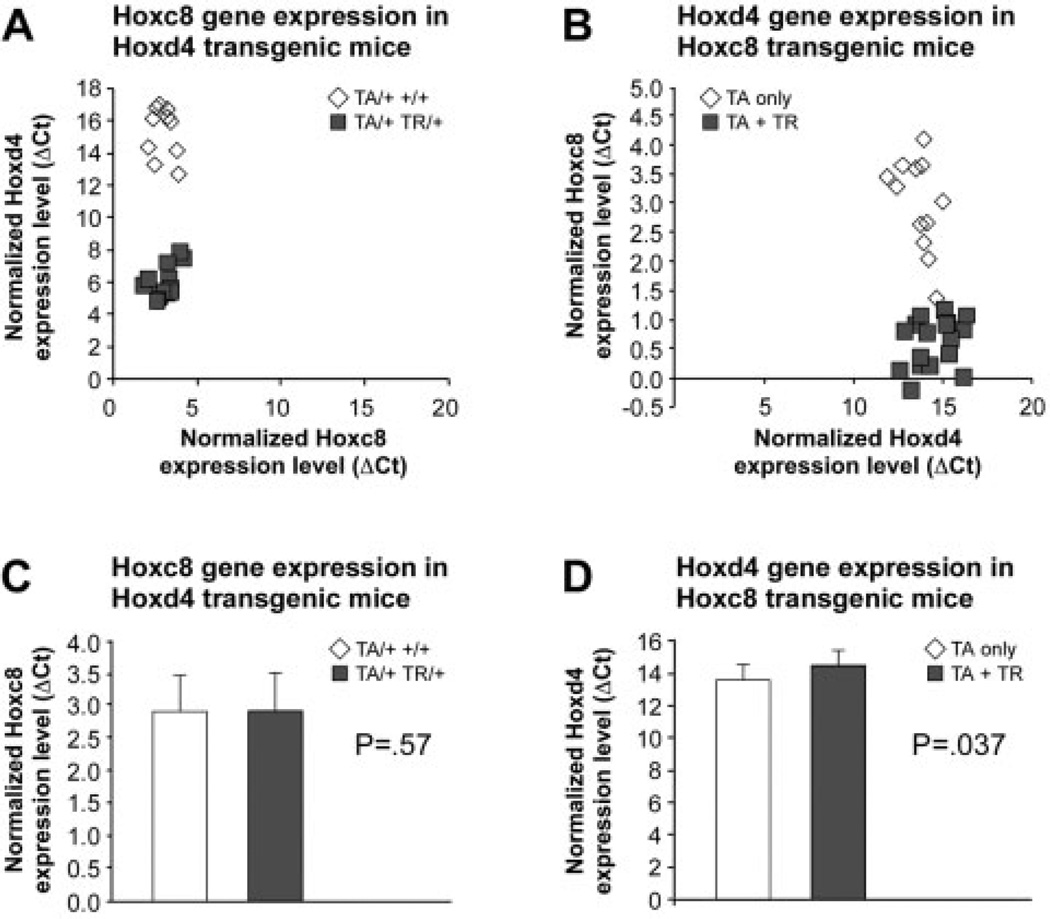
We also assessed the expression of each Hox gene in the rib cage chondrocytes from the reciprocal transgenic strain. Hoxc8 gene expression in Hoxd4 transgenic samples and littermate controls (Fig. 4A) did not reveal significant differences (P = .57; Fig. 4C). Hoxd4 expression levels in Hoxc8 transgenic mice were very low as indicated by a high mean ΔCt, and slightly lower on average in the transgenic chondrocytes compared to littermate controls (Fig. 4B and D). While this result reached statistical significance of P = .037, its biological significance is unclear at present given that the standard deviations for transgenic and control groups broadly overlap (Fig. 4C and D).
Figure 4.
Expression of Hoxd4 and Hoxc8 in transgenic chondrocytes. Expression of the Hoxc8 gene was measured in individual Hoxd4 transgenic and control samples (A), and Hoxd4 expression was assayed in individual Hoxc8 transgenic and control chondrocytes (B). Open symbols = controls; filled symbols = TR-containing samples. The results are plotted as Gapdhnormalized Hoxc8 and Hoxd4 expression levels relative to normalized levels of Hoxd4 and Hoxc8, respectively. Statistical evaluation by Student t test of control and transgenic groups (C, D) indicates a possible difference of Hoxd4 levels (P = .037) in Hoxc8 transgenic samples.
