Abstract
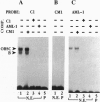
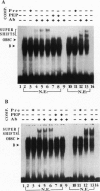
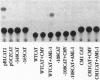
Tissue and cell-type specific expression of the rat osteocalcin (rOC) gene involves the interplay of multiple transcriptional regulatory factors. In this report we demonstrate that AML-1 (acute myeloid leukemia-1), a DNA-binding protein whose genes are disrupted by chromosomal translocations in several human leukemias, interacts with a sequence essential for enhancing tissue-restricted expression of the rOC gene. Deletion analysis of rOC promoter-chloramphenicol acetyltransferase constructs demonstrates that an AML-1-binding sequence within the proximal promoter (-138 to -130 nt) contributes to 75% of the level of osteocalcin gene expression. The activation potential of the AML-1-binding sequence has been established by overexpressing AML-1 in osteoblastic as well as in nonosseous cell lines. Overexpression not only enhances rOC promoter activity in osteoblasts but also mediates OC promoter activity in a nonosseous human fibroblastic cell line. A probe containing this site forms a sequence specific protein-DNA complex with nuclear extracts from osteoblastic cells but not from nonosseous cells. Antisera supershift experiments indicate the presence of AML-1 and its partner protein core-binding factor beta in this osteoblast-restricted complex. Mutations of the critical AML-1-binding nucleotides abrogate formation of the complex and strongly diminish promoter activity. These results indicate that an AML-1 related protein is functional in cells of the osteoblastic lineage and that the AML-1-binding site is a regulatory element important for osteoblast-specific transcriptional activation of the rOC gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen J. M., Forbush K. A., Perlmutter R. M. Functional dissection of the lck proximal promoter. Mol Cell Biol. 1992 Jun;12(6):2758–2768. doi: 10.1128/mcb.12.6.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronow M. A., Gerstenfeld L. C., Owen T. A., Tassinari M. S., Stein G. S., Lian J. B. Factors that promote progressive development of the osteoblast phenotype in cultured fetal rat calvaria cells. J Cell Physiol. 1990 May;143(2):213–221. doi: 10.1002/jcp.1041430203. [DOI] [PubMed] [Google Scholar]
- Bae S. C., Yamaguchi-Iwai Y., Ogawa E., Maruyama M., Inuzuka M., Kagoshima H., Shigesada K., Satake M., Ito Y. Isolation of PEBP2 alpha B cDNA representing the mouse homolog of human acute myeloid leukemia gene, AML1. Oncogene. 1993 Mar;8(3):809–814. [PubMed] [Google Scholar]
- Banerjee C., Stein J. L., Van Wijnen A. J., Frenkel B., Lian J. B., Stein G. S. Transforming growth factor-beta 1 responsiveness of the rat osteocalcin gene is mediated by an activator protein-1 binding site. Endocrinology. 1996 May;137(5):1991–2000. doi: 10.1210/endo.137.5.8612540. [DOI] [PubMed] [Google Scholar]
- Bidwell J. P., Van Wijnen A. J., Fey E. G., Dworetzky S., Penman S., Stein J. L., Lian J. B., Stein G. S. Osteocalcin gene promoter-binding factors are tissue-specific nuclear matrix components. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3162–3166. doi: 10.1073/pnas.90.8.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P., Karsenty G. Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol Cell Biol. 1995 Apr;15(4):1858–1869. doi: 10.1128/mcb.15.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill L. L., Zaninetta D., Karjalainen K. A transcriptional enhancer of the mouse T cell receptor delta gene locus. Eur J Immunol. 1991 Mar;21(3):807–810. doi: 10.1002/eji.1830210339. [DOI] [PubMed] [Google Scholar]
