Abstract
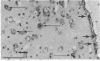
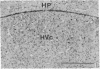
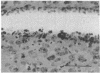
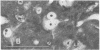
The vocal control nucleus designated HVc (hyperstriatum ventrale, pars caudalis) of adult female canaries expands in response to systemic testosterone administration, which also induces the females to sing in a male-like manner. We became interested in the possibility of neurogenesis as a potential basis for this phenomenon. Intact adult female canaries were injected with [3H]thymidine over a 2-day period. Some birds were given testosterone implants at various times before thymidine. The birds were sacrificed 5 wk after hormone implantation, and their brains were processed for autoradiography. In parallel control experiments, some birds were given implants of cholesterol instead of testosterone. All birds showed considerable numbers of labeled neurons, glia, endothelia, and ventricular zone cells in and around HVc. Ultrastructural analysis confirmed the identity of these labeled neurons. Cholesterol- and testosterone-treated birds had similar neuronal labeling indices, which ranged from 1.8% to 4.0% in HVc. Thus, neurogenesis occurred in these adults independently of exogenous hormone treatment. Conversely, both glial and endothelial proliferation rates were markedly stimulated by exogenous testosterone treatment. We determined the origin of the thymidine-incorporating neurons by sacrificing two thymidine-treated females soon after their thymidine injections, precluding any significant migration of newly labeled cells. Analysis of these brains revealed no cells of neuronal morphology present in HVc but a very heavily labeled ventricular zone overlying HVc. We conclude that neuronal precursors exist in the HVc ventricular zone that incorporate tritiated thymidine during the S phase preceding their mitosis; after division these cells migrate into, and to some extent beyond, HVc. This ventricular zone neurogenesis seems to be a normally occurring phenomenon in intact adult female canaries.
Full text
PDF


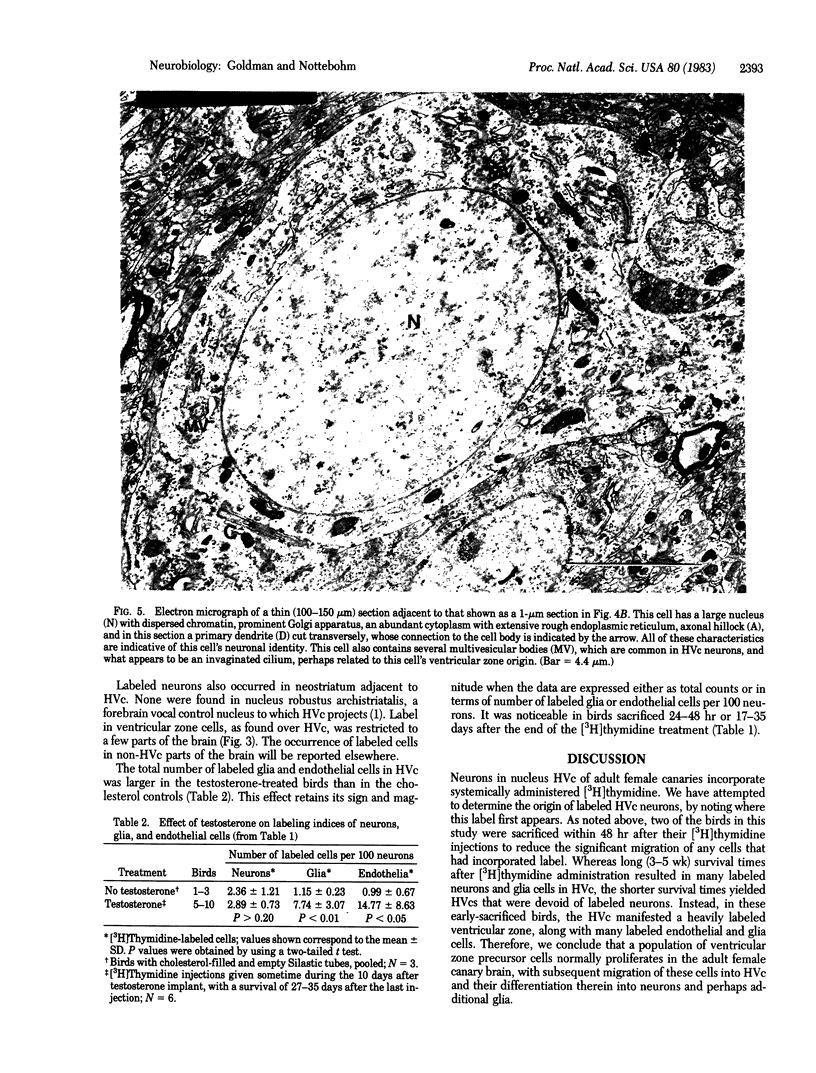

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969 Dec;137(4):433–457. doi: 10.1002/cne.901370404. [DOI] [PubMed] [Google Scholar]
- Altman J., Das G. D. Post-natal origin of microneurones in the rat brain. Nature. 1965 Aug 28;207(5000):953–956. doi: 10.1038/207953a0. [DOI] [PubMed] [Google Scholar]
- Bayer S. A. Changes in the total number of dentate granule cells in juvenile and adult rats: a correlated volumetric and 3H-thymidine autoradiographic study. Exp Brain Res. 1982;46(3):315–323. doi: 10.1007/BF00238626. [DOI] [PubMed] [Google Scholar]
- Bayer S. A., Yackel J. W., Puri P. S. Neurons in the rat dentate gyrus granular layer substantially increase during juvenile and adult life. Science. 1982 May 21;216(4548):890–892. doi: 10.1126/science.7079742. [DOI] [PubMed] [Google Scholar]
