Abstract
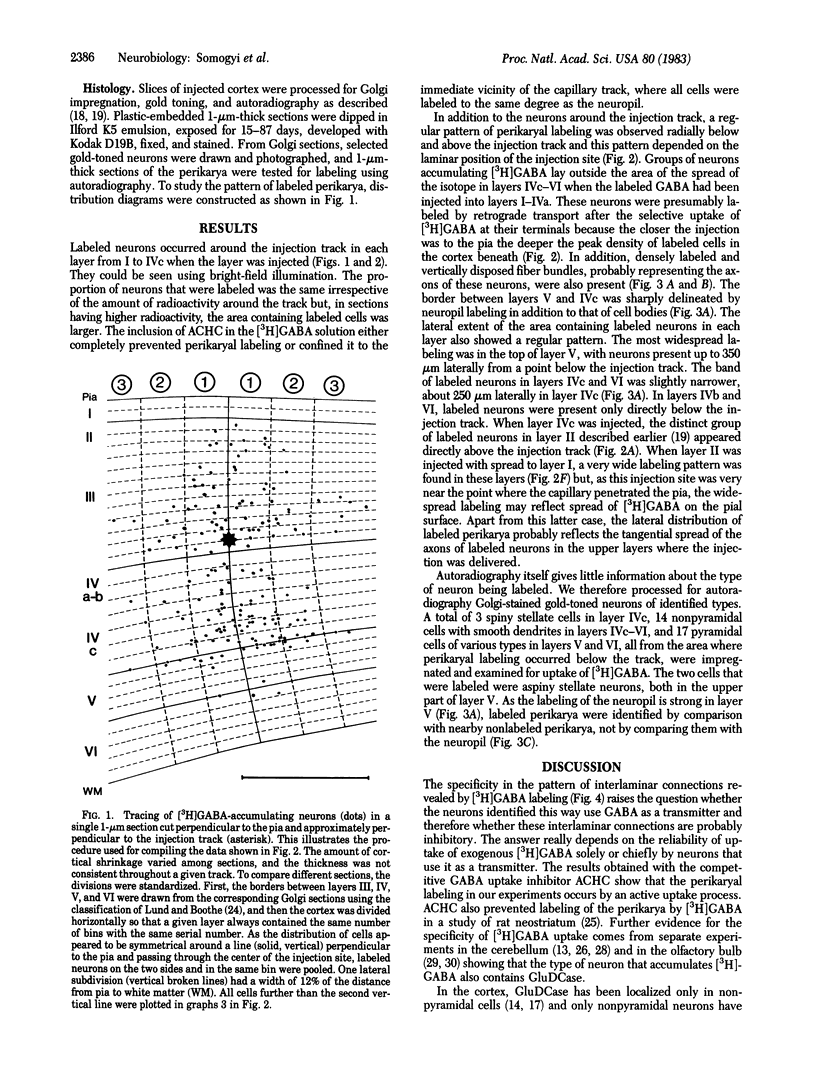
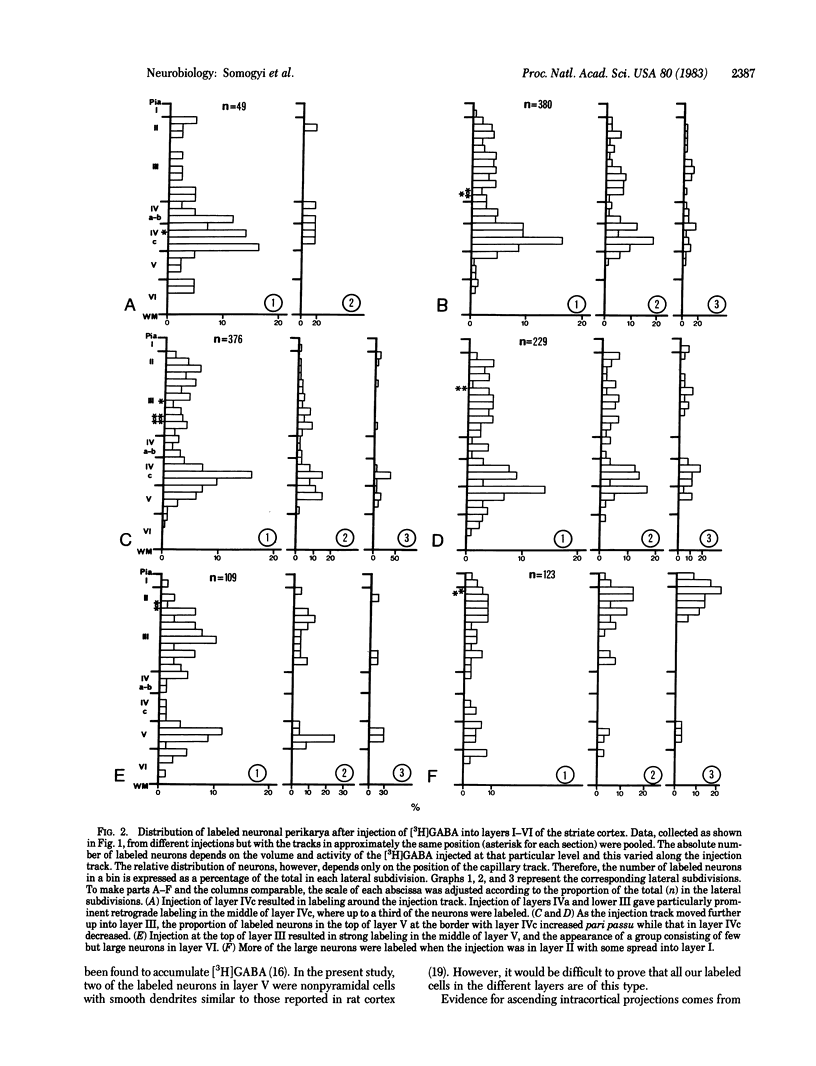
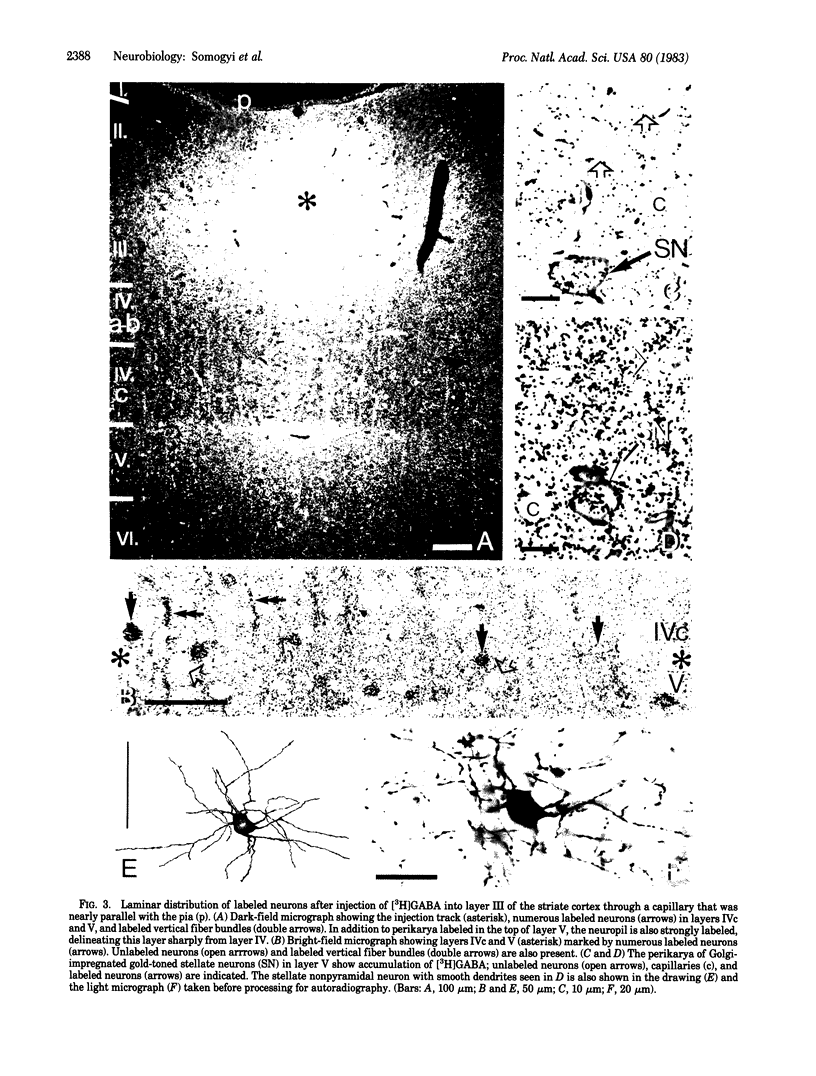
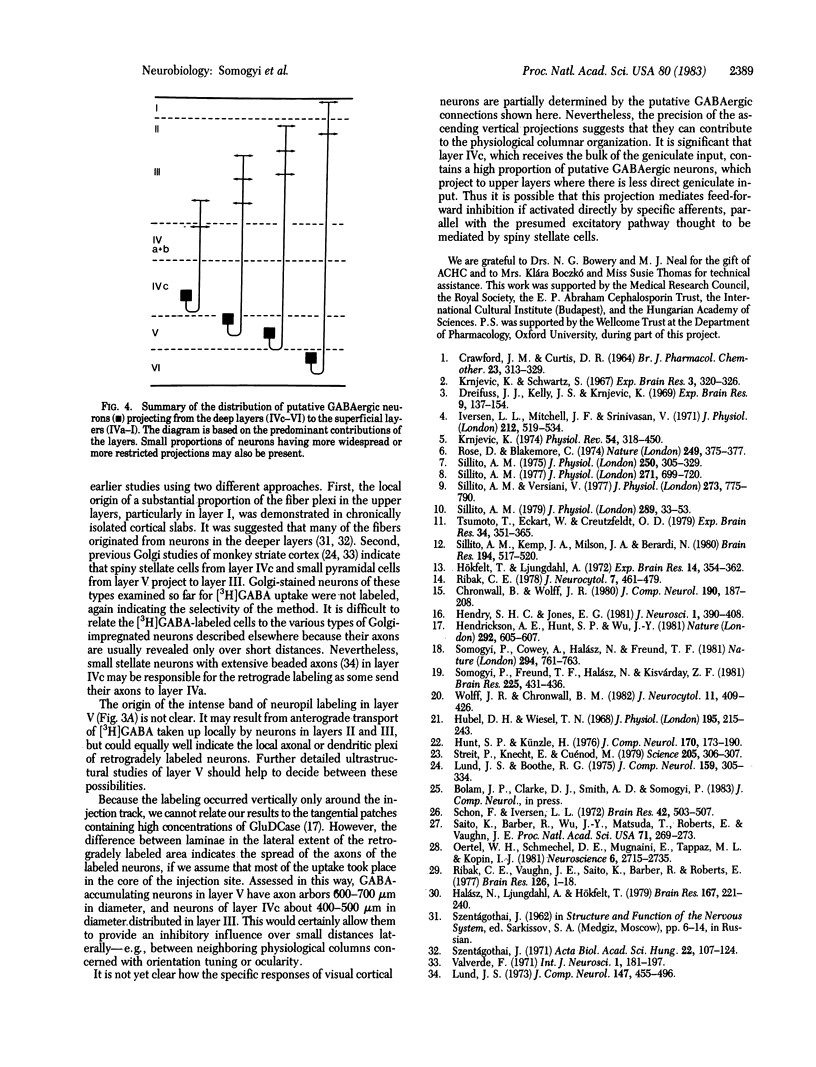
Several lines of evidence suggest that gamma-aminobutyric acid is an inhibitory neurotransmitter in the cerebral cortex. To study the intracortical projection of neurons that selectively accumulate this amino acid, we injected radioactive gamma-aminobutyric acid into the upper layers of the striate cortex of monkeys along tracks at an oblique angle to the pia. Sections from the injected area were then processed by a combination of autoradiography and Golgi impregnation to reveal the distribution of labeled neurons and their morphological characteristics. Labeled neurons always occurred around the injection site in each layer. In addition, a consistent radial pattern of perikaryal labeling was observed in layers IVc-VI below the injection track in layers I-IVa. The closer the injection track was to the pia the deeper the peak density of labeled cells appeared. After injection in layers IVa and the lower part of III, the highest number of labeled neurons was in layer IVc; after injection in the upper part of layer III, most labeled neurons were in layer V; and, after injection in layers I and II, the proportion of labeled neurons increased in the lower part of layer V and in layer VI. All these neurons in the infragranular layers are presumably labeled by retrograde axonal transport via the labeled fiber bundles that extended from upper to lower layers. Thirty-four Golgi-stained neurons of various types were also examined for retrograde labeling. Two were labeled, and both were aspiny stellate cells in layer V. The arrangement of these putative GABAergic neurones, with axons that ascend from lower to upper layers in a regular pattern and arborize locally, would enable them to mediate inhibition within cortical columns and between neighboring columns.