DISCUSSION
The goal of this study was to elucidate consequences of Hox transgene expression in chondrocytes at the transcriptional level. After discovering a beneficial effect of folate on cartilage development in Hoxd4 transgenic animals, we hypothesized that Hox transgene expression might affect the expression of folate pathway genes in the chondrocytes of Hoxd4 or Hoxc8 transgenic mice. We therefore set out to quantitatively measure Hox gene and transgene expression levels in Hoxc8 and Hoxd4 transgenic chondrocytes from individual animals and correlate these with the levels of expression of folate pathway genes in the same samples.
Quantitative real-time PCR was used to measure the relative level of mRNA present in chondrocyte samples from individual rib cages of transgenic and control animals. All assays were done in triplicate, giving generally high reproducibility for each sample. Between samples, there was considerable variability in gene expression levels, even within the same family. Nevertheless, transgenic groups were clearly distinguishable from control groups, as they showed very little overlap (up to 8.6% of the samples) between ΔCt values. Thus, we establish highly significant correlations between genotype for the respective Hox transgene and its expression level.
For Hoxc8 transgenic chondrocyte samples, we determined a 4.6-fold elevated level of Hoxc8 expression compared to controls. This is in very good agreement with our previous estimates by semiquantitative PCR and reporter gene assays that showed overexpression of Hoxc8 by 3.6-fold and 5.7-fold in the posterior region of Hoxc8 transgenic embryos at midgestation (Rundle et al., 1998). Our current results thus support the conclusion that a similar level of overexpression is achieved in transgenic chondrocytes at late stages of development. In Hoxd4 transgenic chondrocytes, 15.4-fold increased expression of Hoxd4 was found compared to controls. This apparently higher magnitude of transgene overexpression compared to the Hoxc8-transgenic chondrocytes may result from the fact that Hoxd4 expression levels in normal chondrocytes are exceedingly low. Alternatively, it is possible that the Hoxd4 transresponder transgene could be activated to a greater degree than the Hoxc8 transresponder transgene. However, in a previous study (Rundle et al., 1998), we have shown that efficacy of VP16-mediated transactivation is less dependent on the transresponder transgene than on the levels of activity of the transactivator and genotype status for the transactivator transgene (homozygous TA/TA versus hemizygous TA/+). The greater magnitude of Hoxd4 transgene overexpression is consistent with the finding that the defective cartilage phenotype is more pronounced in Hoxd4 transgenics that are hemizygous for the transactivator transgene (Kappen et al., 2004), whereas the most severe phenotype in Hoxc8-transgenic cartilage was only found in animals homozygous for the transactivator transgene locus (Yueh et al., 1998). Taken together, these considerations support the notion derived from genetic evidence (Condie and Capecchi, 1993, 1994; Fromental-Ramain et al., 1996) that the level of Hox gene expression is critical to normal as well as pathological development.
It is noteworthy that the measurements of Hox gene expression levels generally follow normal distributions. The only exceptions are a group of 3 samples (out of 29) in Figure 2G, and a group of 4 samples (out of 78) in Figure 2F; graphs in both figures display bimodal distributions that form a minor peak towards the direction of reduced expression. The 3 samples from Figure 2G are shown in Figure 2K to be outside of the regression line, and, correspondingly, the 4 samples from Figure 2F also deviate from the regression line in Figure 2J. The reasons for these deviations are unknown, but apply to only a small number of samples. The most plausible explanation involves technical considerations: for low gene expression levels in rib chondrocytes, experimental errors are expected to have larger impact on normalization calculations.