- Gottschalk L. R., Leiden J. M. Identification and functional characterization of the human T-cell receptor beta gene transcriptional enhancer: common nuclear proteins interact with the transcriptional regulatory elements of the T-cell receptor alpha and beta genes. Mol Cell Biol. 1990 Oct;10(10):5486–5495. doi: 10.1128/mcb.10.10.5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann H. M., Catron K. M., van Wijnen A. J., McCabe L. R., Lian J. B., Stein G. S., Stein J. L. Transcriptional control of the tissue-specific, developmentally regulated osteocalcin gene requires a binding motif for the Msx family of homeodomain proteins. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12887–12891. doi: 10.1073/pnas.91.26.12887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt K. H., Olson L., Moye-Rowley W. S., Pessin J. E. Phosphatidylinositol 3-kinase activation is mediated by high-affinity interactions between distinct domains within the p110 and p85 subunits. Mol Cell Biol. 1994 Jan;14(1):42–49. doi: 10.1128/mcb.14.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagoshima H., Shigesada K., Satake M., Ito Y., Miyoshi H., Ohki M., Pepling M., Gergen P. The Runt domain identifies a new family of heteromeric transcriptional regulators. Trends Genet. 1993 Oct;9(10):338–341. doi: 10.1016/0168-9525(93)90026-e. [DOI] [PubMed] [Google Scholar]
- Kamachi Y., Ogawa E., Asano M., Ishida S., Murakami Y., Satake M., Ito Y., Shigesada K. Purification of a mouse nuclear factor that binds to both the A and B cores of the polyomavirus enhancer. J Virol. 1990 Oct;64(10):4808–4819. doi: 10.1128/jvi.64.10.4808-4819.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeska R. J., Rodan S. B., Rodan G. A. Parathyroid hormone-responsive clonal cell lines from rat osteosarcoma. Endocrinology. 1980 Nov;107(5):1494–1503. doi: 10.1210/endo-107-5-1494. [DOI] [PubMed] [Google Scholar]
- Markose E. R., Stein J. L., Stein G. S., Lian J. B. Vitamin D-mediated modifications in protein-DNA interactions at two promoter elements of the osteocalcin gene. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1701–1705. doi: 10.1073/pnas.87.5.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnikova I. N., Crute B. E., Wang S., Speck N. A. Sequence specificity of the core-binding factor. J Virol. 1993 Apr;67(4):2408–2411. doi: 10.1128/jvi.67.4.2408-2411.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merriman H. L., van Wijnen A. J., Hiebert S., Bidwell J. P., Fey E., Lian J., Stein J., Stein G. S. The tissue-specific nuclear matrix protein, NMP-2, is a member of the AML/CBF/PEBP2/runt domain transcription factor family: interactions with the osteocalcin gene promoter. Biochemistry. 1995 Oct 10;34(40):13125–13132. doi: 10.1021/bi00040a025. [DOI] [PubMed] [Google Scholar]
- Meyers S., Downing J. R., Hiebert S. W. Identification of AML-1 and the (8;21) translocation protein (AML-1/ETO) as sequence-specific DNA-binding proteins: the runt homology domain is required for DNA binding and protein-protein interactions. Mol Cell Biol. 1993 Oct;13(10):6336–6345. doi: 10.1128/mcb.13.10.6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers S., Lenny N., Hiebert S. W. The t(8;21) fusion protein interferes with AML-1B-dependent transcriptional activation. Mol Cell Biol. 1995 Apr;15(4):1974–1982. doi: 10.1128/mcb.15.4.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecino M., Pockwinse S., Lian J., Stein G., Stein J. DNase I hypersensitive sites in promoter elements associated with basal and vitamin D dependent transcription of the bone-specific osteocalcin gene. Biochemistry. 1994 Jan 11;33(1):348–353. doi: 10.1021/bi00167a045. [DOI] [PubMed] [Google Scholar]
- Nuchprayoon I., Meyers S., Scott L. M., Suzow J., Hiebert S., Friedman A. D. PEBP2/CBF, the murine homolog of the human myeloid AML1 and PEBP2 beta/CBF beta proto-oncoproteins, regulates the murine myeloperoxidase and neutrophil elastase genes in immature myeloid cells. Mol Cell Biol. 1994 Aug;14(8):5558–5568. doi: 10.1128/mcb.14.8.5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa E., Inuzuka M., Maruyama M., Satake M., Naito-Fujimoto M., Ito Y., Shigesada K. Molecular cloning and characterization of PEBP2 beta, the heterodimeric partner of a novel Drosophila runt-related DNA binding protein PEBP2 alpha. Virology. 1993 May;194(1):314–331. doi: 10.1006/viro.1993.1262. [DOI] [PubMed] [Google Scholar]