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CRAWFORD J. M., CURTIS D. R. THE EXCITATION AND DEPRESSION OF MAMMALIAN CORTICAL NEURONES BY AMINO ACIDS. Br J Pharmacol Chemother. 1964 Oct;23:313–329. doi: 10.1111/j.1476-5381.1964.tb01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronwall B., Wolff J. R. Prenatal and postnatal development of GABA-accumulating cells in the occipital neocortex of rat. J Comp Neurol. 1980 Mar 1;190(1):187–208. doi: 10.1002/cne.901900113. [DOI] [PubMed] [Google Scholar]
- Dreifuss J. J., Kelly J. S., Krnjević K. Cortical inhibition and gamma-aminobutyric acid. Exp Brain Res. 1969;9(2):137–154. doi: 10.1007/BF00238327. [DOI] [PubMed] [Google Scholar]
- Halasz N., Ljungdahl A., Hökfelt T. Transmitter histochemistry of the rat olfactory bulb III. Autoradiographic localization of [3H]GABA. Brain Res. 1979 May 11;167(2):221–240. doi: 10.1016/0006-8993(79)90818-7. [DOI] [PubMed] [Google Scholar]
- Hendrickson A. E., Hunt S. P., Wu J. Y. Immunocytochemical localization of glutamic acid decarboxylase in monkey striate cortex. Nature. 1981 Aug 13;292(5824):605–607. doi: 10.1038/292605a0. [DOI] [PubMed] [Google Scholar]
- Hendry S. H., Jones E. G. Sizes and distributions of intrinsic neurons incorporating tritiated GABA in monkey sensory-motor cortex. J Neurosci. 1981 Apr;1(4):390–408. doi: 10.1523/JNEUROSCI.01-04-00390.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubel D. H., Wiesel T. N. Receptive fields and functional architecture of monkey striate cortex. J Physiol. 