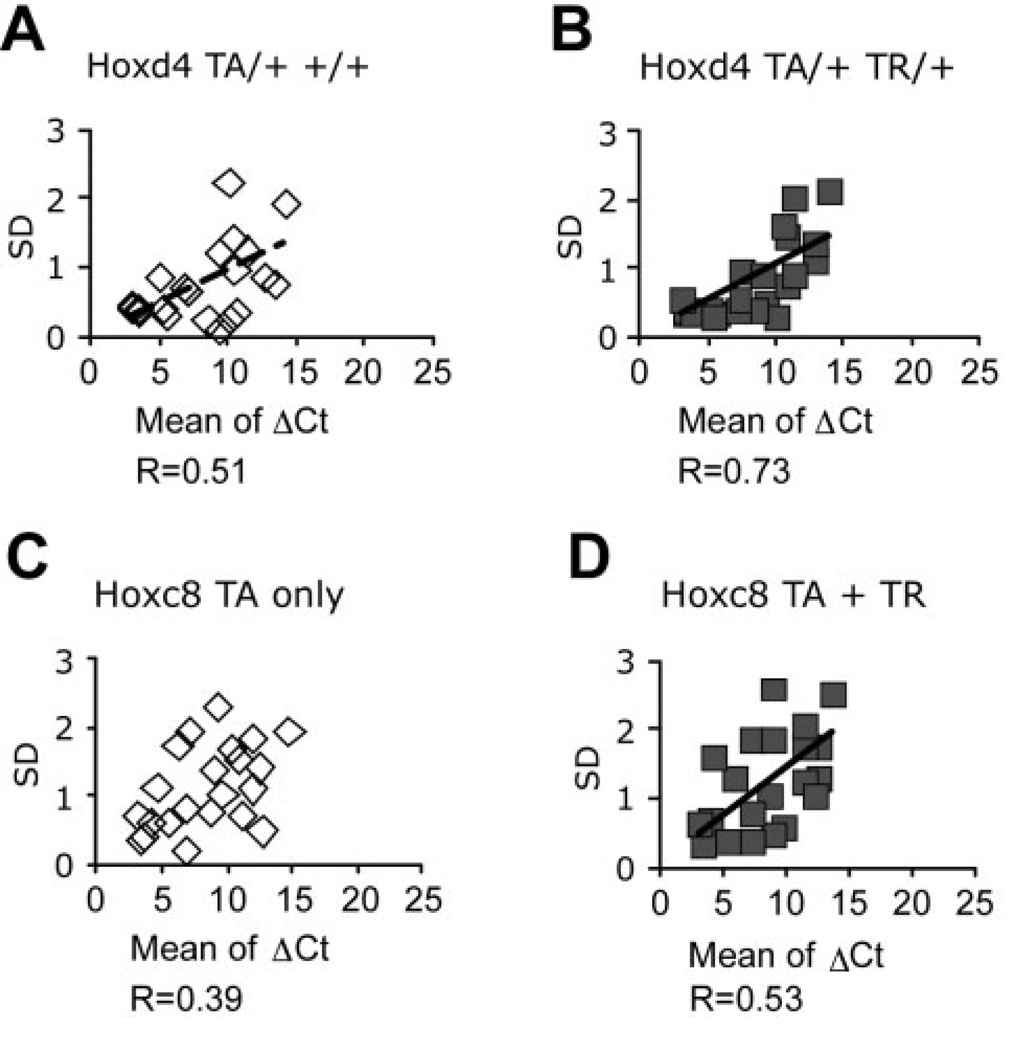
This proposition is borne out when we analyze the size of standard deviations for all gene measurements. For samples with very low mRNA prevalence of the gene of interest, and hence higher ΔCt values, we find larger standard deviations for the triplicate measurements for each sample (Fig. 5A and B, for Hoxd4 and Hoxc8, respectively). This trend is also confirmed for the means of all individuals and the standard deviation for each folate pathway gene measurement (Fig. 5C and D).
Figure 5.
Biological variability in folate pathway gene expression is similar in control and Hox transgenic animals. Graphic display of results in Table 3. Generally, larger standard deviations were found in cases of lower expression level (high ΔCt value) with correlation coefficients noted below each panel.
The normal distributions of gene expression for transgene as well as endogenous Hox gene expression levels indicate that even in fully inbred genetic strains with defined genetic background (here FVB), there exists variability in gene expression levels. The range from lowest to highest ΔCt value covers 8 cycles within the control groups and 6 cycles within the transgenic groups. It should be noted, however, that each range includes the most extreme values. Considering the majority of data points (75%), the expression levels of Hoxd4 in normal primary chondrocytes vary by 2.4-fold, and within transgenic samples, by 1.5-fold. Thus, even though the animals are genetically identical in their transgene configuration and inbred genetic background, the respective populations vary in Hoxd4 expression levels. This is also evident for Hoxc8 expression, both in control as well as in Hoxc8 transgenic samples. In the control group, 75% of the samples fall in a range of up to 4.6-fold difference; measurements between transgenic samples vary by 3.4-fold. These measurements are, to our knowledge, the first demonstration of variation in Hox gene expression levels in populations of genetically identical animals.