- Burholt D. R., Schultze B., Maurer W. Autoradiographic confirmation of the mitotic division of every mouse jejunal crypt cell labelled with 3 H-thymidine. Evidence against the existence of cells synthesizing metabolic DNA. Cell Tissue Kinet. 1973 May;6(3):229–237. [PubMed] [Google Scholar]
- Fujita S. DNA constancy in neurons of the human cerebellum and spinal cord as revealed by Feulgen cytophotometry and cytofluorometry. J Comp Neurol. 1974 May 15;155(2):195–202. doi: 10.1002/cne.901550205. [DOI] [PubMed] [Google Scholar]
- Graziadei P. P., Graziadei G. A. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neurons. J Neurocytol. 1979 Feb;8(1):1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- Kaplan M. S., Hinds J. W. Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science. 1977 Sep 9;197(4308):1092–1094. doi: 10.1126/science.887941. [DOI] [PubMed] [Google Scholar]
- Korr H. Proliferation of different cell types in the brain. Adv Anat Embryol Cell Biol. 1980;61:1–72. doi: 10.1007/978-3-642-67577-5. [DOI] [PubMed] [Google Scholar]
- Luine V., Nottebohm F., Harding C., McEwen B. S. Androgen affects cholinergic enzymes in syringeal motor neurons and muscle. Brain Res. 1980 Jun 16;192(1):89–107. doi: 10.1016/0006-8993(80)91011-2. [DOI] [PubMed] [Google Scholar]
- Mares V., Schultze B., Maurer W. Stability of DNA in Purkinje cell nuclei of the mouse. An autoradiographic study. J Cell Biol. 1974 Nov;63(2 Pt 1):665–674. [PMC free article] [PubMed] [Google Scholar]
- Mundinger P. C. Vocal imitation and individual recognition of finch calls. Science. 1970 Apr 24;168(3930):480–482. doi: 10.1126/science.168.3930.480. [DOI] [PubMed] [Google Scholar]
- Nottebohm F., Kasparian S., Pandazis C. Brain space for a learned task. Brain Res. 1981 May 25;213(1):99–109. doi: 10.1016/0006-8993(81)91250-6. [DOI] [PubMed] [Google Scholar]
- Nottebohm F., Stokes T. M., Leonard C. M. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976 Feb 15;165(4):457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Testosterone triggers growth of brain vocal control nuclei in adult female canaries. Brain Res. 1980 May 12;189(2):429–436. doi: 10.1016/0006-8993(80)90102-x. [DOI] [PubMed] [Google Scholar]
- SIDMAN R. L., MIALE I. L., FEDER N. Cell proliferation and migration in the primitive ependymal zone: an autoradiographic study of histogenesis in the nervous system. Exp Neurol. 1959 Oct;1:322–333. doi: 10.1016/0014-4886(59)90024-x. [DOI] [PubMed] [Google Scholar]
- Stevenson J. A., Yoon M. G. Mitosis of radial glial cells in the optic tectum of adult goldfish. J Neurosci. 1981 Aug;1(8):862–875. doi: 10.1523/JNEUROSCI.01-08-00862.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]