1968 Mar;195(1):215–243. doi: 10.1113/jphysiol.1968.sp008455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt S. P., Künzle H. Selective uptake and transport of label within three identified neuronal systems after injection of 3H-GABA into the pigeon optic tectum: an autoradiographic and Golgi study. J Comp Neurol. 1976 Nov 15;170(2):173–189. doi: 10.1002/cne.901700204. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Ljungdahl A. Autoradiographic identification of cerebral and cerebellar cortical neurons accumulating labeled gamma-aminobutyric acid ( 3 H-GABA). Exp Brain Res. 1972;14(4):354–362. doi: 10.1007/BF00235032. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Mitchell J. F., Srinivasan V. The release of gamma-aminobutyric acid during inhibition in the cat visual cortex. J Physiol. 1971 Jan;212(2):519–534. doi: 10.1113/jphysiol.1971.sp009339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjević K., Schwartz S. The action of gamma-aminobutyric acid on cortical neurones. Exp Brain Res. 1967;3(4):320–336. doi: 10.1007/BF00237558. [DOI] [PubMed] [Google Scholar]
- Lund J. S. Organization of neurons in the visual cortex, area 17, of the monkey (Macaca mulatta). J Comp Neurol. 1973 Feb 15;147(4):455–496. doi: 10.1002/cne.901470404. [DOI] [PubMed] [Google Scholar]
- Oertel W. H., Schmechel D. E., Mugnaini E., Tappaz M. L., Kopin I. J. Immunocytochemical localization of glutamate decarboxylase in rat cerebellum with a new antiserum. Neuroscience. 1981;6(12):2715–2735. doi: 10.1016/0306-4522(81)90115-9. [DOI] [PubMed] [Google Scholar]
- Ribak C. E. Aspinous and sparsely-spinous stellate neurons in the visual cortex of rats contain glutamic acid decarboxylase. J Neurocytol. 1978 Aug;7(4):461–478. doi: 10.1007/BF01173991. [DOI] [PubMed] [Google Scholar]