It is unlikely that this variation is entirely due to potential technical error: although it is possible that our chondrocyte preparations could contain some nonchondrocytic cells, this proportion consistently was less than 5% (Cormier et al., 2003), and all cell preparations for this study were performed by the same experienced individual. Furthermore, all measurements were normalized to an internal reference gene, Gapdh, excluding differences in cell yield as a confounding variable. Therefore, we believe that the distributions of relative expression levels reflect genuine biological variability in gene expression or in the frequency of Hoxc8-expressing cells within the cartilage. It is noteworthy in this context that the range of variability (variance) in control groups statistically is not significantly different from the variances exhibited in transgenic groups (data not shown), further supporting the notion that variability is not associated with the technical system, but is rather a biological phenomenon. While in transgenic cartilage, the range of expression levels may be associated with the severity of cartilage defects, the functional relevance of such variation in Hox gene expression levels in normal mice remains to be investigated.
Our studies show that Hoxd4 and Hoxc8 gene expression levels were significantly upregulated in transgenic animals compared to controls, consistent with the cartilage defects that manifest postnatally (Yueh et al., 1998; Kappen et al., 2004). However, measuring the gene expression of 18 different enzymes involved in the folate pathway as well as the Folate receptors 1, 2, and 4 and the Reduced folate carrier 1 (Table 2) in chondrocytes from rib cages of individual Hoxd4 and Hoxc8 transgenic mice, we found no significant differences in mRNA levels compared to littermate controls. Thus, our results do not provide evidence in support of the notion of local misregulation of folate pathway genes. This still leaves open the possibility that enzymatic activities affecting folate metabolism could be altered. Furthermore, it is conceivable that supplementation of folate affects pathways other than its own metabolism, a proposition we are currently investigating by performing quantitative RT-PCR assays for genes known to be involved in chondrocyte maturation as well as microarray studies.
Our Hoxc8 transgenic mice overexpress Hoxc8 under control of its own promoter. Thus, overexpression of the Hoxc8 transgene occurs in cells that express Hoxc8 normally during skeletal development, namely chondrocytes. Hoxd4 transgene expression within the Hoxc8 domain is achieved by using the same Hoxc8 regulatory sequences as for the Hoxc8 transgene. Transgenesis results in significantly elevated Hoxd4 expression in Hoxd4 transgenic chondrocytes. Yet, there is no effect on expression of the Hoxc8 gene in these animals. Consistent with expression in normal chondrocytes, Hoxc8 was detectable at normal levels in Hoxd4 transgenic rib cage chondrocytes. In contrast, Hoxd4 is expressed at very low levels in normal rib chondrocytes; in Hoxc8 transgenic cells, Hoxd4 levels appeared to be somewhat lower (albeit with marginal statistical significance). These results indicate that changes in Hoxd4 expression have no effect on the level of Hoxc8 expression, and vice versa, overexpression of Hoxc8 does not affect Hoxd4 mRNA levels to a considerable degree. Therefore, we conclude that it is unlike that Hoxc8 and Hoxd4 regulate each other’s gene expression; instead, each transgene acts independently.
The expression of folate pathway enzymes in cartilage or chondrocytes has not previously been investigated. Specific folate transport proteins, the folate receptors, are required for the uptake of folate into cells (Antony, 1996). We showed recently that Folate receptor 2 is expressed in mouse chondrocytes (Kappen et al., 2004). From the current data, mRNAs for Folate receptors 1 and 4 are also detectable, albeit at very low levels. The reduced folate carrier (Rfc1) is a typical member of the major facilitator superfamily and mediates the transport of folate in mammalian cells (Sirotnak and Tolner, 1999). This carrier system generates uphill folate transport through an exchange with organic phosphates concentrated within the intracellular compartment (Henderson and Zevely, 1983; Yang et al., 1984). We find that the Rfc gene is transcribed in neonatal chondrocytes, consistent with the postulated widespread expression of Rfc1 in the developing embryo (Maddox et al., 2003). Overall, we found no alterations in the expression of any of the folate transport proteins in Hoxd4 or Hoxc8 transgenic chondrocytes, suggesting that—at least at the developmental stage analyzed—folate transport in these cells is likely normal.
