Abstract
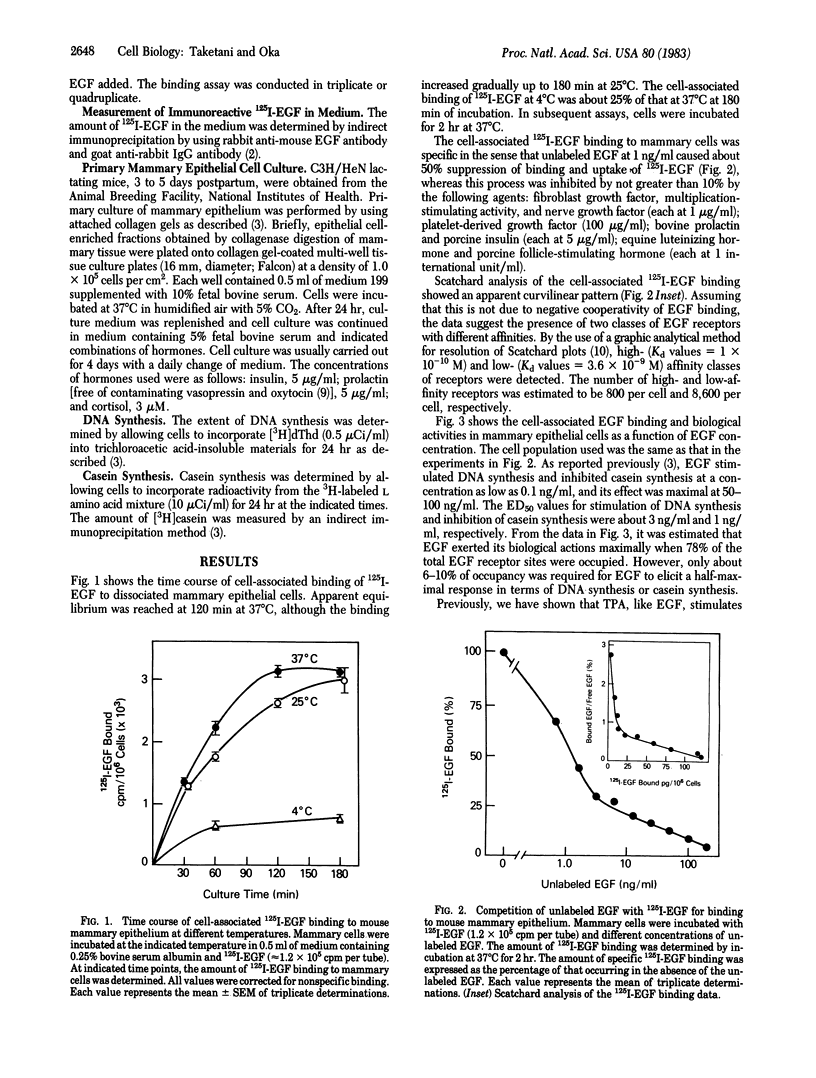
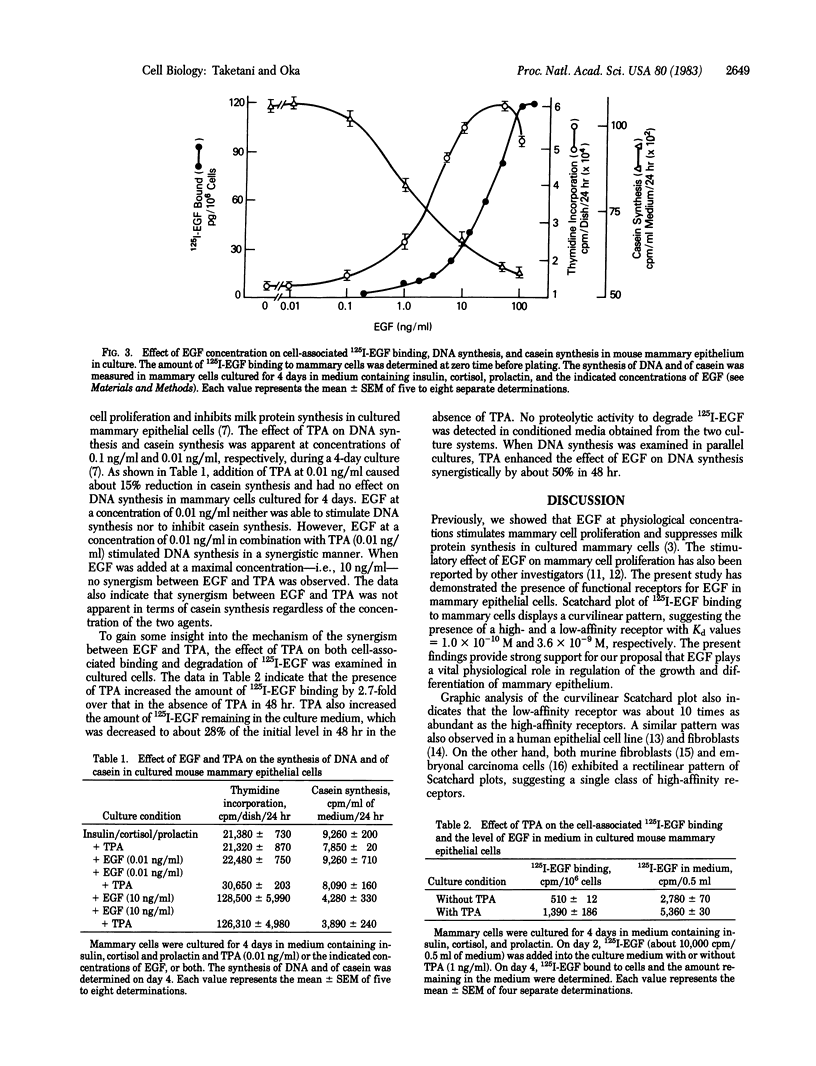
Normal murine mammary epithelial cells possessed the capacity to bind epidermal growth factor (EGF) in a specific and saturable manner. Scatchard plot analysis showed a curvilinear pattern. Assuming that this is not due to negative cooperativity of EGF binding, the data suggest the presence of two classes of receptors with a high and a low affinity: Kd values = 1 x 10(-10)M and 3.6 x 10(-9)M, respectively. The number of high- and low-affinity receptors was estimated to be 800 per cell and 8,600 per cell, respectively. The occupancy of EGF receptors for a half-maximal stimulation of DNA synthesis or inhibition of casein synthesis was about 10% and 6% of total receptors, respectively. A potent tumor promoter, 12-O-tetradecanoylphorbol 13-acetate, acted synergistically with EGF in terms of stimulation of DNA synthesis but not in terms of inhibition of casein synthesis when the two agents were added at a suboptimal concentration. The presence of the tumor promoter increased the amount of EGF bound to mammary cells in culture and also decreased a loss in the amount of EGF in the culture medium. These results indicate that mammary epithelial cells possess functional receptors for EGF, which are modulated by the tumor promoter.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aharonov A., Pruss R. M., Herschman H. R. Epidermal growth factor. Relationship between receptor regulation and mitogenesis in 3T3 cells. J Biol Chem. 1978 Jun 10;253(11):3970–3977. [PubMed] [Google Scholar]
- Ances I. G. Serum concentrations of epidermal growth factor in human pregnancy. Am J Obstet Gynecol. 1973 Feb 1;115(3):357–362. doi: 10.1016/0002-9378(73)90591-7. [DOI] [PubMed] [Google Scholar]
- Baker J. B., Cunningham D. D. Glucocorticoid-mediated alteration in growth factor binding and action: analysis of the binding change. J Supramol Struct. 1978;9(1):69–77. doi: 10.1002/jss.400090108. [DOI] [PubMed] [Google Scholar]