- Ogawa E., Maruyama M., Kagoshima H., Inuzuka M., Lu J., Satake M., Shigesada K., Ito Y. PEBP2/PEA2 represents a family of transcription factors homologous to the products of the Drosophila runt gene and the human AML1 gene. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6859–6863. doi: 10.1073/pnas.90.14.6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen T. A., Bortell R., Yocum S. A., Smock S. L., Zhang M., Abate C., Shalhoub V., Aronin N., Wright K. L., van Wijnen A. J. Coordinate occupancy of AP-1 sites in the vitamin D-responsive and CCAAT box elements by Fos-Jun in the osteocalcin gene: model for phenotype suppression of transcription. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9990–9994. doi: 10.1073/pnas.87.24.9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge N. C., Frampton R. J., Eisman J. A., Michelangeli V. P., Elms E., Bradley T. R., Martin T. J. Receptors for 1,25(OH)2-vitamin D3 enriched in cloned osteoblast-like rat osteogenic sarcoma cells. FEBS Lett. 1980 Jun 16;115(1):139–142. doi: 10.1016/0014-5793(80)80744-7. [DOI] [PubMed] [Google Scholar]
- Pikaart M., Feng J., Villeponteau B. The polyomavirus enhancer activates chromatin accessibility on integration into the HPRT gene. Mol Cell Biol. 1992 Dec;12(12):5785–5792. doi: 10.1128/mcb.12.12.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo J. M., Pfohl J. L., Hernandez-Munain C., Wang S., Speck N. A., Krangel M. S. Indistinguishable nuclear factor binding to functional core sites of the T-cell receptor delta and murine leukemia virus enhancers. Mol Cell Biol. 1992 Nov;12(11):4817–4823. doi: 10.1128/mcb.12.11.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake M., Inuzuka M., Shigesada K., Oikawa T., Ito Y. Differential expression of subspecies of polyomavirus and murine leukemia virus enhancer core binding protein, PEBP2, in various hematopoietic cells. Jpn J Cancer Res. 1992 Jul;83(7):714–722. doi: 10.1111/j.1349-7006.1992.tb01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satake M., Nomura S., Yamaguchi-Iwai Y., Takahama Y., Hashimoto Y., Niki M., Kitamura Y., Ito Y. Expression of the Runt domain-encoding PEBP2 alpha genes in T cells during thymic development. Mol Cell Biol. 1995 Mar;15(3):1662–1670. doi: 10.1128/mcb.15.3.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speck N. A., Renjifo B., Golemis E., Fredrickson T. N., Hartley J. W., Hopkins N. Mutation of the core or adjacent LVb elements of the Moloney murine leukemia virus enhancer alters disease specificity. Genes Dev. 1990 Feb;4(2):233–242. doi: 10.1101/gad.4.2.233. [DOI] [PubMed] [Google Scholar]
- Suzow J., Friedman A. D. The murine myeloperoxidase promoter contains several functional elements, one of which binds a cell type-restricted transcription factor, myeloid nuclear factor 1 (MyNF1). Mol Cell Biol. 1993 Apr;13(4):2141–2151. doi: 10.1128/mcb.13.4.2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornell A., Hallberg B., Grundström T. Differential protein binding in lymphocytes to a sequence in the enhancer of the mouse retrovirus SL3-3. Mol Cell Biol. 1988 Apr;8(4):1625–1637. doi: 10.1128/mcb.8.4.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topp W. C. Normal rat cell lines deficient in nuclear thymidine kinase. Virology. 1981 Aug;113(1):408–411. doi: 10.1016/0042-6822(81)90168-9. [DOI] [PubMed] [Google Scholar]
- Wang S. W., Speck N. A. Purification of core-binding factor, a protein that binds the conserved core site in murine leukemia virus enhancers. Mol Cell Biol. 1992 Jan;12(1):89–102. doi: 10.1128/mcb.12.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Wang Q., Crute B. E., Melnikova I. N., Keller S. R., Speck N. A. Cloning and characterization of subunits of the T-cell receptor and murine leukemia virus enhancer core-binding factor. Mol Cell Biol. 1993 Jun;13(6):3324–3339. doi: 10.1128/mcb.13.6.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. E., Fujioka K., Hetherington C. J., Shapiro L. H., Chen H. M., Look A. T., Tenen D. G. Identification of a region which directs the monocytic activity of the colony-stimulating factor 1 (macrophage colony-stimulating factor) receptor promoter and binds PEBP2/CBF (AML1). Mol Cell Biol. 1994 Dec;14(12):8085–8095. doi: 10.1128/mcb.14.12.8085. [DOI] [PMC free article] [PubMed] [Google Scholar]