Our data also provide no evidence that any of the transcripts encoding folate-metabolizing enzymes are affected in expression levels by Hoxd4 or Hoxc8 overexpression. Widespread expression in many tissues has been reported for Dhfr, Sahh, Mthfr, Msr, Cbs, Ms, Tysy, and Amd, with apparently differential levels of expression for the latter four genes in different adult tissues (Robert et al., 2003). Interestingly, targeted disruption of Cbs was recently reported to affect endochondral ossification (Robert et al., 2005) through impaired cartilage differentiation primarily of the long bones. We did not detect obvious changes in the expression of Cbs at the transcript level in our transgenic models, suggesting that the mechanisms for impaired cartilage differentiation are different from hyperhomocysteinemia. Mat1 expression is believed to be restricted to liver, with Mat2 expressed in the embryo and non-liver tissues (Horikawa et al., 1993; Gil et al., 1996). Consistent with this notion, although both Mat1 and Mat2 could be measured, the level of detection of Mat1 in chondrocytes was very low. S-adenosylhomocysteine hydrolase (Sahh) is the only enzyme that cleaves S-adenosylhomocysteine, and detection of mRNA in chondrocytes is consistent with the reported ubiquitous expression (Hershfield and Krodich, 1987; Chiang et al., 1996) in proliferating cells (Radomski et al., 1999). Bhmt expression appears to be restricted to liver and kidney (Sunden et al., 1997), which also express Bhmt 2 (Chadwick et al., 2000), although in embryos, somewhat wider distribution was noted (Chadwick et al., 2000). Both Nat1 and Nat2 genes are expressed early in mouse development in the embryo-placental complex, as well as in the placental tissue (Stanley et al., 1998; Mitchell et al., 1999), with overlapping expression in many adult tissues (Windmill et al., 2000). It is believed that these acetyltransferases are involved in folate catabolism (Upton et al., 2001). We detected transcripts for all of these folate pathway genes in primary chondrocytes. DNA methylation is essential for development, and Dnmt1, Dnmt3a, and Dnmt3b are responsible for de novo methylation of the mouse genome during early embryonic development (Okano et al., 1998).
Expression of all Dnmt transcripts was detected in our assays. Thymidylate synthase (Tysy) catalyses the conversion of dUMP and 5–10-methylene-tetrahydrofolate to dTMP and dihydrofolate. This is the de novo source of deoxythymidylate for DNA replication (Zhao and Goldman, 2003). The levels of cellular folate metabolites are thus important for nucleotide synthesis (Herbig et al., 2002). Tysy and dihydrofolate reductase (Dhfr) are expressed in many tissues (Baker, 1969; Priest et al., 1981), some at low levels (Mudd et al., 1965), and Tysy expression is cell-cycle dependent. In our transgenic mice, no changes were found in Tysy gene expression, making it unlikely that DNA synthesis is directly affected by Hox transgene expression. Expression levels for Dhfr, whose product reduces folate to tetrahydrofolate, as well as Mthfr—both important candidates in the folate pathway—or gene for enzymes involved in the methionine remethylation cycle, such as Ms, Msr, Bhmt, Sahh, and Mat, show no alterations in transgenic chondrocytes. Interestingly, both the Tysy and Dhfr enzymes can bind to their cognate mRNAs and in this way autoregulate expression via translational repression (Tai et al., 2004). Thus, in addition to transcriptional regulation, cell- or tissue-specific mechanisms control the availability of enzymes in the folate pathway for folate metabolism.
Targeted gene disruption in mice demonstrated that Folbp1 (Piedrahita et al., 1999), Rfc (Zhao et al., 2001), Amd (Nishimura et al., 2002), Dmnt1 (Li et al., 1992), Sahh (Miller et al., 1994), and Ms (Swanson et al., 2001) all are essential, while Folbp2 (Piedrahita et al., 1999), Nat1 and 2 (Sugamori et al., 2003), are not required for embryonic development. No skeletal abnormalities have been reported for those mutants that survive postnatally. We have previously shown (Kappen et al., 2004) that folate metabolism is required for chondrocyte proliferation and differentiation, and that defects in cartilage maturation in Hoxd4 transgenic mice can be rescued by folate supplementation (Kappen et al., 2004), but which specific pathways involve folate in chondrocytes remains to be investigated. From our current studies, we conclude that overexpression of Hoxd4 and Hoxc8, respectively, does not perturb folate pathway gene expression. The response to folate in Hoxd4 transgenic mice therefore likely involves mechanisms other than the transcriptional regulation of folate metabolizing enzymes.
A second important implication from our results is that the expression of enzymes that metabolize folate does not appear to be regulated by Hox transcription factors. Thus, folate pathway genes may only be indirect targets of Hox transgene overexpression in our transgenic system, and other targets of Hox transcription factors must be responsible for the cartilage defects. Finally, our results allow us to determine whether Hox transgene expression affects the expression of other Hox genes. The measurements of Hoxc8 and Hoxd4 in the reciprocal transgenics strains do not provide evidence for such cross-regulation, and thus exclude Hox genes themselves as potential target genes.
To our knowledge, the analyses we present here constitute the first comprehensive assessment of folate pathway metabolic genes in primary mouse tissue of late stage embryos. Other tissues have not yet been evaluated in a systematic fashion, but the reagents and methodology established here make such assays straightforward. Our expression studies thus provide a baseline for future studies into the role of folate metabolism in skeletal development, and in embryonic tissues in general.