- Ribak C. E., Vaughn J. E., Saito K., Barber R., Roberts E. Glutamate decarboxylase localization in neurons of the olfactory bulb. Brain Res. 1977 Apr 22;126(1):1–18. doi: 10.1016/0006-8993(77)90211-6. [DOI] [PubMed] [Google Scholar]
- Rose D., Blakemore C. Effects of bicuculline on functions of inhibition in visual cortex. Nature. 1974 May 24;249(455):375–377. doi: 10.1038/249375a0. [DOI] [PubMed] [Google Scholar]
- Schon F., Iversen L. L. Selective accumulation of ( 3 H)GABA by stellate cells in rat cerebellar cortex in vivo. Brain Res. 1972 Jul 20;42(2):503–507. doi: 10.1016/0006-8993(72)90550-1. [DOI] [PubMed] [Google Scholar]
- Sillito A. M. Inhibitory mechanisms influencing complex cell orientation selectivity and their modification at high resting discharge levels. J Physiol. 1979 Apr;289:33–53. doi: 10.1113/jphysiol.1979.sp012723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito A. M. Inhibitory processes underlying the directional specificity of simple, complex and hypercomplex cells in the cat's visual cortex. J Physiol. 1977 Oct;271(3):699–720. doi: 10.1113/jphysiol.1977.sp012021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito A. M., Kemp J. A., Milson J. A., Berardi N. A re-evaluation of the mechanisms underlying simple cell orientation selectivity. Brain Res. 1980 Aug 4;194(2):517–520. doi: 10.1016/0006-8993(80)91234-2. [DOI] [PubMed] [Google Scholar]
- Sillito A. M. The contribution of inhibitory mechanisms to the receptive field properties of neurones in the striate cortex of the cat. J Physiol. 1975 Sep;250(2):305–329. doi: 10.1113/jphysiol.1975.sp011056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillito A. M., Versiani V. The contribution of excitatory and inhibitory inputs to the length preference of hypercomplex cells in layers II and III of the cat's striate cortex. J Physiol. 