Abstract
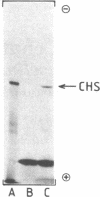
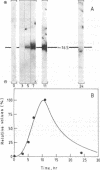
DNAs complementary to poly(A)+ mRNAs from UV-irradiated cell suspension cultures of parsley (Petroselinum hortense) were inserted into pBR322 and used to transform Escherichia coli strain RR1. A clone containing a DNA complementary to chalcone synthase mRNA was identified by hybrid-selected and hybrid-arrested translation. Large and rapid changes in the amount of chalcone synthase mRNA in response to irradiation of the cells was detected by RNA blot hybridization experiments. The pattern of changes coincided with that previously determined for the rate of chalcone synthase synthesis as measured either in vivo or with polyribosomal mRNA in vitro. The results are consistent with the hypothesis that induction of chalcone synthase by UV light is due to a transient increase in the rate of synthesis of chalcone synthase mRNA.
Keywords: cDNA clones, flavonoid biosynthesis, phytochrome control
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Gardiner S. E., Schröder J., Matern U., Hammer D., Hahlbrock K. mRNA-dependent regulation of UDP-apiose synthase activity in irradiated plant cells. J Biol Chem. 1980 Nov 25;255(22):10752–10757. [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller W., Egin-Bühler B., Gardiner S. E., Knobloch K. H., Matern U., Ebel J., Hahlbrock K. Enzymes of General Phenylpropanoid Metabolism and of Flavonoid Glycoside Biosynthesis in Parsley: Differential Inducibility by Light during the Growth of Cell Suspension Cultures. Plant Physiol. 1979 Sep;64(3):371–373. doi: 10.1104/pp.64.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller W., Hahlbrock K. Highly purified "flavanone synthase" from parsley catalyzes the formation of naringenin chalcone. Arch Biochem Biophys. 1980 Apr 1;200(2):617–619. doi: 10.1016/0003-9861(80)90395-1. [DOI] [PubMed] [Google Scholar]
- Kreuzaler F., Ragg H., Heller W., Tesch R., Witt I., Hammer D., Hahlbrock K. Flavanone synthase from Petroselinum hortense. Molecular weight, subunit composition, size of messenger RNA, and absence of pantetheinyl residue. Eur J Biochem. 1979 Aug 15;99(1):89–96. doi: 10.1111/j.1432-1033.1979.tb13235.x. [DOI] [PubMed] [Google Scholar]
- Law S., Tamoaki T., Kreuzaler F., Dugaiczyk A. Molecular cloning of DNA complementary to a mouse alpha-fetoprotein mRNA sequence. Gene. 1980 Jun;10(1):53–61. doi: 10.1016/0378-1119(80)90143-2. [DOI] [PubMed] [Google Scholar]
- Mandel M., Higa A. Calcium-dependent bacteriophage DNA infection. J Mol Biol. 1970 Oct 14;53(1):159–162. doi: 10.1016/0022-2836(70)90051-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E., Kuff E. L. Structural gene identification and mapping by DNA-mRNA hybrid-arrested cell-free translation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4370–4374. doi: 10.1073/pnas.74.10.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Ragg H., Hahlbrock K. Messenger RNA coding for phenylalanine ammonia-lyase. Characterization and partial purification from cell suspension cultures of Petroselinum hortense. Eur J Biochem. 1980 Jan;103(2):323–330. doi: 10.1111/j.1432-1033.1980.tb04318.x. [DOI] [PubMed] [Google Scholar]
- Ragg H., Kuhn D. N., Hahlbrock K. Coordinated regulation of 4-coumarate:CoA ligase and phenylalanine ammonia-lyase mRNAs in cultured plant cells. J Biol Chem. 1981 Oct 10;256(19):10061–10065. [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder J., Kreuzaler F., Schäfer E., Hahlbrock K. Concomitant induction of phenylalanine ammonia-lyase and flavanone synthase mRNAs in irradiated plant cells. J Biol Chem. 1979 Jan 10;254(1):57–65. [PubMed] [Google Scholar]
- Sim G. K., Kafatos F. C., Jones C. W., Koehler M. D., Efstratiadis A., Maniatis T. Use of a cDNA library for studies on evolution and developmental expression of the chorion multigene families. Cell. 1979 Dec;18(4):1303–1316. doi: 10.1016/0092-8674(79)90241-1. [DOI] [PubMed] [Google Scholar]
- Vogt V. M. Purification and further properties of single-strand-specific nuclease from Aspergillus oryzae. Eur J Biochem. 1973 Feb 15;33(1):192–200. doi: 10.1111/j.1432-1033.1973.tb02669.x. [DOI] [PubMed] [Google Scholar]