ACKNOWLEDGMENTS
We thank Diane Costanzo, Scott Hansen, and Andrew Wall for taking care of the mice and their husbandry, Lynette Smith for consultation with statistical analysis, and J. Michael Salbaum for critical discussions.
Grant sponsor: Phillip Morris External Research Program.
REFERENCES
- Antony AC. Folate receptors. Annu Rev Nutr. 1996;16:501–521. doi: 10.1146/annurev.nu.16.070196.002441. [DOI] [PubMed] [Google Scholar]
- Baker B. Tissue-specific irreversible inhibitors of dihydrofolic reductase. Acc Chem Res. 1969;2:129–136. [Google Scholar]
- Capecchi MR. Function of homeobox genes in skeletal development. Ann NY Acad Sci. 1996;785:34–37. doi: 10.1111/j.1749-6632.1996.tb56241.x. [DOI] [PubMed] [Google Scholar]
- Chadwick LH, McCandless SE, Silverman GL, et al. Betaine-homocysteine methyltransferase-2: cDNA cloning, gene sequence, physical mapping, and expression of the human and mouse genes. Genomics. 2000;70:66–73. doi: 10.1006/geno.2000.6319. [DOI] [PubMed] [Google Scholar]
- Chiang PK, Gordon RK, Tal J, et al. S-adenosylmethionine and methylation. FASEB J. 1996;10:471–480. [PubMed] [Google Scholar]
- Condie BG, Capecchi MR. Mice homozygous for a targeted disruption of hoxd-3 (hox-4.1) exhibit anterior transformations of the first and second cervical vertebrae, the atlas and the axis. Development. 1993;119:579–595. doi: 10.1242/dev.119.3.579. [DOI] [PubMed] [Google Scholar]
- Condie BG, Capecchi MR. Mice with targeted disruptions in the paralogous genes hoxa-3 and hoxd-3 reveal synergistic interactions. Nature. 1994;370:304–307. doi: 10.1038/370304a0. [DOI] [PubMed] [Google Scholar]
- Cormier S, Mello MA, Kappen C. Normal proliferation and differentiation of Hoxc-8 transgenic chondrocytes in vitro. BMC Dev Biol. 2003;3:4. doi: 10.1186/1471-213X-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeizel AE. Folic acid and prevention of birth defects. JAMA. 1996;275:1635–1636. [PubMed] [Google Scholar]
- Czeizel AE, Timar L, Sarkozi A. Dose-dependent effect of folic acid on the prevention of orofacial clefts. Pediatrics. 1999;104:e66. doi: 10.1542/peds.104.6.e66. [DOI] [PubMed] [Google Scholar]
- Fromental-Ramain C, Warot X, Lakkaraju S, et al. Specific and redundant functions of the paralogous Hoxa-9 and Hoxd-9 genes in forelimb and axial skeleton patterning. Development. 1996;122:461–472. doi: 10.1242/dev.122.2.461. [DOI] [PubMed] [Google Scholar]
- Gil B, Casado M, Pajares MA, et al. Differential expression pattern of S-adenosylmethionine synthetase isoenzymes during rat liver development. Hepatology. 1996;24:876–881. doi: 10.1002/hep.510240420. [DOI] [PubMed] [Google Scholar]
- Henderson GB, Zevely EM. Structural requirements for anion substrates of the methotrexate transport system in L1210 cells. Arch Biochem Biophys. 1983;221:438–446. doi: 10.1016/0003-9861(83)90162-5. [DOI] [PubMed] [Google Scholar]
- Herbig K, Chiang EP, Lee LR, et al. Cytoplasmic serine hydroxy-methyltransferase mediates competition between folate-dependent deoxyribonucleotide and S-adenosylmethionine biosyntheses. J Biol Chem. 2002;277:38381–38389. doi: 10.1074/jbc.M205000200. [DOI] [PubMed] [Google Scholar]
- Hershfield MS, Krodich NM. S-adenosylhomocysteine hydrolase is an adenosine-binding protein: a target for adenosine toxicity. Science. 1987;202:757–760. doi: 10.1126/science.715439. [DOI] [PubMed] [Google Scholar]
- Horikawa S, Kobayashi Y, Sugiyama T, et al. Expression of non-hepatic-type S-adenosylmethionine synthetase isozyme in rat hepatomas induced by 3′-methyl-4-dimethylaminoazobenzene. FEBS Lett. 1993;334:69–71. doi: 10.1016/0014-5793(93)81682-p. [DOI] [PubMed] [Google Scholar]