1977 Dec;273(3):775–790. doi: 10.1113/jphysiol.1977.sp012123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P., Cowey A., Halász N., Freund T. F. Vertical organization of neurones accumulating 3H-GABA in visual cortex of rhesus monkey. Nature. 1981 Dec 24;294(5843):761–763. doi: 10.1038/294761a0. [DOI] [PubMed] [Google Scholar]
- Somogyi P., Freund T. F., Halász N., Kisvárday Z. F. Selectivity of neuronal [3H]GABA accumulation in the visual cortex as revealed by Golgi staining of the labeled neurons. Brain Res. 1981 Nov 30;225(2):431–436. doi: 10.1016/0006-8993(81)90849-0. [DOI] [PubMed] [Google Scholar]
- Streit P., Knecht E., Cuenod M. Transmitter-specific retrograde labeling in the striato-nigral and raphe-nigral pathways. Science. 1979 Jul 20;205(4403):306–308. doi: 10.1126/science.451602. [DOI] [PubMed] [Google Scholar]
- Szentágothai J. Some geometrical aspects of the neocortical neuropil. Acta Biol Acad Sci Hung. 1971;22(2):107–124. [PubMed] [Google Scholar]
- Tsumoto T., Eckart W., Creutzfeldt O. D. Modification of orientation sensitivity of cat visual cortex neurons by removal of GABA-mediated inhibition. Exp Brain Res. 1979 Jan 15;34(2):351–363. doi: 10.1007/BF00235678. [DOI] [PubMed] [Google Scholar]
- Valverde F. Short axon neuronal subsystems in the visual cortex of the monkey. Int J Neurosci. 1971 Feb;1(3):181–197. doi: 10.3109/00207457109146970. [DOI] [PubMed] [Google Scholar]
- Wolff J. R., Chronwall B. M. Axosomatic synapses in the visual cortex of adult rat. A comparison between GABA-accumulating and other neurons. J Neurocytol. 1982 Jun;11(3):409–425. doi: 10.1007/BF01257986. [DOI] [PubMed] [Google Scholar]