- Cohen S., Savage C. R., Jr Recent studies on the chemistry and biology of epidermal growth factor. Recent Prog Horm Res. 1974;30(0):551–574. doi: 10.1016/b978-0-12-571130-2.50018-3. [DOI] [PubMed] [Google Scholar]
- Gorden P., Carpentier J. L., Cohen S., Orci L. Epidermal growth factor: morphological demonstration of binding, internalization, and lysosomal association in human fibroblasts. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5025–5029. doi: 10.1073/pnas.75.10.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Haigler H. T., McKanna J. A., Cohen S. Direct visualization of the binding and internalization of a ferritin conjugate of epidermal growth factor in human carcinoma cells A-431. J Cell Biol. 1979 May;81(2):382–395. doi: 10.1083/jcb.81.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee L. S., Weinstein I. B. Epidermal growth factor, like phorbol esters, induces plasminogen activator in HeLa cells. Nature. 1978 Aug 17;274(5672):696–697. doi: 10.1038/274696a0. [DOI] [PubMed] [Google Scholar]
- Lee L. S., Weinstein I. B. Mechanism of tumor promoter inhibition of cellular binding of epidermal growth factor. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5168–5172. doi: 10.1073/pnas.76.10.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono M., Oka T. The differential actions of cortisol on the accumulation of alpha-lactalbumin and casein in midpregnant mouse mammary gland in culture. Cell. 1980 Feb;19(2):473–480. doi: 10.1016/0092-8674(80)90522-x. [DOI] [PubMed] [Google Scholar]
- Rees A. R., Adamson E. D., Graham C. F. Epidermal growth factor receptors increase during the differentiation of embryonal carcinoma cells. Nature. 1979 Sep 27;281(5729):309–311. doi: 10.1038/281309a0. [DOI] [PubMed] [Google Scholar]
- Rosenthal H. E. A graphic method for the determination and presentation of binding parameters in a complex system. Anal Biochem. 1967 Sep;20(3):525–532. doi: 10.1016/0003-2697(67)90297-7. [DOI] [PubMed] [Google Scholar]