- Kappen C. The VP16-dependent binary system for inducible gene expression in transgenic mice. In: Accili D, editor. Genetic manipulation of receptor expression and function. New York: John Wiley & Sons; 1999. pp. 69–92. [Google Scholar]
- Kappen C, Mello MA, Finnell RH, Salbaum JM. Folate modulates cartilage defects in Hoxd-4 transgenic mice. Genesis. 2004;39:115–166. doi: 10.1002/gene.20036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knezevic V, DeSanto R, Schughart K, et al. Hoxd-12 differentially affects preaxial and postaxial chondrogenic branches in the limb and regulates Sonic hedgehog in a positive feedback loop. Development. 1997;124:4523–4536. doi: 10.1242/dev.124.22.4523. [DOI] [PubMed] [Google Scholar]
- Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–926. doi: 10.1016/0092-8674(92)90611-f. [DOI] [PubMed] [Google Scholar]
- Maddox DM, Manlapat A, Roon P, et al. Reduced-folate carrier (RFC) is expressed in placenta and yolk sac, as well as in cells of the developing forebrain, hindbrain, neural tube, craniofacial region, eye, limb buds and heart. BMC Dev Biol. 2003;3:6. doi: 10.1186/1471-213X-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MW, Duhl DM, Winkes BM, et al. The mouse lethal nonagouti (a(x)) mutation deletes the S-adenosylhomocysteine hydrolase (Ahcy) gene. EMBO J. 1994;13:1806–1816. doi: 10.1002/j.1460-2075.1994.tb06449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell MK, Futscher BW, McQueen CA. Developmental expression of N-acetyltransferases in C57BI/6 mice. Drug Metab Dispos. 1999;27:261–264. [PubMed] [Google Scholar]
- Mudd SH, Finkelstein JD, Irreverre F, Laster L. Transsulfuration in mammals. Microassays and tissue distributions of three enzymes of the pathway. J Biol Chem. 1965;240:4382–4392. [PubMed] [Google Scholar]
- Nishimura K, Nakatsu F, Kashiwagi K, et al. Essential role of S-adenosylmethionine decarboxylase in mouse embryonic development. Genes Cells. 2002;7:41–47. doi: 10.1046/j.1356-9597.2001.00494.x. [DOI] [PubMed] [Google Scholar]
- Okano M, Xie S, Li E. Dnmt2 is not required for de novo and maintenance methylation of viral DNA in embryonic stem cells. Nucleic Acids Res. 1998;26:2536–2540. doi: 10.1093/nar/26.11.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piedrahita JA, Oemata B, Bennett GD, et al. Mice lacking the folate acid-binding protein Folbp1 are defective in early embryonic development. Nat Genet. 1999;23:228–232. doi: 10.1038/13861. [DOI] [PubMed] [Google Scholar]
- Priest DG, Doig MT, Hynes JB. Purification of mouse liver thymidylate synthetase by affinity chromatography using 10-methyl-5,8-dideazafolate as the affinant. Experientia. 1981;37:119–120. doi: 10.1007/BF01963180. [DOI] [PubMed] [Google Scholar]
- Radomski N, Kaufmann C, Dreyer C. Nuclear accumulation of S-adenosylhomocysteine hydrolase in transcriptionally active cells during development of Xenopus laevis. Mol Biol Cell. 1999;10:4283–4298. doi: 10.1091/mbc.10.12.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert K, Vialard F, Thiery E, et al. Expression of the cystathionine beta synthase (CBS) gene during mouse development and immunolocalization in adult brain. J Histochem Cytochem. 2003;51:363–371. doi: 10.1177/002215540305100311. [DOI] [PubMed] [Google Scholar]
- Robert K, Maurin N, Vaysettes C, et al. Cystathionine beta synthase deficiency affects mouse endochondral ossification. Anat Rec A Discov Mol Cell Evol Biol. 2005;282:1–7. doi: 10.1002/ar.a.20145. [DOI] [PubMed] [Google Scholar]
- Rundle CH, Macias MP, Gardner DP, et al. Transactivation of Hox gene expression in a VP16-dependent binary transgenic mouse system. Biochim Biophys Acta. 1998;1398:164–178. doi: 10.1016/s0167-4781(98)00046-3. [DOI] [PubMed] [Google Scholar]
- Shi X, Yang X, Chen D, et al. Smad1 interacts with homeobox DNA-binding proteins in bone morphogenetic protein signaling. J Biol Chem. 1999;274:13711–13717. doi: 10.1074/jbc.274.19.13711. [DOI] [PubMed] [Google Scholar]
- Sirotnak FM, Tolner B. Carrier-mediated membrane transport of folates in mammalian cells. Annu Rev Nutr. 1999;19:91–122. doi: 10.1146/annurev.nutr.19.1.91. [DOI] [PubMed] [Google Scholar]
- Stanley LA, Copp AJ, Pope J, et al. Immunochemical detection of arylamine N-acetyltransferase during mouse embryonic development and in adult mouse brain. Teratology. 1998;58:174–182. doi: 10.1002/(SICI)1096-9926(199811)58:5<174::AID-TERA3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Sugamori KS, Wong S, Gaedigk A, et al. Generation and functional characterization of arylamine N-acetyltransferase Nat1/Nat2 double-knockout mice. Mol Pharmacol. 2003;64:170–179. doi: 10.1124/mol.64.1.170. [DOI] [PubMed] [Google Scholar]
- Sunden SL, Renduchintala MS, Park EI, et al. Betaine-homocysteine methyltransferase expression in porcine and human tissues and chromosomal localization of the human gene. Arch Biochem Biophys. 1997;345:171–174. doi: 10.1006/abbi.1997.0246. [DOI] [PubMed] [Google Scholar]
- Swanson DA, Liu ML, Baker PJ, et al. Targeted disruption of the methionine synthase gene in mice. Mol Cell Biol. 2001;21:1058–1065. doi: 10.1128/MCB.21.4.1058-1065.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai N, Schmitz JC, Liu J, et al. Translational autoregulation of thymidylate synthase and dihydrofolate reductase. Front Biosci. 2004;9:2521–2526. doi: 10.2741/1413. [DOI] [PubMed] [Google Scholar]
- Upton A, Johnson N, Sandy J, Sim E. Arylamine N-acetyltransferases—of mice, men and microorganisms. Trends Pharmacol Sci. 2001;22:140–146. doi: 10.1016/s0165-6147(00)01639-4. [DOI] [PubMed] [Google Scholar]
- Windmill KF, Gaedigk A, Hall PM, et al. Localization of N-acetyltransferases NAT1 and NAT2 in human tissues. Toxicol Sci. 2000;54:19–29. doi: 10.1093/toxsci/54.1.19. [DOI] [PubMed] [Google Scholar]
- Yang CH, Sirotnak FM, Dembo M. Interaction between anions and the reduced folate/methotrexate transport system in L1210 cell plasma membrane vesicles: directional symmetry and anion specificity for differential mobility of loaded and unloaded carrier. J Membr Biol. 1984;79:285–292. doi: 10.1007/BF01871067. [DOI] [PubMed] [Google Scholar]
- Yang X, Ji X, Shi X, Cao X. Smad1 domains interacting with Hoxc-8 induce osteoblast differentiation. J Biol Chem. 2000;275:1065–1072. doi: 10.1074/jbc.275.2.1065. [DOI] [PubMed] [Google Scholar]
- Yokouchi Y, Nakazato S, Yamamoto M, et al. Misexpression of Hoxa-13 induces cartilage homeotic transformation and changes cell adhesiveness in chick limb buds. Genes Dev. 1995;9:2509–2522. doi: 10.1101/gad.9.20.2509. [DOI] [PubMed] [Google Scholar]
- Yueh YG, Gardner DP, Kappen C. Evidence for regulation of cartilage differentiation by the homeobox gene Hoxc-8. Proc Natl Acad Sci USA. 1998;95:9956–9961. doi: 10.1073/pnas.95.17.9956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Behringer RR, de Crombrugghe B. Prenatal folic acid treatment suppresses acrania and meroanencephaly in mice mutant for the Cart1 homeobox gene. Nat Genet. 1996;13:275–283. doi: 10.1038/ng0796-275. [DOI] [PubMed] [Google Scholar]
- Zhao R, Russell RG, Wang Y, et al. Rescue of embryonic lethality in reduced folate carrier-deficient mice by maternal folic acid supplementation reveals early neonatal failure of hematopoietic organs. J Biol Chem. 2001;276:10224–10228. doi: 10.1074/jbc.c000905200. [DOI] [PubMed] [Google Scholar]
- Zhao R, Goldman ID. Resistance to antifolates. Oncogene. 2003;22:7431–7457. doi: 10.1038/sj.onc.1206946. [DOI] [PubMed] [Google Scholar]