- Shechter Y., Hernaez L., Cuatrecasas P. Epidermal growth factor: biological activity requires persistent occupation of high-affinity cell surface receptors. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5788–5791. doi: 10.1073/pnas.75.12.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., De Larco J. E., Todaro G. J. Biologically active phorbol esters specifically alter affinity of epidermal growth factor membrane receptors. Nature. 1979 May 31;279(5712):387–391. doi: 10.1038/279387a0. [DOI] [PubMed] [Google Scholar]
- Taketani Y., Oka T. Possible physiological role of epidermal growth factor in the development of the mouse mammary gland during pregnancy. FEBS Lett. 1983 Feb 21;152(2):256–260. doi: 10.1016/0014-5793(83)80391-3. [DOI] [PubMed] [Google Scholar]
- Taketani Y., Oka T. Tumor promoter 12-O-tetradecanoylphorbol 13-acetate, like epidermal growth factor, stimulates cell proliferation and inhibits differentiation of mouse mammary epithelial cells in culture. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1646–1649. doi: 10.1073/pnas.80.6.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takigawa M., Verma A. K., Simsiman R. C., Boutwell R. K. Polyamine biosynthesis and skin tumor promotion: inhibition of 12-O-tetradecanoylphorbol-13-acetate-promoted mouse skin tumor formation by the irreversible inhibitor of ornithine decarboxylase alpha-difluoromethylornithine. Biochem Biophys Res Commun. 1982 Apr 14;105(3):969–976. doi: 10.1016/0006-291x(82)91065-8. [DOI] [PubMed] [Google Scholar]
- Tonelli Q. J., Sorof S. Epidermal growth factor requirement for development of cultured mammary gland. Nature. 1980 May 22;285(5762):250–252. doi: 10.1038/285250a0. [DOI] [PubMed] [Google Scholar]
- Yang J., Guzman R., Richards J., Imagawa W., McCormick K., Nandi S. Growth factor- and cyclic nucleotide-induced proliferation of normal and malignant mammary epithelial cells in primary culture. Endocrinology. 1980 Jul;107(1):35–41. doi: 10.1210/endo-107-1-35. [DOI] [PubMed] [Google Scholar]


